Abstract
Major histocompatibility complex (MHC) class I chain-related protein A (MICA), which is a ligand for human NKG2D, is expressed by a variety of epithelial tumor cells and promotes the activation of natural killer (NK), CD8+ and γδ-T cells. Although ectopic expression of MICA on tumor cells elicits anti-tumor responses, soluble MICA downregulates the activities of lymphocytes. In this study, we showed that recombinant, immobilized MICA (iMICA) molecules coated on plastic wells weakly promote peripheral NK cell activation, secretion of interferon (IFN)-γ and degranulation without inducing apoptosis. In addition, iMICA synergized with IL-15 and soluble 4-1BB ligand (s4-1BBL) to expand NK cells 25- to 42-fold in a 13-day culture, whereas NK cells stimulated only with IL-15 and s4-1BBL expanded 10- to 16-fold. In contrast to NK cells expanded by IL-15 and s4-1BBL stimulation, NK cells expanded long term in the presence of iMICA exhibited increased cytotoxicity against leukemia cells. These results suggest that large numbers of NK cells with high cytotoxicity can be generated by stimulation with IL-15 and s4-1BBL in the presence of iMICA and that these cells can be used for adoptive cancer immunotherapy.
Keywords: expansion, IL-15, MICA, natural killer, 4-1BBL
Introduction
Major histocompatibility complex (MHC) class I chain-related protein A (MICA), MICB, and cytomegalovirus glycoprotein UL16 binding proteins are human ligands for NKG2D, which is expressed by natural killer (NK) cells, CD8+ αβ-T cells, γδ-T cells and some NKT cells. As an activating receptor, engagement of NKG2D triggers both cytotoxicity and interferon (IFN)-γ secretion in human and mouse NK cells.1, 2 Natural or engineered expression of NKG2D ligands (NKG2DLs) on tumor cells greatly enhances their sensitivity to killing by NK cells in vitro, even when the tumor cells express normal levels of MHC class I molecules.3 However, the shedding of soluble MICA promotes downregulation of NKG2D on NK and CD8+ T cells due to NKG2D internalization.4, 5 The impact of solid-phase MICA protein on the expansion and function of NK cells remains unclear.
Studies on the 4-1BB signaling pathway have shown that ligation of 4-1BB promotes the survival and multiplication of CD8+ T and NK cells.6 IL-15 is essential for the development and function of NK cells.7 Coculture of NK cells with K562 cells ectopically coexpressing 4-1BB ligand (4-1BBL) and membrane-bound IL-15 effectively promotes the expansion of NK cells in vitro and mediates their cytotoxicity against some leukemia cells.8, 9 In addition, IL-15 upregulates the expression of NKG2D on NK and T cells, even in the presence of soluble NKG2DL.10 IL-15 and NKG2D signals cross-regulate each other and work together to influence the development and function of NK cells.11, 12 Furthermore, IL-15 augments NKG2DL-mediated anti-tumor responses by promoting the accumulation of NK, NK1.1 and T cells in tumors.13
NK cells kill certain tumor and virus-infected cells directly. NK cell-based adoptive immunotherapeutic strategies are effective against certain cancers, particularly against cancer cells that express low levels of MHC class I molecules and in allogeneic hematopoietic stem cell transplants.14, 15 Nevertheless, because NK cells represent a small fraction of peripheral blood mononuclear cells (PBMCs), generating them in a sufficient number to meet clinical requirements is challenging.16 The proliferation, survival and function of NK cells are improved by engagement of NKG2D with solid-phase immobilized chimeric molecules that resemble NKG2DLs, such as MICA-Fc and ULBP1-Fc.17 In this study, we investigated whether immobilized MICA (iMICA) synergizes with soluble 4-1BBL (s4-1BBL) and IL-15 to expand NK cells efficiently.
