Abstract
Vertically transmitted hepatitis B virus (HBV) usually causes chronic infection. While combined active–passive immunoprophylaxis in neonates of hepatitis B surface antigen-positive (HBsAg+) mothers at birth prevents vertical transmission, it is not yet clear whether neonates encounter the virus or its products in the absence of hepatitis B e antigen (HBeAg). This study was undertaken to investigate HBV antigen-specific T-cell responses in vaccinated neonates of HBsAg+/HBeAg− mothers. Blood was collected from 46 HBsAg+ mothers and their neonates (subjects) as well as 24 age-matched controls. All neonates of HBsAg+ mothers received appropriate immunoprophylaxis, and HBsAg and hepatitis B surface antibody (anti-HBs) antibody titers were determined after completion of the vaccination course. Peripheral blood mononuclear cells (PBMCs) from infants at birth, 1 and 6 months of age were stimulated with recombinant HBsAg, hepatitis B core antigen (HBcAg) and mitogen, and interferon (IFN)-γ concentrations were determined by ELISA. HBsAg-induced production of IL-2, IL-5, IL-6 and IL-10 was assessed using a cytometric bead array kit on cells from 6-month-old neonates post-vaccination. All neonates were HBsAg− and responded to vaccination. Increased IFN-γ production following HBcAg stimulation was seen in 30.4% of neonates born to HBsAg+/HBeAg− mothers. Subjects demonstrated significantly higher IL-2 production post-HBsAg stimulation, whereas IL-5, IL-6 and IL-10 cytokine responses were not significantly different. Almost one-third of uninfected neonates developed viral antigen-induced IFN-γ production, suggesting that they had been exposed to virions or viral derivatives. This encounter, however, did not impair their T-cell responses to vaccination.
Keywords: HBV, neonates, HBcAg, vaccine, cytokine, PBMC
Introduction
Hepatitis B virus (HBV) infection remains a major public health problem affecting 350 million people worldwide despite important achievements in prevention and treatment. Mother-to-infant transmission is crucial in the maintenance of the hepatitis B surface antigen (HBsAg) chronic carrier reservoir because the age of infection is the most determining factor in developing chronicity. If infection occurs after vertical transmission, there is a 95% risk of the patient developing a chronic infection.1, 2 In the absence of vaccination, neonates born to HBsAg+ or hepatitis B e antigen-positive (HBeAg+) mothers have a 70–90% risk of infection, while neonates born to HBsAg+/HBeAg− mothers have a 10–30% risk of infection.3, 4 Although the exact mechanism of vertical transmission is still unclear, it has been postulated that passage of HBV virions or infected PBMCs through the placenta or during the perinatal period is involved.5, 6, 7 Identified risk factors for the development of infection include high maternal perinatal viremia and the transplacental passage of HBeAg.8, 9 Both infected and uninfected infants have been shown to encounter HBeAg in utero, but exposure to hepatitis B core antigen (HBcAg), which would indicate possible exposure to virions, has not been documented in babies of either HBeAg+ or HBeAg− mothers.8, 10 Prevention of mother-to-infant transmission has been largely achieved by the administration of passive hepatitis B immunoglobulin and active immunoprophylaxis to all neonates born to carrier mothers. However, the added benefit of passive immunoprophylaxis in newborns of HBsAg+/HBeAg− carrier mothers has recently been questioned. Currently, the recommended schedules for the prevention of vertical transmission vary significantly among countries and depend mainly on the HBeAg prevalence.
