Abstract
Increasing evidence indicates a role for regulatory T cells (Tregs) in the immune response and in autoimmune diseases, but the role of Tregs and cytokines in autoimmune hepatic diseases remains largely unclear and controversial, especially in patients with primary biliary cirrhosis (PBC). This study was undertaken to investigate Tregs and different cytokines in the liver and peripheral blood of PBC patients. We found that these patients demonstrated a reduction of CD4+CD25+ T cells but elevated CD4+Foxp3+ T cells in peripheral blood mononuclear cells (PBMCs) and CD4+ T cells. The percentage of CD4+CD25+ T cells in PBMCs was negatively correlated with elevated plasma interferon (IFN)-γ levels. A liver-specific analysis showed that the frequency of Foxp3+ Tregs, transforming growth factor (TGF)-β1 and IFN-γ were increased in PBC patients. Our findings suggest that an imbalance between CD4+CD25+ Tregs and cytotoxic cytokines plays a crucial role in the pathogenesis of PBC while the role of Foxp3 needs further investigation.
Keywords: cytokines, forkhead box P3, primary biliary cirrhosis, regulatory T cells
Introduction
Primary biliary cirrhosis (PBC) is an autoimmune disease of the liver. It is characterized by the immune-mediated destruction of small intrahepatic bile ducts histologically referred to as chronic non-suppurative destructive cholangitis, portal inflammation and the presence of antimitochondrial antibodies (AMAs) in the serum that lead to the development of liver fibrosis, cirrhosis and eventual liver failure. PBC affects up to 1 in 700 women in the Western population, the female to male ratio is approximately 10∶1, and the disease typically presents in patients over 40 years old.1, 2 Although the pathogenesis of PBC is not completely understood, immunological aspects are considered to play a key role.3, 4 There is an important role for cytokines and the microenvironment in the regulation and propagation of inflammatory responses in PBC patients,5 and the presence of AMAs and autoreactive T and B cells contribute to liver inflammation.6, 7
A subset of CD4+ T cells called regulatory T cells (Tregs) are crucial for the establishment and maintenance of immunological self-tolerance, and they negatively control many immune responses to non-self antigens.8, 9 Treg deficiency may bring about the progression of various autoimmune diseases.10, 11, 12 The most widely known surface marker for Tregs is CD25; but recently, a large body of data has confirmed that the best marker for Tregs is the intercellular protein forkhead box P3 (Foxp3), which is crucial for Treg development.8 The precise mechanism of Treg function possibly involves contact with target cells or the release of immunoregulatory cytokines that lead to the suppression of autoreactive lymphocyte proliferation and cytokine production after specific T-cell receptor activation.13
In addition to Tregs, the other two T-cell subsets, Th1 and Th2 cells, play important roles in the maintenance of the immune system. An imbalance between these two T-cell subsets has been observed in PBC patients, such as the abnormal distribution of Th1/Th2 cytokine, for example, interferon-γ (IFN-γ), tumor necrosis factor-α and interleukin-4 (IL-4) in peripheral blood and bone marrow.14, 15 However, the association between different T-cell subsets in PBC patients is unclear.
In this study, we determined the frequency of Tregs and levels of different cytokines in peripheral blood and liver tissue of PBC patients. In addition, we analyzed the correlation between Treg and cytokine expression to further understand the pathogenesis of PBC.
Materials and methods
Peripheral blood samples
Peripheral blood samples were obtained from 21 PBC patients and 10 healthy controls from September 2007 to February 2009. All patients fulfilled the diagnostic criteria of PBC based on internationally accepted standards,16 including clinical features and laboratory examinations of bile siltation, positive results for serum AMA presence and pathological confirmation of chronic non-suppurative destructive cholangitis with other causes eliminated. Twelve patients were in the advanced stage of the disease and had no treatment with glucocorticoid, immunosuppressants or other drugs. The other nine patients were in the remission stage after treatment with either ursodeoxycholic acid alone or in conjunction with glucocorticoid. None of the patients examined showed any other autoimmune disorders.