Materials and methods
Reagents and antibodies
Recombinant soluble MICA protein was purchased from the ABGAB Corporation (Chicago, Illinois, USA). IL-2, IL-15 and s4-1BBL were purchased from Peprotec (Rocky Hill, NJ, USA). Recombinant soluble beta 2 microglobulin (β2m) protein was produced and purified in our lab, as previously described.18 The following antibodies were used for immunophenotypic characterization of activated and expanded cells: phycoerythrin (PE)-conjugated anti-CD3; fluorescein isothiocyanate (FITC)-, Alexa 647- and PE-Cy5-conjugated anti-CD56; PE-conjugated anti-CD69; PE-conjugated anti-NKG2D; FITC-conjugated anti-CD16; PE- and FITC-conjugated anti-IFN-γ Alexa 647-conjugated anti-CD107a; allophycocyanin-conjugated anti-NKG2A; Alexa 647 anti-NKp46; PE-conjugated anti-CD226; FITC-conjugated anti-CXCR3; and FITC-conjugated anti-CCR7. All antibodies were purchased from eBioscience (San Diego, CA, USA) or Biolegend (San Diego, CA, USA). Cell-surface antibody staining was evaluated using a FACSAria instrument (Becton Dickinson, San Jose, CA, USA).
Stimulation of PBMCs
PBMCs from healthy donors were isolated on a Ficoll gradient. For analysis of the expression of CD69 and NKG2D on NK cells, PBMCs were cultured overnight in empty, flat-bottomed 96-well tissue culture plates or in plates coated with 4 µg of iMICA protein, 4 µg immobilized recombinant β2m (iβ2m) or 40 ng/ml IL-2 (as a positive control). To evaluate the variation of NKG2D expression within 1 week, a low concentration of IL-2 (10 ng/ml) was added to the medium. The cells were next stained with directly conjugated antibodies against CD3, CD56 and CD69 or NKG2D and analyzed by flow cytometry. For analysis, NK cells were gated on CD3−CD56+ cells.
Expansion of PBMCs
PBMCs were seeded into 24-well tissue culture plates at a density of 1×104 cells/well and cultured alone or in the presence of 4 µg iMICA; 50 ng/ml IL-15 and 50 ng/ml s4-1BBL; or iMICA, IL-15 and s4-1BBL simultaneously. Fresh media containing soluble s4-1BBL and IL-15 was added to the appropriate wells every 3 days. Additionally, fresh media containing 10 ng/ml IL-2 was added to the wells regularly throughout the culture period. The frequencies of NK cells under the different treatment conditions were evaluated on days 1, 8, 15 and 21 by flow cytometry.
Analysis of NK cell degranulation
The stimulated PBMCs were cocultured with K562 cells at a 3∶1 ratio in a final volume of 200 µl in round-bottomed 96-well tissue culture plates at 37 °C and an atmosphere of 5% CO2 for 4 h. Fluorochrome-conjugated anti-CD107a or isotype control monoclonal antibody (mAb) was added to the wells at the beginning of the assay. After 1 h of coculture, monensin (GolgiStop; BD Biosciences) was added to the wells at a 1∶100 dilution. Afterward, the cells were stained with directly conjugated mAbs against CD3 and CD56 for 30 min on ice, washed, resuspended in phosphate-buffered saline, and analyzed by flow cytometry.19
IFN-γ secretion assay
CD3−CD56+ NK cells were sorted on a FACSAria instrument at a purity of over 90%. The sorted cells were cultured and stimulated as described above. Forty-eight hours later, the culture supernatants were collected and used to determine the concentrations of IFN-γ by ELISA (Jingmei Biotech, Shenzhen, China) according to the manufacturer's instructions. The secretion of IFN-γ by the expanded NK cells was evaluated by intracellular cytokine staining (eBioscience).
Proliferation and apoptosis assays
The MTS/PMS assay (Promega, Madison, WI, USA) was used to evaluate the proliferation of NK cells. Briefly, the purified NK cells were cultured in triplicate in 96-well tissue culture plates and stimulated with different concentrations of iMICA and 10 ng/ml IL-2. NK cells cultured alone were used as a control. On days 3 and 5, MTS/PMS was added to each well, and 4 h later, the absorbance of each sample at 490 nm was measured. Apoptosis of the NK cells was also evaluated using an annexin V- and propidium iodide (PI)-based assay (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions.
Expansions of pure NK cells
Purified total NK cells, CD56dimCD16bright or CD56birghtCD16dim cells were cultured with IL-15 and s4-BBL or iMICA, IL-15 and s4-1BBL. On days 1, 4, 7, 10 and 13, the cells were counted by trypan blue exclusion. On days 1 and 13, the cytotoxicity of NK cells against K562, U937 and HL60 leukemia cells was evaluated by measuring the release of lactate dehydrogenase by the target cells using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega). In addition, we also examined the expression of NKG2D, NKp46, CD27, CD226, NKG2A, CXCR3 and CCR7 on the expanded NK cells by flow cytometry.