The establishment of chronicity after vertical transmission has been mainly attributed to the physiologically immature immune system of the neonate. However, the hepatitis B vaccine induces a vigorous antibody response early in most infants that correlates with the protective efficacy of vaccination against mother-to-infant transmission.11 This protection is achieved by the production of HBsAg-specific T helper 1 (Th1) and Th2 cells. A few reports have shown that uninfected infants born to HBsAg+/HBeAg− mothers have impaired HBsAg-specific T-cell responses that correlate with immunization failure and the presence of HBV DNA in PBMCs.7, 12
The aim of the present study was to investigate the T-cell immune responses of uninfected neonates born to HBsAg+/HBeAg− mothers against HBV antigens and vaccination. Th1 and Th2 cytokine production was assessed following HBcAg and HBsAg stimulation in infants at birth, 1 and 6 months of age. Because HBcAg is not included in the vaccine, any HBcAg-induced responses could be due to a previous encounter with HBV virions or viral derivatives. Furthermore, adaptive immune responses to HBsAg were compared to that of control infants to investigate whether potential exposure influences the response to vaccination.
Materials and methods
Subjects and vaccination scheme
All subjects selected for this study were born to HBsAg+/HBeAg−, HCV- and HIV-negative mothers who had no clinical or laboratory signs of active hepatitis B at the time of delivery and had not received any antiviral treatment during pregnancy. All newborns were born at term (37–41 gestation weeks) and birth weight was greater than 2500 g. In accordance with national recommendations, subjects received one dose of 200 IU of hepatitis B immunoglobulin and their first hepatitis B vaccine (Engerix; GlaxoSmithKline Biologicals, Rixensart, Belgium) within 24 h of birth. The hepatitis B vaccination was completed by another two doses of monovalent vaccine at 1 and 6 months of age. Blood samples from neonates were collected by peripheral vein puncture at birth (before the administration of passive–active immunoprophylaxis); at 1 month of age (before the second vaccination dose); and at 6 months of age (10–14 days after the last vaccination dose). Because infants were not examined at all three time points, data were analyzed as a cross-sectional study (Supplementary Figure 1). All vaccinated neonates were re-examined at 7 months of age for hepatitis B surface antibody (anti-HBs) quantification.
The control group consisted of infants born to HBV-, HCV- and HIV-negative mothers who were followed in our outpatient clinic. In accordance with national guidelines, they received three doses of hepatitis B vaccine using combined vaccine (Infarix-Hexa; GlaxoSmithKline Biologicals) at 2, 4 and 6 months of age. Blood sampling in control infants took place at the same time points as the subject infants and was coupled with routine hematologic work-ups.
This research protocol was approved by the Ethics Committees of both Alexandra Maternity General Hospital and the Second Pediatric Clinic of the University of Athens (P&A Kyriakou Children's Hospital). All mothers included in the study signed informed consent forms.
Serological HBV markers and HBV DNA
Hepatitis B serological markers and HBV DNA levels were determined in all mothers enrolled in the study on the day of delivery. Plasma HBsAg and HBeAg were assayed by ELISA Kits (AXSYM; Abbot Laboratories, Abbott Park, IL, USA). HBV DNA was quantified using an ultrasensitive in-house real-time detection PCR (RTD-PCR) assay. The HBV DNA was extracted from 0.5 ml serum using the QIAamp UltraSens Virus Kit (QIAGEN Inc., Valencia, CA, USA) and eluted in 60 µl elution buffer. The RTD-PCR was carried out in the LightCycler II apparatus using RTD-PCR conditions described previously.13 The 95% HBV DNA detection end point of the assay was 22 IU/ml or 60 copies/ml.14
Hepatitis B antigens
A purified preparation of yeast-derived recombinant HBsAg without vaccine additives (GlaxoSmithKline Biologicals) and two purified, truncated, Escherichia coli-derived recombinant hepatitis B core antigens (rHBcAgs) corresponding to amino acid sequences 1–127 (rHBcAg127) and 1–145 (rHBcAg145) (Rhein Biotech Dynavax, Düsseldorf, Germany) were used. Both antigen and peptide variants were >95% pure. The peptide concentration for the stimulation of HBsAg- and HBcAg-specific T cells was optimized at 10 µg/ml (data not shown) and was used throughout the study.