Liver specimens
Needle biopsies or surgical resections were performed to obtain liver tissues from 15 individuals including 10 patients with PBC (nine females and one male; age range: 34–55 years, median: 44 years) and five healthy donors for intravital liver transplantation (all females; age range: 35–49 years, median: 42 years). The study was approved by the institutional Ethics Committee for the Affiliated Drum Tower Hospital of Nanjing University Medical School in Nanjing, China. Written consent from subjects was obtained according to the Declaration of Helsinki. No differences in clinical background were observed between PBC patients and the control group, and all patients were clinically, serologically and histologically diagnosed. PBC livers were staged histologically by Scheuer's classification and there were six in the early stages (stages I–II) and four in the advanced stages (stages III–IV) of disease. All tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin, and 4-µm-thick serial sections were cut from each paraffin block.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were prepared from 2 ml peripheral blood mixed with preservative-free heparin (10 U/ml), resuspended in 100 µl staining buffer and divided into two aliquots (one for detection and the other for hemotype control). The cells were stained with antihuman ffluorescein isothiocyanate-conjugated CD4, allophycocyanin-conjugated CD25 (clone PRA-T4/BC96; eBioscience, San Diego, CA, USA) and intracellular phycoerythrin-conjugated antihuman Foxp3 (clone PCH101; eBioscience). Phycoerythrin-conjugated rat IgG2a (clone eBR2a; eBioscience) was used as an isotype control. Data were acquired on a FACSCalibur (BD Bioscience, San Jose, CA, USA) and analyzed using CellQuest software.
Immunohistochemistry
To investigate the distribution and frequency of cells expressing Foxp3 and the cytokines transforming growth factor (TGF)-β1 and IFN-γ, liver tissue sections were deparaffinized and fixed with different concentrations of alcohol. Antigen retrieval was achieved by pressure cooking for 5 min in 10 mM sodium citrate buffer (pH 6.0). All sections were soaked in 3% H2O2 (Maxim Biological Technology Ltd, Fuzhou, China) for 5 min to inhibit endogenous peroxidase activity, rinsed and incubated with normal rabbit serum (Maxim Biological Technology Ltd) for 10 min at ambient temperature to block non-specific binding. Mouse antihuman monoclonal anti-Foxp3 antibody (NO320201; Biolegend, San Diego, CA, USA) was diluted 1∶50, rabbit-derived polyclonal anti-IFN-γ (sc-702; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was diluted 1∶400 and rabbit polyclonal anti-TGF-β1 (RAB0238; Maxim Biological Technology Ltd) was used directly. The primary antibodies listed above were applied to the specimens in a wet chamber at 4 °C overnight. Biotinylated rabbit antigoat IgG (Envision Kit; Dako, Carpinteria, CA, USA) and Ultrasensitive TM S-P Kit (antirabbit; Maxim Biological Technology Ltd) were used as secondary antibodies for Foxp3 and cytokines respectively, and 3,3′-diaminobenzidine (Sigma, St Louis, MO, USA) was applied as a substrate for coloration. The sections were counterstained with hematoxylin and cover-slipped for observation under a light microscope.
Evaluation of the frequency of Foxp3+ Tregs and different cytokines
The number of Foxp3+ Tregs was counted in six portal tract areas at ×400 magnification in each specimen. The proportion of Foxp3+ Tregs was determined as the number of Foxp3+ Tregs within the total number of mononuclear cells. The localization and frequency of TGF-β1 and IFN-γ were measured and semiquantified by Image-Plus 6.0 software (Media Cybernetics Ltd. San Diego, CA, USA) evaluating the area (S) and accumulative optical density (IOD) of positive signals. One pathologist completed all of the counting at the same time.
Plasma cytokine analysis
Concentrations of IFN-γ and IL-4 were measured using a human ELISA Kit (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
The differences in Treg numbers and cytokine concentration between PBC patients and the control group in the peripheral blood or target liver were determined by independent t test. Correlation between different indexes was analyzed by Spearman's rank correlation test by SPSS version 13.0 software. All analyses were two-sided and a P value of <0.05 was taken to represent significance.