Statistical analysis
The differences between the various treatments were analyzed using the ANOVA test. P values of <0.05, indicated by asterisks throughout the figures, were considered statistically significant.
Results
iMICA alone weakly activates NK cells
To determine whether iMICA protein alone can activate NK cells, the PBMCs were cultured with plate-bound iMICA overnight. To evaluate NK cell activation, we examined cell-surface expression of the early activation marker CD69, the degranulation marker CD107a and the production of IFN-γ. In contrast to iβ2m, iMICA increased the expression of CD69 in NK cells; however, this effect was weak because the percentage of CD69+ NK cells activated with iMICA was lower than when activated with IL-2 (Figure 1a). Similarly, iMICA alone weakly stimulated NK cell degranulation, whereas activation with phorbol myristate acetate (PMA)/ionomycin stimulated higher levels of degranulation (Figure 1b and c). Additionally, IFN-γ production in NK cells was only increased when PBMCs were stimulated with 4 µg of iMICA protein (Figure 1d).
Figure 1.
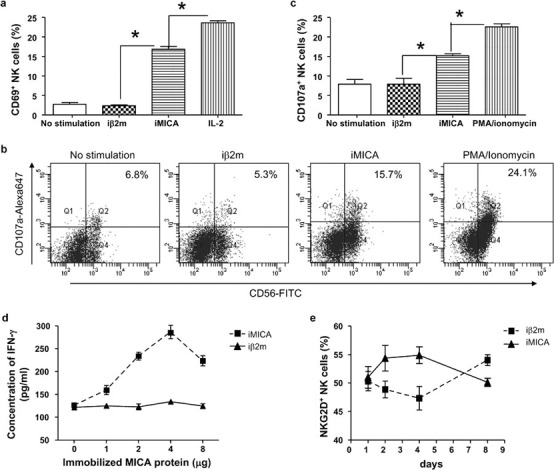
Immobilized MICA weakly promoted fresh NK cell activation. (a) Freshly isolated PBMCs were cultured in flat-bottomed 96-well plates alone, or with iMICA (4 µg), or with iβ2m (4 µg) or with IL-2 (40 ng/ml) overnight. Then cells were harvested and labeled with anti-CD3, anti-CD56 and anti-CD69 mAbs, and the expression of CD69 on NK cells was analyzed by flow cytometry. (b) PBMCs were cultured alone, or with iMICA (4 µg), or with iβ2m (4 µg), or with PMA (10 ng/ml)/ionomycin (0.5 µM) overnight, then were harvested and coincubated with K562 cells at a ratio of 3∶1 in round-bottomed 96-well plates. Fluorochrome-conjugated anti-CD107a mAb was added at the same time. After 1 h of coincubation, monensin (2 µM) was added. Three hours later, surface staining was done by incubating cells with anti-CD3 and anti-CD56 mAbs for 30 min. Lastly frequencies of CD3−CD56+CD107a+ cells were analyzed by flow cytometry. One representative example was shown. All data from were collected, analyzed and shown in (c). (d) After sorted by flow cytometry, NK cells were stimulated with iMICA or with iβ2m at different quantities for 48 h. Then culture supernatants were collected, and detected for IFN-γ concentrations by an ELISA assay according to the manufacturer's instructions. (e) Fresh PBMCs were cultured with iMICA (4 µg) or with iβ2m (4 µg). Low concentration of IL-2 (10 ng/ml) was added into the culture medium. On days 1, 2, 4 and 8, these cultured cells were respectively labeled with anti-CD3, anti-CD56 and anti-NKG2D mAbs. The NKG2D expression on NK cells was analyzed by flow cytometry. Data represented mean±SD for triplicate wells from a typical experiment. *P<0.05. iβ2m, immobilized recombinant beta 2 microglobulin; IFN, interferon; iMICA, immobilized major histocompatibility complex class I chain-related protein A; mAb, monoclonal antibody; MICA, major histocompatibility complex class I chain-related protein A; NK, natural killer; PBMC, peripheral blood mononuclear cell; PMA, phorbol myristate acetate.