In vitro lymphocyte responses to HBV antigens
PBMCs were isolated by Ficoll density gradient centrifugation (Sigma, St Louis, MO, USA) and resuspended in complete RPMI-1640 medium (Cambrex Bio Sciences Walkersville, Inc., Walkersville, MD, USA) supplemented with 2 mM L-glutamine (Cambrex Bio Sciences Walkersville, Inc.), 1% non-essential amino acids (Cambrex Bio Sciences Walkersville, Inc.), 10% human AB serum (Sigma) and antibiotics penicillin (100 U/ml) and streptomycin (100 µg/ml) (both from Cambrex Bio Sciences Walkersville, Inc.). PBMCs were seeded at 1×106 cells/ml in a 48-well culture plate (Corning Inc., Corning, NY, USA) with 10 µg/ml phytohemagglutinin mitogen (PHA-L; Sigma), 10 µg/ml recombinant HBsAg, 10 µg/ml rHBcAg127 and 10 µg/ml rHBcAg145 (in the same well) or medium alone. Following a 72-h incubation at 37 °C in a humidified CO2 (5%) incubator, culture supernatants were collected and stored at −20 °C until use.
Cytokine quantification
The levels of IFN-γ were determined by sandwich ELISA using a commercial kit (BD Biosciences, San Diego, CA, USA) according to the manufacturer's instructions. The detection limit of the assay was 4 pg/ml.
The levels of IL-2, IL-5, IL-6 and IL-10 in the supernatants of cultured PBMCs were assessed by flow cytometry using the human cytometric bead array as per the manufacturer's instructions (BD Biosciences, San Jose, CA, USA). Briefly, capture bead populations with distinct fluorescence intensities were coated with cytokine-specific capture antibodies. Volumes of 50 µl of the recombinant standards or supernatants, 50 µl of phycoerythrin-conjugated detection antibodies and 50 µl of the mixed-bead population were mixed and incubated for 3 h. The newly formed sandwich complexes were then washed, and the sample data were acquired on a FACSCalibur with BD Cytometric Bead Array Software (BD Biosciences). Cytokine concentrations were quantified using FACSDiva and FCAP Array software.
Statistical analysis
Differences in numeric variables between groups were analyzed by the non-parametric Mann–Whitney test with two-tailed P values. Results were expressed as mean±standard error of the mean, and a P value of <0.05 was considered statistically significant. Statistical calculations were performed using the GraphPad Prism Software (version 3) program.
Results
Population description
The study population consisted of 23 neonatal subjects born to HBsAg+/HBeAg− mothers who were followed during their first 7 months of life for a total of 46 observations (Supplementary Figure 1). Neonates of 24 healthy mothers served as controls. Perinatal viremia was detected in 54.3% of carrier mothers with a median viral load of 3.6±3.4 log10 copies/ml on the day of delivery. All infants remained HBsAg− and had anti-HBs antibody levels above 10 IU/l at the age of 7 months.
Cytokine responses against HBV antigens
Spontaneous and mitogen (PHA)-induced cytokine secretion was comparable among subjects and controls at all ages (Figure 1a). Cytokine responses against PHA, HBcAg and HBsAg were similar between neonates born to viremic and non-viremic mothers as well as to control neonates (P>0.005).
Figure 1.
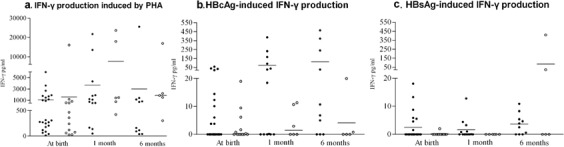
In vitro IFN-γ production from PBMCs in subjects and controls. PBMC secretion of IFN-γ after a 72-h incubation with PHA (a), HBcAg (b) and HBsAg (c) from 23 neonatal subjects born to HBsAg+ mothers (•) and 24 neonatal controls (○) at birth, 1 and 6 months of age. The data were compared by Mann–Whitney test: no significant differences were found between the two groups (P>0. 005). Each dot represents a single donor and horizontal bars represent the mean values. HBcAg, hepatitis B core antigen; HBsAg, hepatitis B surface antigen; IFN, interferon; PBMC, peripheral blood mononuclear cell; PHA, phytohemagglutinin.