Results
Decreased CD4+CD25+ but increased CD4+Foxp3+ T cells in peripheral blood of PBC patients
The clinical characteristics of PBC patients are shown in Table 1. There were no significant differences in sex, age and autoantibody levels between patients in the advanced and remission stage of the disease. We defined the frequency of the Treg population as either the percentage of CD4+CD25+ T cells or CD4+Foxp3+ T cells in a total of 1×105 peripheral blood lymphocytes (PBL) or total CD4+ T cells see Supplementary Figure 1. We found that the percentage of CD4+Foxp3+ T cells in PBL increased significantly in PBC patients compared to the control group (3.83±0.21% versus 2.66±0.17%, P<0.01), but the percentage of CD4+CD25+ T cells in PBL was much lower in PBC patients compared to the control group (1.79±0.15% versus 3.24±0.16%, P<0.001; Figure 1a and c). In addition, the ratio of CD4+Foxp3+ to CD4+ T cells increased significantly in PBC patients (11.41±6.3% versus 5.74±2.76%, P<0.05), while the ratio of CD25+ to CD4+ T cells decreased significantly (5.84±3.78% versus 10.88±4.24%, P<0.01; Figure 1b and Supplementary Table 1) compared to the control group. Furthermore, patients in the remission stage of the disease had increased levels of CD4+CD25+ T cells in PBL compared to patients with advanced stage PBC (2.23±0.70% versus 1.45±0.50%, P<0.01), while there were no differences in the percentages of CD4+Foxp3+ T cell in PBL or of Treg in CD4+ T cells between the two groups (data not shown).
Table 1. Baseline characteristics of PBC patients.
| Group (n) | Sex (F/M) | Age (years) | ALT (U/l) | AST (U/l) | ALP (U/l) | γ-GGT (U/l) | TBil (umol/l) | AMA (+/−) | ANA (+/−) |
|---|---|---|---|---|---|---|---|---|---|
| Advanced (12) | 11/1 | 51.0±9.5 | 115.5±114.2 | 130.5±110.9 | 479.8±325.7 | 669.5±424.9 | 66.6±72.4 | 10/2 | 9/3 |
| Remission (9) | 7/2 | 62.1±13.2 | 41.0±24.8 | 63.3±41.8 | 146.9±49.7* | 110.4±86.5* | 29.8±29.7 | 8/1 | 6/3 |
*P<0.01 versus patients with advanced stage PBC; values are mean±standard deviation. PBC patients were divided into two groups: advanced stage (ALP>2UNL, and γ-GGT>3UNL) and remission stage (ALP≤2UNL, and γ-GGT≤3UNL) of the disease.
Abbreviations: ALP, alkali phosphatase; ALT, alanine aminotransferase; AMA, antimitochondrial antibody; ANA, antinuclear antibody; AST, aspartate aminotransferase; PBC, primary biliary cirrhosis; TBil, total bilirubin; UNL, upper normal limit; γ-GGT, γ-glutamyltranspeptidase.
Figure 1.
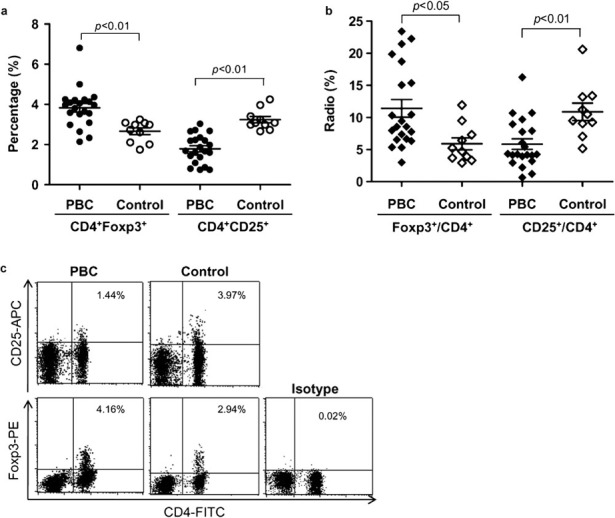
Increased CD4+Foxp3+ cells and decreased CD4+CD25+ cells in PBC patients. (a) The percentage of CD4+Foxp3+ or CD4+CD25+ cells in PBL of PBC patients and controls was analyzed by flow cytometry. (b) The ratios of Foxp3+ or CD25+ to CD4+ T cells in PBC patients were calculated. (c) Representative plots of CD4+Foxp3+ or CD4+CD25+ cells in PBL of PBC patients and controls. Foxp3, forkhead box P3; PBC, primary biliary cirrhosis; PBL, peripheral blood lymphocytes.