It has been shown that soluble MICA decreases the activity of NK cells by inducing the internalization of NKG2D4 because sustained ligation of NKG2D leads to separation of NKG2D from its adaptor, DAP10, which downregulates the function of NK cells.20, 21 Thus, it is possible that sustained engagement of NKG2D by iMICA protein might decrease the expression of NKG2D on the surface of NK cells. However, we found that the expression of NKG2D in NK cells activated for 1 week did not decrease when compared with freshly isolated NK cells (Figure 1e).
iMICA in combination with IL-2 promotes the proliferation of NK cells but has no effect on apoptosis
Although iMICA weakly stimulated the activation of freshly isolated NK cells, ligation of NKG2D in NK cells activated with IL-2 and membrane-bound MICA or NKG2D antibody significantly increased the biological function of NK cells.17 The addition of low concentrations of IL-2 (20 ng/ml) to the culture medium significantly increased the numbers of NK cells stimulated with 4 or 8 µg of iMICA protein for 3 or 5 days (Figure 2a). Previous reports have shown that soluble HLA class I molecules induce the apoptosis of NK cells through the engagement of CD822 or killing immunoglobulin-like receptors (KIR)23 Because of the similarities between the α3 domains of classical HLA I molecules and MICA proteins, we investigated whether iMICA could promote the apoptosis of NK cells. As observed with iβ2m, iMICA had no impact on the apoptosis of NK cells after 3 or 5 days of stimulation (Figure 2b).
Figure 2.
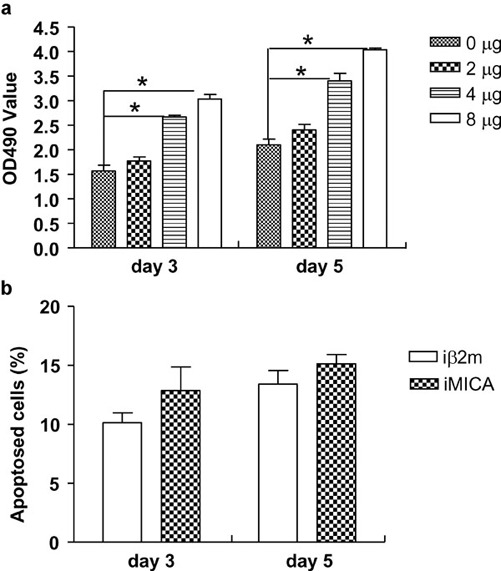
Immobilized MICA promoted proliferation of NK cells, whereas it had no effects on apoptosis. (a) After different yields of recombinant MICA protein were coated on plastic wells, NK cells sorted as CD3−CD56+ cells by flow cytometry were added to the required wells. IL-2 (10 ng/ml) was also added. On days 3 and 5, the MTS/PMS reagent was added to each well. Four hours later, absorbent data at 490 nm were detected. (b) Sorted NK cells were stimulated with iMICA (4 µg) or with iβ2m (4 µg). IL-2 (10 ng/ml) was also added. On days 3 and 5, the cultured NK cells were double-labeled with FITC-annexin V and PI to observe their apoptosis by flow cytometry. Results were shown as mean±SD of three replicates. Experiments were repeated three times. *P<0.05. iβ2m, immobilized recombinant beta 2 microglobulin; iMICA, immobilized major histocompatibility complex class I chain-related protein A; MICA, major histocompatibility complex class I chain-related protein A; NK, natural killer; PI, propidium iodide.
iMICA synergizes with s4-1BBL and IL-15 to efficiently expand highly functional NK cells from PBMCs
To determine whether iMICA could synergize with s4-1BB ligand and IL-15 to expand NK cells in vitro, PBMCs isolated from four donors were cultured under a variety of conditions: iMICA alone; IL-15 and s4-1BBL; iMICA, IL-15 and s4-1BBL; or unstimulated as a negative control. After 3 weeks of culture, only iMICA in combination with IL-15 and s4-1BBL promoted the expansion of NK cells, with NK cells representing approximately 16% (range: 8–25%) of the cells in the culture population. In contrast, stimulation of the PBMCs under the other conditions only generated a minimal number of NK cells (Figure 3a).
Figure 3.