IFN-γ production following HBcAg stimulation in the subject group was higher than that in the control group, but this difference was not significant (P>0.05). Although controls were born to HBV-negative mothers, low but detectable IFN-γ secretion in HBcAg-treated cells was seen in eight out of 24 individuals; the magnitude of these responses was below 20 pg/ml (Figure 1b). This phenomenon was attributed to bacterial contamination because HBcAg was E. coli derived and was >95% pure. To avoid false-positive results, only subjects that responded with >20 pg/ml IFN-γ production were considered to have positive HBcAg-specific responses. Figure 2 shows IFN-γ production from the subjects that responded to HBcAg. When these responses were compared to those of age-matched controls, differences were statistically significant at birth, 1 and 6 months of age (P=0.0039, P=0.0022 and P=0.0129, respectively). The responses to HBcAg were not correlated with maternal viremia in the subject group.
Figure 2.
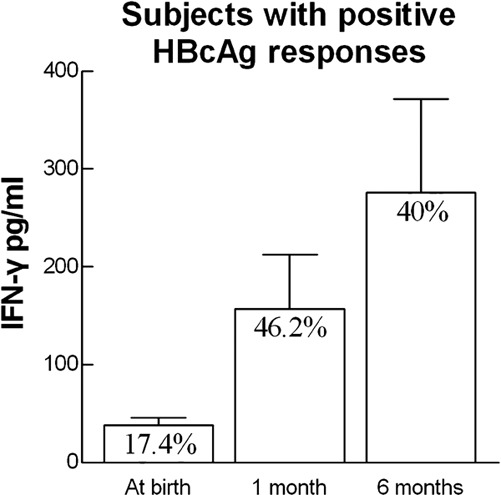
IFN-γ secretion by PBMCs of subjects that responded to HBcAg stimulation. The percentages of responders as well as the secreted IFN-γ levels from PBMCs after a 72-h incubation with HBcAg at birth, 1 and 6 months of age are shown. HBcAg, hepatitis B core antigen; IFN, interferon; PBMC, peripheral blood mononuclear cell.
IFN-γ concentrations following HBsAg stimulation were below 25 pg/ml, except for one 6-month-old control subject who responded with 400 pg/ml. There were no significant differences between subjects and controls at all ages examined (Figure 1c).
Secretion of IL-2, IL-5, IL-6 and IL-10 was assessed at 6 months of age and after HBsAg stimulation in eight neonates, six of which were born to viremic mothers (4.2±4 log10 vc/ml) and five controls (Figure 3). A significantly increased production of IL-2 was observed in the subject group compared to the control group (P=0.02). IL-5 and IL-6 secretions were also higher in the subject group, but this difference was not statistically significant (P=0.17 and P=0.09, respectively). Cytokine production was not associated with the maternal levels of HBV DNA, and IL-10 secretion was comparably low in both groups (P=0.94).
Figure 3.