Elevated plasma IFN-γ levels and the correlation with Tregs
The plasma IFN-γ level in PBC patients was much higher than in the control group (238.6±164.5 pg/ml versus 46.6±9.4 pg/ml, P<0.05), but there was no difference in IL-4 levels between the two groups (42.31±7.74 pg/ml versus 48.11±3.50 pg/ml, P>0.05). In addition, the plasma IFN-γ level was negatively correlated with the percentage of CD4+CD25+ T cells in PBL (R=−0.84, P=0.01; Figure 2a) for PBC patients. We also found a negative correlation between IFN-γ expression and the ratio of CD4+CD25+ to CD4+ T cells in patients (R=−0.46, P<0.05; Figure 2b), but there was no significant correlation between either cytokines and the percentage of CD4+Foxp3+ T cells.
Figure 2.
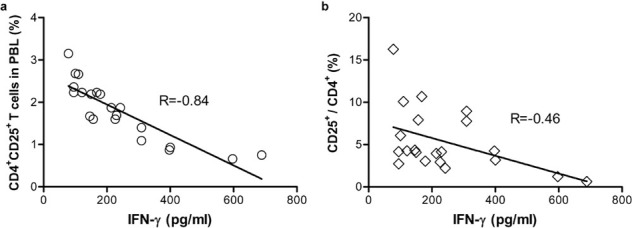
Negative correlation between the percentage of CD4+CD25+ cells and the expression of plasma IFN-γ. In PBC patients, correlation between the percentage of CD4+CD25+ cells (a) or the ratio of CD25+ to CD4+ T cells (b), and the level of plasma IFN-γ was analyzed by Superman correlation test. IFN, interferon.
Frequency of Foxp3+ Tregs increased in the liver of PBC patients
The clinical data of 10 PBC patients who underwent liver biopsy are shown in Table 2. All the patients were confirmed by typical pathologic characteristics for PBC (see Supplementary Figure 2). In addition to measuring the percentage of Tregs in peripheral blood, we examined Foxp3+ T cells in target liver tissue. As shown in Figure 3a, we found that Foxp3 localized in the nucleus of portal area mononuclear cells in the liver of PBC patients, but there were no obvious Foxp3+ T cells in liver tissues of the control group (×400; Figure 3b). PBC livers contained a significantly higher percentage of Foxp3+ Tregs than control livers (3.67±2.52% versus 1.53±0.93%, P<0.05). While patients in the advanced stage of the disease showed a lower frequency of Foxp3+ Tregs compared to those in the early stage, the results were not significant (2.50±0.59% versus 4.46±3.06%, P>0.05).
Table 2. Clinical data of 10 PBC patients who underwent liver biopsy.
| Group (n) | Early stage (6) | Advanced stage (4) |
|---|---|---|
| Sex (F/M) | 5/1 | 4/0 |
| Age (years) | 44.3±6.5 | 43.2±6.6 |
| ALT (U/l) | 103.0±63.0 | 239.5±93.7* |
| AST (U/l) | 104.6±53.4 | 192.9±126.2 |
| ALP (U/l) | 304.9±239.0 | 533.4±306.5 |
| γ-GGT (U/l) | 252.6±286.8 | 687.3±427.6 |
| TBil (umol/l) | 25.0±13.7 | 72.4±56.8 |
| DBil (umol/l) | 11.5±8.7 | 50.1±47.2 |
| IgM (g/l) | 5.7±3.1 | 3.9±1.3 |
| AMA (+/−) | 6/0 | 1/3 |
| ANA (+/−) | 4/2 | 3/1 |
*P<0.05 versus early stage of the disease (Scheuer's classification stages I and II); values are mean±standard deviation.