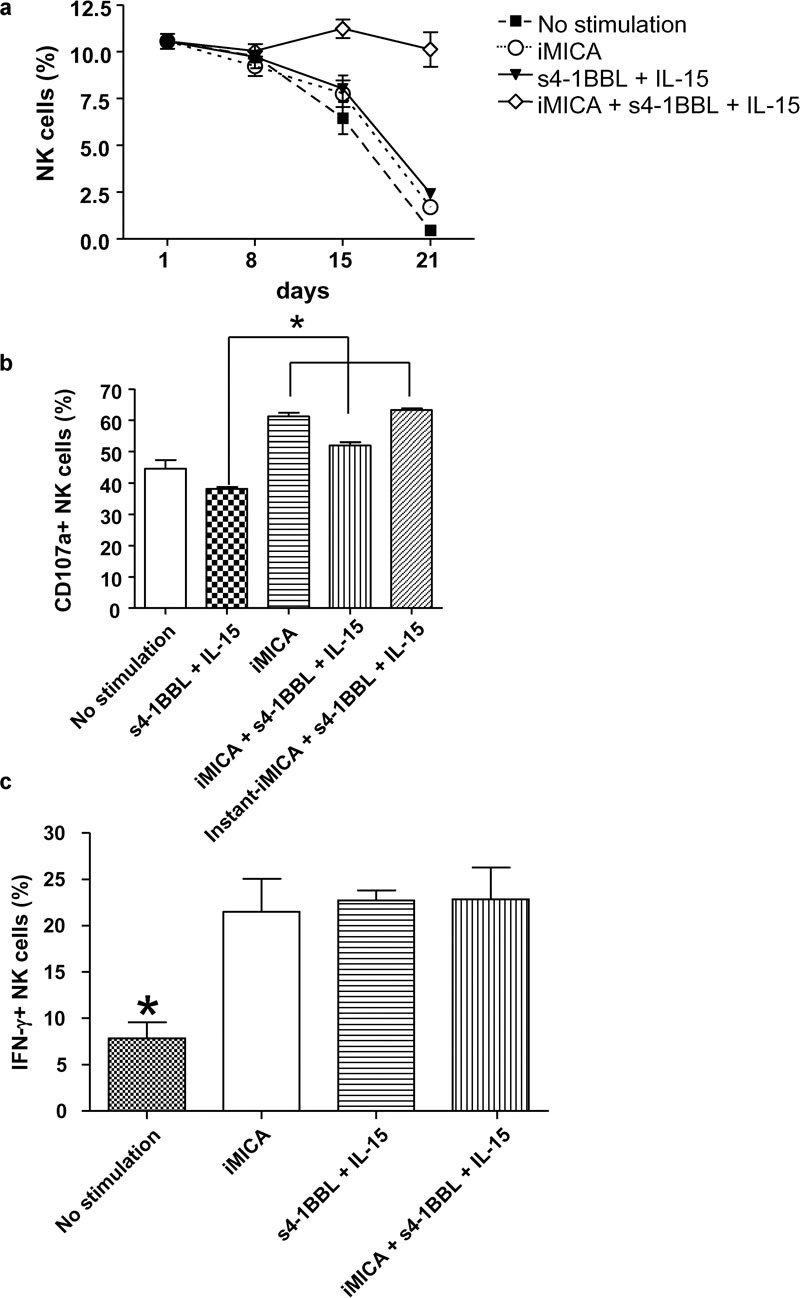
Immobilized MICA synergized with s4-1BBL and IL-15 to expand NK cells of PBMCs with high function. (a) Fresh PBMCs were plated into 24-well plates at 1×104 cells/well and cultured alone, or in stimulation with iMICA (4 µg), or with IL-15 (50 ng/ml) and s4-1BBL (50 ng/ml), or with iMICA, IL-15 and s4-1BBL simultaneously. Fresh media containing s4-1BBL and IL-15 were put into the required wells every 3 days. IL-2 (10 ng/ml) was added regularly throughout the culturing period. On days 1, 8, 15 and 21, these cultured cells were labeled with anti-CD3 and anti-CD56 mAbs, and frequencies of NK cells were analyzed by flow cytometry. (b) On day 21, PBMCs cultured as above were mixed with K562 cells at a ratio of 3∶1 for 4 h. Then CD107a expressions on NK cells were analyzed by flow cytometry after anti-CD107a mAb, monensin, anti-CD3 and anti-CD56 mAbs were added into the culture system sequentially. In addition, on day 20, long-term cultured PBMCs with s4-1BBL and IL-15 were additionally stimulated with iMICA (4 µg) overnight to evaluate their degranulation simultaneously. This stimulation was called ‘instant-iMICA+s4-1BBL+IL-15'. (c) On day 21, all PBMCs expanded as above were treated with brefeldin A (10 g/ml) for 4 h. Next, these cells were labeled with anti-CD3 and anti-CD56 mAbs, fixed and permeabilized, and labeled with anti-IFN-γ mAb. Frequencies of CD3−CD56+IFN-γ+ cells were analyzed by flow cytometry. Results were shown as mean±SD of three replicate wells from a typical experiment. *P<0.05. IFN, interferon; iMICA, immobilized major histocompatibility complex class I chain-related protein A; mAb, monoclonal antibody; MICA, major histocompatibility complex class I chain-related protein A; NK, natural killer; PBMC, peripheral blood mononuclear cell; s4-1BBL, soluble 4-1BB ligand.