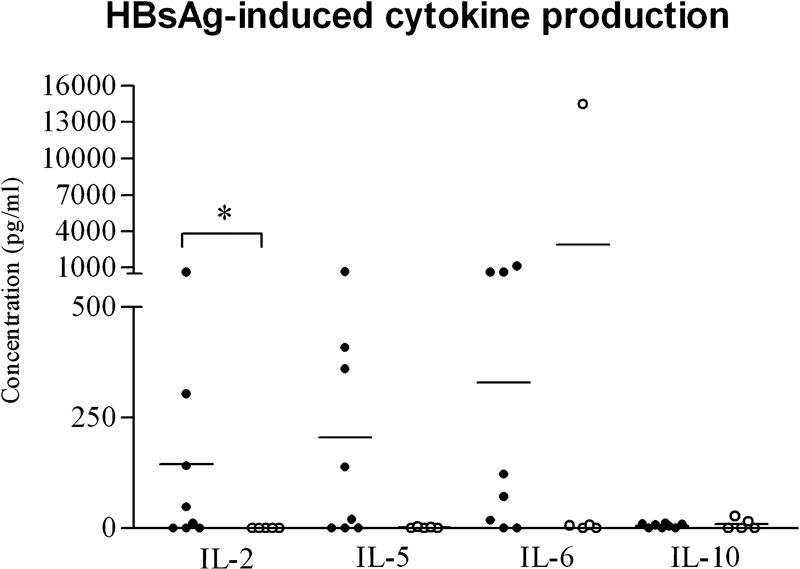
In vitro cytokine production from PBMCs after HBsAg stimulation in 6-month-old subjects and controls. PBMC secretion of IL-2, IL-5, IL-6 and IL-10 after a 72-h incubation with HBsAg from eight neonatal subjects born to HBsAg+ mothers (•) and five neonatal controls (○) at 6 months of age. The data were compared by Mann–Whitney test: IL-2 expression was significantly higher in the subject group compared to the control group (P<0.005). Each dot represents a single donor and horizontal bars represent the mean values. HBsAg, hepatitis B surface antigen; PBMC, peripheral blood mononuclear cell.
Discussion
T helper cells can be categorized as Th1 or Th2 cells according to the cytokines they produce. Th1 cells are characterized by IL-2, IFN-γ and transforming growth factor-β production, whereas Th2 cells secrete mainly IL-4, IL-5 and IL-6. Both Th1 and Th2 responses against HBV antigens are required for successful immunoprophylaxis or clearance of the virus. The high incidence of infection and the development of chronic infection in infants born to HBsAg-carrier mothers have been attributed to viral antigen-specific unresponsiveness of both Th1 and Th2 cells.7, 15, 16 In neonates born to HBsAg+/HBeAg+ mothers, immune tolerance is further induced by transplacental passage of HBeAg that can result in specific tolerance of T helper cells to both HBeAg and HBcAg because these two antigens are crossreactive.6, 17 However, intrauterine infection and immunoprophylactic failure have also been reported in infants of HBsAg+/HBeAg− mothers.7, 18
Most studies of neonatal T-cell responses against HBV have focused on the immune responses elicited by HBsAg in vaccinated healthy infants.15, 16, 19 In the present study, all subjects were born to HBsAg+/HBeAg− mothers, of which 54.3% had detectable HBV viremia on the day of delivery. All subject neonates remained uninfected post-immunoprophylaxis and responded to vaccination, with anti-HBs titers above 10 IU/l.20 To evaluate the effect of the HBV-infected maternal environment on neonatal immune responses, we assessed HBcAg-induced IFN-γ production at birth, 1 and 6 months of age. Although there was detectable cytokine production in the control group, the magnitude of these responses was significantly higher in almost one-third (30.4%) of the patients (Figure 1b). Because we used an E. coli-derived recombinant HBcAg preparation that was >95% pure to stimulate the PBMCs throughout the study, a <0.5 µg/ml concentration of bacterial protein may have contributed to the low but detectable IFN-γ production in the control group. Although not using a control antigen derived from E. coli limits the study, the magnitude of the HBcAg-induced responses in the patients was increased by up to 20-fold, and we used a threshold point to avoid false-positive results (Figures 1b and 2). In addition, differences in antigen-induced cytokine production did not correlate to spontaneous or mitogen-stimulated cell responses (Figure 1a); therefore, cytokine secretion was not caused by a non-specific reaction or by generalized immune dysfunction.