Abbreviations: ALP, alkali phosphatase; ALT, alanine aminotransferase; AMA, antimitochondrial antibody; ANA, antinuclear antibody; AST, aspartate aminotransferase; DBil, direct bilirubin; IgM, immunoglobulin M; PBC, primary biliary cirrhosis; TBil, total bilirubin; γ-GGT, γ-glutamyltranspeptidase.
Figure 3.
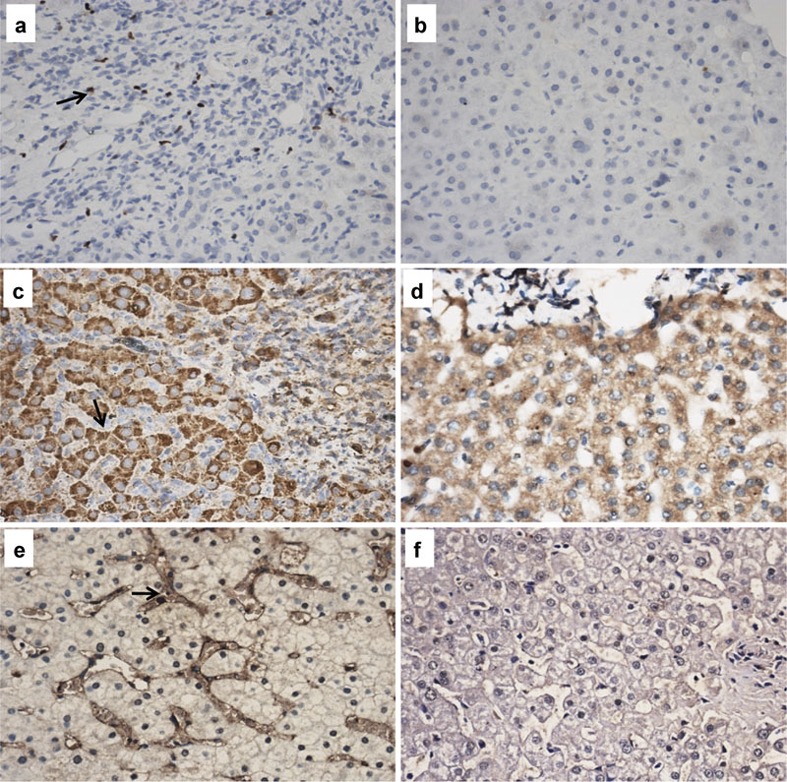
Increased expression of Foxp3, TGF-β1 and IFN-γ in liver tissues of PBC patients (×400). Foxp3+ T cells were found to be scattered among the lymphocytes infiltration in the portal tracts of PBC patients (a), but no significant Foxp3+ T cells were found in healthy livers (b). TGF-β1 mainly localized in the cytoplasm of hepatic cells in PBC patients (c), and also with lower expression in control livers (d). The expression of IFN-γ was found in the sinus space of hepatic cells and surrounding small bile ducts in PBC livers (e), while with no significant expression in healthy livers (f). Foxp3, forkhead box P3; IFN, interferon; PBC, primary biliary cirrhosis; TGF, transforming growth factor; Treg, regulatory T cell.
Immunostaining of cytokines in the liver tissues
Immunohistochemistry staining showed that TGF-β1 localized in the cytoplasm of hepatic cells, but not in the portal areas of monocyte infiltration. Liver sections from PBC patients showed higher levels of TGF-β1 than controls (×400; Figure 3c and d). IFN-γ expression was seen in the sinus space of hepatic cells and also surrounding small bile ducts in the livers of PBC patients, but not in the portal areas. There was no significant IFN-γ expression in the liver tissues of normal individuals (×400; Figure 3e and f). The expression of these two cytokines was significantly higher in PBC patients than in the control group (Table 3).