Compared with freshly isolated NK cells, NK cells expanded in culture by stimulation with iMICA, IL-15 and s4-1BBL maintained their degranulation capacity after 21 days of culture, whereas NK cells stimulated with IL-15 and s4-1BBL exhibited decreased degranulation. Despite the generation of a small number of cells, NK cells stimulated by iMICA alone exhibited a high degranulation capacity. The degranulation ability of NK cells stimulated with IL-15 and s4-1BBL increased when these cells were additionally cultured with iMICA for 24 h (referred to as instant iMICA stimulation) (Figure 3b). The production of IFN-γ by the expanded NK cells was enhanced under all the conditions tested (Figure 3c). Collectively, these results demonstrate that iMICA synergizes with IL-15 and s4-1BBL to efficiently promote the expansion of functional NK cells.
NK cells sorted to high purity are expanded by stimulation with iMICA, IL-15 and s4-1BBL
To confirm the ability of iMICA, IL-15 and s4-1BBL to expand NK cells in vitro, CD3−CD56+ cells were sorted to high purity and expanded by stimulation with iMICA, IL-15 and s4-1BBL or with IL-15 and s4-1BBL. After 13 days of stimulation, the numbers of NK cells increased 32-fold (range: 25- to 42-fold) when cultured with iMICA, whereas cells cultured with IL-15 and s4-1BBL increased 13-fold (range: 10- to 16-fold) (Figure 4a). In addition, NK cells stimulated with iMICA, IL-15 and s4-1BBL exhibited high cytotoxicity against K562, U937 and HL60 leukemia cells (67.4, 58.6 and 62.3%, respectively), especially at the low effector: target ratio of 2.5∶1 (Figure 4b–d).
Figure 4.
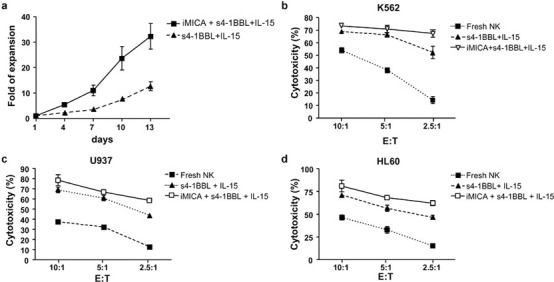
Immobilized MICA, s4-1BBL and IL-15 expanded pure NK cells efficiently with high cytotoxicity against leukemic cells. (a) Growth curve of pure NK cells within the different culture environment. Purified NK cells (1×103 cells/well) were cultured with iMICA (4 µg), IL-15 (50 ng/ml) and s4-1BBL (50 ng/ml), or with IL-15 and s4-1BBL. Cell numbers were directly counted with trypan blue dye staining. IL-2 (10 ng/ml) were added throughout the culturing period. (b–d) Cytotoxicities of fresh and 13-day differently expanded NK cells against K562, U937 and HL60 leukemic cells. Fresh or expanded NK cells were mixed with target cells for 4 h at the ratios of 10∶1, 5∶1 and 2.5∶1. Cytotoxicities were assessed by levels of lactate dehydrogenase released by targets in the culture medium according to the manufactures' guide. Results were shown as mean±SD of three replicates. Experiments were repeated three times. iMICA, immobilized major histocompatibility complex class I chain-related protein A; MICA, major histocompatibility complex class I chain-related protein A; NK, natural killer; s4-1BBL, soluble 4-1BB ligand.
Peripheral blood NK cells are divided into two subsets: CD56dimCD16bright and CD56brightCD16dim. CD56dimCD16bright cells represent approximately 90% of the peripheral blood cell pool and mainly act against target cells via their cytotoxic activity. In contrast, CD56brightCD16dim cells represent 10% of the peripheral blood cell pool and produce IFN-γ to regulate both innate and adaptive immune function. We sought to determine if and which of these NK cell subsets preferentially expands in our culture system. To this end, the two subsets were sorted to high purity by flow cytometry (Figure 5a) and independently expanded under different stimulatory conditions. Stimulation with iMICA, IL-15 and s4-1BBL highly expanded both NK cell subsets; however, the CD56dimCD16bright cells proliferated more efficiently (24.4-fold for CD56dimCD16bright cells versus 14.6-fold for CD56brightCD16dim cells; Figure 5b).