To explain these observed specific IFN-γ responses, one could postulate that neonates were exposed to virions in utero or during the delivery. In the newborn subjects that elicited HBcAg-specific T-cell responses at birth, viral encounter must have taken place in utero, because if this encounter was during birth there could not have been sufficient time for the development of antigen specific immune responses. However, neonates that developed responses at 1 month of age or older might have been exposed at birth. The fact that HBcAg-specific IFN-γ secretion in newborn subjects was not associated with maternal perinatal HBV DNA levels could be due to the small number of subjects analysed. One may wonder why these neonates did not get infected, because they could not have been protected by perinatal administration of immunoprophylaxis. The absence of infection in this group was most likely due to low maternal viral load (3.6±3.4 log10 copies/ml), the absence of HBeAg and/or the efficiency of innate and adaptive immune responses. The finding that HBcAg-induced IFN-γ production increases with age and that proportionally more neonates responded at 1 or 6 months of age may be due to the physiologic functional T-cell and dendritic cell maturational process during infancy. Alternatively, the finding may be explained by the higher probability that encounter with virions or viral derivatives took place during the delivery rather than in utero.
The responses to vaccination were assessed by measuring cytokine secretion following HBsAg stimulation. At birth, although subjects were unvaccinated, HBsAg-specific IFN-γ production was observed in four neonates in the subject group, which did not correlate with mitogen responses or with maternal viremia. Interestingly, these four neonates also had HBcAg-specific IFN-γ production. This finding further suggests the possibility that these newborns were exposed to virions or viral derivatives in utero.21
HBV DNA presence in the serum and PBMCs of uninfected infants born to HBsAg+/HBeAg− mothers has been shown in previous reports.7, 18 Interestingly, PBMCs have been found to be persistently positive for HBV DNA during the first year of life but the serum is negative for HBV DNA and HBsAg. Similarly to HBV, specific T-cell responses have been detected in uninfected but in-utero-exposed infants born to HIV-positive mothers during the first year of life.22, 23 However, it is not clear whether antigen-specific responses observed in neonates are due to encounter with free particles. HBV-specific T-cell responses could have been induced by maternal anti-idiotypic antibodies crossing the placenta24 or by the passage of small quantities of HBV virions and free HBV proteins that are unable to establish a productive infection but could prime HBV-specific T cells, as seen in other infections.
To evaluate the influence of HBV maternal infection on neonatal vaccination outcomes, HBsAg-induced Th1 and Th2 cytokine production was assessed 10 to 14 days after the completion of the vaccination course.19 IFN-γ production in 6-month-old subjects was as low as the age-matched controls. The level of IL-2 was significantly higher in subjects than in controls. IL-2 plays a crucial role in the development of anti-HBs antibody responses: it further induces IFN-γ secretion and promotes the proliferation and differentiation of B lymphocytes. Levels of the Th2 cytokines IL-5 and IL-6 were also higher in the subject group, although not to a statistically significant level, and IL-10 was comparably low in both groups. Cytokine responses were not correlated with anti-HBs antibody titers or maternal viremia. Although all neonates responded to vaccination, the production of IL-2, IL-5 and IL-6 following HBsAg stimulation in the control group as well as IFN-γ and IL-10 production in both groups resembled what would be expected in non-responder neonates.19 Therefore, other immunological pathways are involved in the development of anti-HBs antibody production in neonatal life. Jafarzadeh et al. have shown that HBsAg-specific Th1 and Th2 production in vaccinated neonates varies significantly among individuals and does not correlate with anti-HBs antibody production. In our present study, the differences observed in HBsAg responses between subjects and controls may be attributed to the different vaccination courses they received. Neonates from the subject group received active and passive immunoprophylaxis at birth and some may have encountered HBsAg before birth.
The main weaknesses of this study are the small number of subjects and the cross-sectional design. Although parents were asked to bring neonates back to the clinic for follow-up, not all children were examined at all three time points. All control infants were examined at one time point during their vaccination course and another one after its completion during routine examination in our outpatient department.