Table 3. Comparison of TGF-β1 and IFN-γ expression in the liver of patients and controls.
| Index | PBC (n=10) | Control (n=5) | t | P | |
|---|---|---|---|---|---|
| TGF-β1 | S (µm2) | 7779.81±4421.16 | 3888.00±1314.55 | 2.481 | 0.032 |
| IOD | 2773.57±896.60 | 1651.12±494.70 | 2.516 | 0.031 | |
| IFN-γ | S (µm2) | 16 131.61±3567.93 | 7937.88±2186.07 | −5.488 | 0.000 |
| IOD | 5458.45±1907.45 | 2869.76±858.78 | −3.620 | 0.003 | |
Expression of TGF-β1 and IFN-γ in the liver of PBC patients and healthy controls was analyzed by Image-Plus 6.0 software. Positive signals were evaluated by area (S) and accumulated optical density (IOD). The mean values were calculated from 10 independent areas by one pathologist using a high power lens (×400).
Abbreviations: IFN, interferon; PBC, primary biliary cirrhosis; TGF, transforming growth factor. t, t test.
Superman analysis confirmed that the S of IFN-γ expression was negatively correlated with the percentage of Foxp3+ T cells in PBL (R=−0.665, P<0.05), but no correlation was found between IOD of IFN-γ expression and the percentage of Foxp3+ T cells in PBL (R=−0.406, P>0.05). In addition, we did not find significant correlation between S or IOD of TGF-β1 expression and Foxp3+ T cell frequency in PBL (S: R=0.083, P>0.05, IOD: R=0.083, P>0.05).
Correlation between frequency of Foxp3+ T cells and liver functional indexes
No correlation was found between the Foxp3+ T cell percentage in liver tissue and serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkali phosphatase (ALP), γ-glutamyltranspeptidase (γ-GGT), total bilirubin (TBil), direct bilirubin (DBil) and immunoglobulin M (IgM) levels (p>0.05).
Discussion
First demonstrated by Sakaguchi in 1995, natural occurring CD4+CD25+ Tregs have been described in both humans and mice.17 Subsequent studies have shown that transcription factor Foxp3 appears to play a key role in the ontogeny of CD4+CD25+ Tregs.18, 19 It is now clear that Tregs play an important part in controlling aberrant immune responses, including autoimmunity, tumor immunity, infection, allergic reaction and transplantation tolerance, and their clinical application would cure many human diseases.20
In 2006, Lan et al.21 first reported a deficiency of CD4+CD25high and Foxp3+ T cells in PBC patients, although they concluded that Treg functionality was not affected. Consistent with the studies of Lan et al.21 and Liu et al.,22 our results showed that PBC patients displayed significantly lower percentages of CD4+CD25+ Tregs in PBMCs and in CD4+ T cells compared to control subjects, which suggests the breakdown of peripheral tolerance in PBC patients. Recently, Sakaki et al. detected high expression of Foxp3 only in PBC livers.23, 24 They explained it as peripheral immune migration to the target tissue, and they concluded that Treg deficiency had no bearing on the development of chronic non-suppurative destructive cholangitis via loss of self-tolerance in PBC. In this study, we found elevated expression of Foxp3 in both the periphery and the liver tissue of patients, which was not fully explained by immune migration but the absolute number in these patients.
While Foxp3 has been considered a defining marker for the Treg subset25 and used for many years in identifying Tregs, current studies have found that overactivated CD4+ T cells can also transiently express Foxp3, and these Foxp3+ T cells have no immunosuppressive activity.26, 27 The fact that Foxp3 expression is necessary for Treg development but not sufficient to confer regulatory functions might render doubt on using Foxp3 as a definite marker for Tregs.28 Previous studies have shown that PBC patients have many autoreactive CD4+ and CD8+ T cells, and increased Foxp3 expression may simply reflect T-cell activation in PBC patients, which is also seen in patients with systemic lupus erythematosus29 and rheumatoid arthritis.30 We did not find marked differences in Foxp3+ T cells in the liver or peripheral blood between patients in advanced and remission stages of the disease, which could be attributed to the limited number of samples collected.