Figure 5.
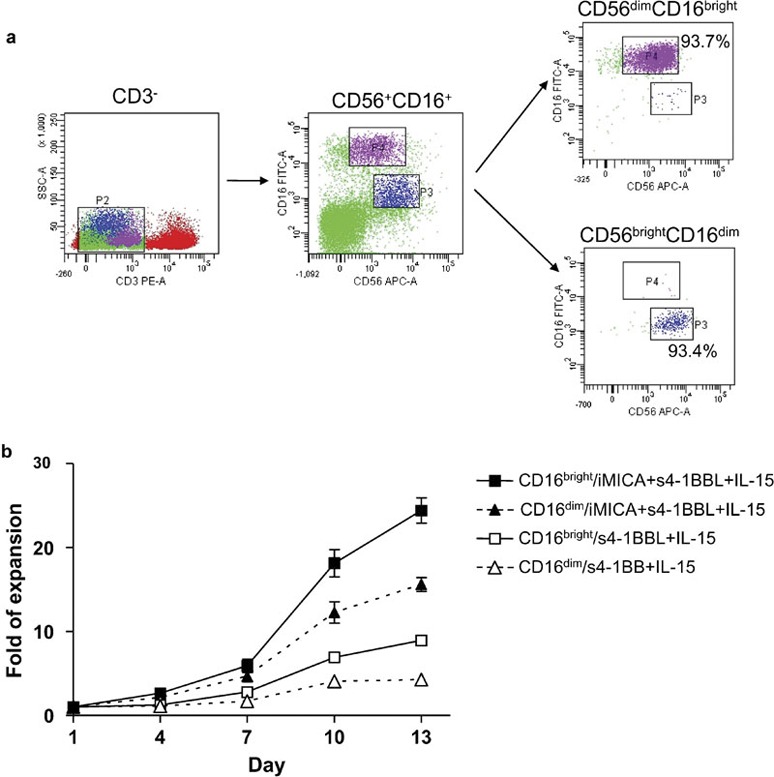
Immobilized MICA, IL-15 and s4-1BBL expanded both CD56brightCD16dim and CD56dimCD16bight cells. (a) NK cells from freshly isolated PBMCs were sorted into CD56brightCD16dim and CD56dimCD16bight subsets with polychromatic flow cytometry gating. (b) Growth curves of NK cell subsets within the different culture environments for 13 days. Both CD56brightCD16dim (0.5×103 cells/well) and CD56dimCD16bight (1×103 cells/well) subsets were cultured with iMICA (4 µg), IL-15 (50 ng/ml) and s4-1BBL (50 ng/ml), or with IL-15 and s4-1BBL. IL-2 (10 ng/ml) were added throughout the culturing period. Cell numbers were directly counted with trypan blue dye staining. Data represented the average±SD for triplicate wells from a typical experiment of three tests. iMICA, immobilized major histocompatibility complex class I chain-related protein A; MICA, major histocompatibility complex class I chain-related protein A; NK, natural killer; PBMC, peripheral blood mononuclear cell; s4-1BBL, soluble 4-1BB ligand.
Phenotypic characterization of the expanded NK cells
We evaluated the expression of relevant receptors, including NKG2D, CD226 (DNAM-1), NKp46, CD27, NKG2A, CXCR3 and CCR7, on the expanded NK cells (Figure 6). The activating receptors NKG2D, NKp46 and CD27 were upregulated on NK cells after stimulation, but expression of the costimulatory receptor CD226 did not change significantly. The expression of the inhibitory receptor NKG2A significantly decreased in the expanded NK cells. CCR7 is a chemokine receptor involved in the recruitment of lymphocytes to the lymph nodes,24 and CXCR3 is associated with the accumulation of lymphocytes in tumors.25 NK cells cultured for long term decreased their expression of CCR7 but increased their expression of CXCR3, indicating that the expanded NK cells have the potential to preferentially migrate to tumor sites. In addition, iMICA in combination with IL-15 and s4-1BBL preferentially stimulated NK cells to express NKp46 and CXCR3.