Taken together, our results indicate that even in the absence of maternal HBeAg and in low maternal viremia, one-third of uninfected neonates show evidence of specific T helper cell responses. These responses could be due to intrauterine and/or perinatal encounter with virions and/or vital particles. This encounter, however, does not impair neonatal T-cell responses to vaccination. Additional studies from our group and other reports have shown that circulating and cord blood dendritic cells from uninfected neonates of carrier mothers are numerically and functionally efficient to present HBsAg.25, 26 The presence of maternal viremia does not impair neonatal immune responses to vaccination and is not correlated with the development of viral antigen-specific cytokine production. This correlation would have been an oversimplified explanation to a much more complex issue because a single time point measurement of HBV viremia cannot predict viral antigen encounter. More information about the immune responses during mother-to-infant transmission is critical to the understanding of HBV infection and vaccination in neonatal life.
Acknowledgments
The authors thank Glaxo SmithKline Biologicals, Belgium, and Rhein Biotech Dynavax, Germany, for kindly providing the purified preparations of HBsAg and HBc antigens, respectively. We are particularly grateful to the subjects and the mothers who allowed their babies to be enrolled in the study. We also thank Dr Apostolos Bossios and Dr Christina Piperi for their consultation assistance in developing the experimental techniques used in this study. The study was cofunded by the European Social Fund and National Resources—(EPEAEK II) PYTHAGORAS and was also supported by a European Society of Pediatric Diseases Small Grant and a fellowship from the European Society of Clinical Microbiology and Infectious Diseases.
Footnotes
Note: Supplementary information is available on the Cellular & Molecular Immunology website (http://www.nature.com/cmi/).
Supplementary Information
References
- Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992–1000. doi: 10.1093/clinids/20.4.992. [DOI] [PubMed] [Google Scholar]
- McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- Akhter S, Talukder MQ, Bhuiyan N, Chowdhury TA, Islam MN, Begum S. Hepatitis B virus infection in pregnant mothers and its transmission to infants. Indian J Pediatr. 1992;59:411–415. doi: 10.1007/BF02751551. [DOI] [PubMed] [Google Scholar]
- Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771–774. doi: 10.1056/NEJM197504102921503. [DOI] [PubMed] [Google Scholar]
- Lin HH, Lee TY, Chen DS, Sung JL, Ohto H, Etoh T, et al. Transplacental leakage of HBeAg-positive maternal blood as the most likely route in causing intrauterine infection with hepatitis B virus. J Pediatr. 1987;111 (6 Pt 1)::877–881. doi: 10.1016/s0022-3476(87)80210-x. [DOI] [PubMed] [Google Scholar]
- Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero. Proc Natl Acad Sci USA. 1990;87:6599–6603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Meng J, Zhang S. Mechanism of peripheral blood mononuclear cell invasion by HBV on artificial immunization in newborns. Chin Med J (Engl) 2002;115:1380–1382. [PubMed] [Google Scholar]
- Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38:1075–1086. doi: 10.1053/jhep.2003.50453. [DOI] [PubMed] [Google Scholar]
- Ranger-Rogez S, Alain S, Denis F.Hepatitis viruses: mother to child transmission Pathol Biol (Paris) 200250568–575.French. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhang J, Yang H, Li X, Wen S, Guo Y, et al. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J Med Virol. 2003;71:360–366. doi: 10.1002/jmv.10493. [DOI] [PubMed] [Google Scholar]
- Kane M. Global programme for control of hepatitis B infection. Vaccine. 1995;13 (Suppl. 1:S47–S49. doi: 10.1016/0264-410x(95)80050-n. [DOI] [PubMed] [Google Scholar]