Similar to Lan et al., we also examined the Th1-, Th2- and Treg-related cytokines, IFN-γ, IL-4 and TGF-β1 respectively, to further explain the role of Tregs and other pathogenic factors in PBC patients. Previous studies have confirmed that PBC patients have much higher levels of Th1 cytokines.31 PBMCs from PBC patients but not control participants produced IFN-γ whether costimulation-competent or -incompetent antigen-presenting cells were used,32 which suggests a role for IFN-γ in the pathogenesis of PBC. Recent studies have found that Tregs can prevent the proliferation and effector function of autoreactive T cells and downregulate the production of IFN-γ by CD8+ T cells in humans.33 The negative correlation between IFN-γ and CD4+CD25+ Tregs in both PBL and CD4+ T cells in this study possibly reveals an imbalanced immune system in PBC patients. The lower frequency of CD4+CD25+ Tregs in PBC patients may lead to the activation of responsive T cells inducing the secretion of inflammatory cytokines such as IFN-γ, and then result in injury specific to the liver. Consistent with Liu et al.,22 patients in the remission stage of the disease showed higher levels of CD4+CD25+ Tregs in PBL than patients in the advanced stage, suggesting the role of ursodeoxycholic acid therapy in immune regulation of PBC patients. On the other hand, Tregs might represent a small population of immune tolerance in the diseased liver, or they might act in a distal manner.21 The percentage of CD4+CD25+ Tregs was not significantly correlated with liver functional indexes, indicating that liver injury in PBC patients might not completely result from Treg deficiency.
TGF-β1 is required for the induction of Foxp3 in T-cell receptor stimulation in human naive CD4+ T cells.34 High levels of TGF-β1 in the liver might contribute to the induction of Foxp3+ in T cells. On the other hand, the function of TGF-β1 in the liver is complicated. It is an anti-inflammatory cytokine but it can also promote the secretion of extracellular matrix and enhance the formation of liver fibrosis.35 We found that TGF-β1 localized in the cytoplasm of hepatic cells but not in the portal areas of monocyte infiltration, suggesting the importance of TGF-β1 in the microenvironment of the diseased liver.
In conclusion, the decreased level of CD4+CD25+ Tregs might induce the imbalanced microenvironment in PBC patients allowing more secretion of inflammatory cytokines, which plays a direct role in the pathogenesis of PBC. The exact role of Foxp3 in PBC patients needs further investigation.
Acknowledgments
This study was supported by a grant from the Jiangsu Province 135 Talent Foundation (RC2007004).
Footnotes
Note: Supplementary information is available on the Cellular & Molecular Immunology website(http://www.nature.com/cmi/).
Supplementary Information
References
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- Prince M, Chetwynd A, Newman WL, Metcalf JV, James OF. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology. 2002;123:1044–1051. doi: 10.1053/gast.2002.36027. [DOI] [PubMed] [Google Scholar]
- Jones DE. Pathogenesis of primary biliary cirrhosis. J Hepatol. 2003;39:639–648. doi: 10.1016/s0168-8278(03)00270-8. [DOI] [PubMed] [Google Scholar]
- Jones DE. Pathogenesis of primary biliary cirrhosis. Postgrad Med J. 2008;84:23–33. doi: 10.1136/gut.2007.122150. [DOI] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Kita H, Lian ZX, van de Water J, He XS, Matsumura S, Kaplan M, et al. Identification of HLA-A2-restricted CD8+ cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Imawari M, Gershwin ME. Cellular immune response in primary biliary cirrhosis. Hepatol Res. 2004;28:12–17. doi: 10.1016/j.hepres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Setoguchi R, Yagi H, Nomura T. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in self-tolerance and autoimmune disease. Curr Top Microbiol Immunol. 2006;305:51–66. doi: 10.1007/3-540-29714-6_3. [DOI] [PubMed] [Google Scholar]
- Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- Behrens F, Himsel A, Rehart S, Stanczyk J, Beutel B, Zimmermann SY, et al. Imbalance in distribution of functional autologous regulatory T cells in rheumatoid arthritis. Ann Rheum Dis. 2007;66:1151–1156. doi: 10.1136/ard.2006.068320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissier MC, Assier E, Bitona J, Denys A, Falgarone G, Bessis N. Regulatory T cells (Treg) in rheumatoid arthritis. Joint Bone Spine. 2009;76:10–14. doi: 10.1016/j.jbspin.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Yamashiki M, Kosaka Y, Nishimura A, Watanabe S, Nomoto M, Ichida F. Analysis of serum cytokine levels in primary biliary cirrhosis patients and healthy adults. J Clin Lab Anal. 1998;12:77–82. doi: 10.1002/(SICI)1098-2825(1998)12:2<77::AID-JCLA1>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachou K, Rigopoulou EI, Tsikrikoni A, Alexandrakis MG, Passam F, Kyriakou DS, et al. Autoimmune hepatitis type 1 and primary biliary cirrhosis have distinct bone marrow cytokine production. J Autoimmun. 2005;25:283–288. doi: 10.1016/j.jaut.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005–1013. doi: 10.1053/he.2000.5984. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- Jiang SP. Regulatory T cells: from bench to bedside. Int Immunopharmacol. 2009;9:515–517. doi: 10.1016/j.intimp.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, et al. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhsis. Hepatology. 2006;43:729–737. doi: 10.1002/hep.21123. [DOI] [PubMed] [Google Scholar]
- Liu B, Shi XH, Zhang FC, Zhang W, Gao LX. Antimitochondrial antibodidy-negative primary biliary cirrhosis: a subset of primary biliary cirrhosis. Liver Int. 2008;28:233–239. doi: 10.1111/j.1478-3231.2007.01651.x. [DOI] [PubMed] [Google Scholar]
- Sakaki M, Hiroishi K, Baba T, Ito T, Hirayama Y, Saito K, et al. Intrahepatic status of regulatory T cells in autoimmune liver diseases and chronic viral hepatitis. Hepatol Res. 2008;38:354–361. doi: 10.1111/j.1872-034X.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Ikeda H, Sawada S, Sato Y, Nakanuma Y. Naturally-occurring regulatory T cells are increased in inflamed portal tracts with cholangiopathy in primary biliary cirrhosis. J Clin Pathol. 2007;60:1102–1107. doi: 10.1136/jcp.2006.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- Ziegler S. FOXP3: not just for regulatory T cells anymore. Eur J Immunol. 2007;37:21–23. doi: 10.1002/eji.200636929. [DOI] [PubMed] [Google Scholar]
- Bonelli M, von Dalwigk K, Savitskaya A, Smolen JS, Scheinecker C. Foxp3 expression in CD4+ T cells of patients with systemic lupus erythematosus: a comparative phenotypic analysis. Ann Rheum Dis. 2008;67:664–671. doi: 10.1136/ard.2007.074690. [DOI] [PubMed] [Google Scholar]
- Han GM, O'Neil-Andersen NJ, Zurier RB, Lawrence DA. CD4+CD25high T cell numbers are enriched in the peripheral blood of patients with rheumatoid arthritis. Cell Immunol. 2008;253:92–101. doi: 10.1016/j.cellimm.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiki M, Kosaka Y, Nishimura A, Watanabe S, Nomoto M, Ichida F. Analysis of serum cytokine levels in primary biliary cirrhosis patients and healthy adults. J Clin Lab Anal. 1998;12:77–82. doi: 10.1002/(SICI)1098-2825(1998)12:2<77::AID-JCLA1>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda S, Ishikawa F, Kamihira T, Komori A, Niiro H, Baba E, et al. Autoreactive T-cell responses in primary biliary cirrhosis are proinflammatory whereas those of controls are regulatory. Gastroenterology. 2006;131:606–618. doi: 10.1053/j.gastro.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Camara NO, Sebille F, Lechler RI. Human CD4+CD25+ regulatory cells have marked and sustained effects on CD8+ T cell activation. Eur J Immunol. 2003;33:3473–3483. doi: 10.1002/eji.200323966. [DOI] [PubMed] [Google Scholar]
- Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+ FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-β dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