- Lazizi Y. Trasplacental passage of hepatitis B virus DNA from hepatitis B e antigen-negative mothers and delayed immune response in newborns. J Infec Dis. 1994;169:704–706. doi: 10.1093/infdis/169.3.704. [DOI] [PubMed] [Google Scholar]
- Paraskevis D, Haida C, Tassopoulos N, Raptopoulou M, Tsantoulas D, Papachristou H, et al. Development and assessment of a novel real-time PCR assay for quantitation of HBV DNA. J Virol Methods. 2002;103:201–212. doi: 10.1016/s0166-0934(02)00033-2. [DOI] [PubMed] [Google Scholar]
- Paraskevis D, Katsoulidou C, Moschidis Z, Hatzitheodorou E, Varaklioti A, Hatzakis A. Proceeding of XVIIth Regional Congress. Madrid: International Society of Blood Transfusion; 2007. Development of a flexible and sensitive in-house real-time PCR assay for the quantification of HBV DNA. [Google Scholar]
- Jafarzadeh A, Shokri F. The antibody response to HBs antigen is regulated by coordinated Th1 and Th2 cytokine production in healthy neonates. Clin Exp Immunol. 2003;131:451–456. doi: 10.1046/j.1365-2249.2003.02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota MO, Vekemans J, Schlegel-Haueter SE, Fielding K, Whittle H, Lambert PH, et al. Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine. 2004;22:511–519. doi: 10.1016/j.vaccine.2003.07.020. [DOI] [PubMed] [Google Scholar]
- Brunetto MR, Giarin MM, Oliveri F, Chiaberge E, Baldi M, Alfarano A, et al. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc Natl Acad Sci USA. 1991;88:4186–4190. doi: 10.1073/pnas.88.10.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontisso P, Vidalino L, Quarta S, Gatta A. Biological and clinical implications of HBV infection in peripheral blood mononuclear cells. Autoimmun Rev. 2008;8:13–17. doi: 10.1016/j.autrev.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Jafarzadeh A, Kardar G, Khoshnoodi J, Shokri F. Downregulation of IL-12 production in healthy non-responder neonates to recombinant hepatitis B vaccine. Iran Biomed J. 2004;8:41–45. [Google Scholar]
- Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs. 2003;63:1021–1051. doi: 10.2165/00003495-200363100-00006. [DOI] [PubMed] [Google Scholar]
- Chang MH. Hepatitis B virus infection. Semin Fetal Neonatal Med. 2007;12:160–167. doi: 10.1016/j.siny.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Borkowsky W, Krasinski K, Moore T, Papaevangelou V. Lymphocyte proliferative responses to HIV-1 envelope and core antigens by infected and uninfected adults and children. AIDS Res Hum Retroviruses. 1990;6:673–678. doi: 10.1089/aid.1990.6.673. [DOI] [PubMed] [Google Scholar]
- Rich KC, Siegel JN, Jennings C, Rydman RJ, Landay AL. Function and phenotype of immature CD4+ lymphocytes in healthy infants and early lymphocyte activation in uninfected infants of human immunodeficiency virus-infected mothers. Clin Diagn Lab Immunol. 1997;4:358–361. doi: 10.1128/cdli.4.3.358-361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pride MW, Thakur A, Thanavala Y. Mimicry of the a determinant of hepatitis B surface antigen by an antiidiotypic antibody. I. Evaluation in hepatitis B surface antigen responder and nonresponder strains. J Exp Med. 1993;177:127–134. doi: 10.1084/jem.177.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumbi L, Papadopoulos N, Anastassiadou V, Machaira M, Kafetzis D, Papaevangelou V.Dendritic cells in uninfected infants born to hepatitis B virus positive mothers Clin Vaccine Immunol 2010. in press. [DOI] [PMC free article] [PubMed]
- Li RB, Chen HS, Xie Y, Fei R, Cong X, Jiang D, et al. Dendritic cells from chronic hepatitis B patients can induce HBV antigen-specific T cell responses. World J Gastroenterol. 2004;10:1578–1582. doi: 10.3748/wjg.v10.i11.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