Figure 6.
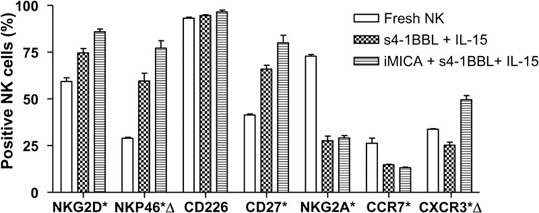
Receptor expression patterns of long-term cultured NK cells. Fresh PBMCs were cultured with iMICA (4 µg), IL-15 (50 ng/ml) and s4-1BBL (50 ng/ml), or with IL-15 and s4-1BBL. IL-2 (10 ng/ml) was added throughout the culturing period. Expression levels of different receptors on fresh or 13-day differently expanded NK cells were assessed by multicolor flow cytometry. For each receptor, analysis was made on receptor-positive subsets within the total NK cell population. Asterisks indicated significant differences between fresh and cultured NK cells (P<0.05). Triangles indicated significant differences between iMICA, IL-15, s4-1BBL stimulated- and IL-15, s4-1BBL stimulated-NK cells (P<0.05). Results were shown as average±SD of three replicates from a typical experiment of five tests. iMICA, immobilized major histocompatibility complex class I chain-related protein A; NK, natural killer; PBMC, peripheral blood mononuclear cell; s4-1BBL, soluble 4-1BB ligand.
Discussion
Here, we showed that iMICA protein alone weakly induced the activation, degranulation and production of IFN-γ in freshly isolated NK cells but had no effects on apoptosis. We also demonstrated that iMICA in combination with IL-2 stimulates NK cell proliferation and, in combination with IL-15 and 4-1BBL, expands highly cytotoxic NK cells from PBMCs after 21 days of culture. Furthermore, we demonstrated that the numbers of purified NK cells increase 32-fold in response to iMICA, IL-15 and 4-1BBL, with both subsets of peripheral blood NK cells proliferating equivalently. The NK cells expanded using our culture system exhibited increased expression of activating receptors and decreased expression of inhibitory receptors. Altogether, these results suggest that, although iMICA alone has a weak stimulatory effect on NK cells, this protein can synergize with IL-15 and 4-1BBL to promote the efficient expansion and cytolytic function of NK cells ex vivo.
Ligation of the hexameric NKG2D-DAP10 complex by its interaction with MICA or other ligands expressed by target cells leads to NK cell activation. Activated DAP10 binds multiple proteins, including the PI3K regulatory subunit p85, resulting in the phosphorylation of ERK, which is required for the generation of cytotoxicity and F-actin polymerization. In addition, NKG2D stimulation leads to PI3K-dependent Akt phosphorylation, which activates various cell survival pathways.26, 27 Recently, IL-15-induced STAT5 phosphorylation was shown to be aborted in DAP10-Ub transgenic NK cells,11 demonstrating that IL-15 signaling requires DAP10-associated proteins. The combined use of iMICA, IL-15 and s4-1BBL in this study not only improved the cytotoxicity of NK cells, an effect mainly mediated by NKG2D signaling, but also additively promoted the expansion of NK cells when compared to cells stimulated only with IL-15 and s4-1BBL. This result suggests that NKG2D and IL-15 receptor signaling are closely associated or synergize to activate NK cells; however, the mechanism underlying this partnership is unknown.
It should be noted that iMICA alone weakly activated freshly isolated NK cells, as shown by the low frequency of CD69+ NK cells and their decreased capacity for degranulation when compared to cells stimulated with 40 ng/ml IL-2 or PMA/ionomycin. The failure of NK cells to expand upon stimulation with iMICA alone for 3 weeks also suggests that iMICA has a weak capacity to promote proliferation (Figure 3a). After 21 days of culture, IL-15- and s4-1BBL-expanded NK cells did not significantly decrease their capacity for degranulation. However, combined use of iMICA, IL-15 and s4-1BBL in our culture system increased the cytotoxicity of NK cells. These results suggest that iMICA can be optionally added to a culture system if high numbers of NK cells with high cytotoxicity are required.
In our culture system, expanding the NK cells beyond 21 days seemed to be unnecessary because we could obtain a high number of CD3−CD56+ cells with enhanced function after a short culture time (approximately 15 days). In addition, purified NK cells were efficiently expanded to kill leukemia cells at a ratio of 2.5∶1, which is a method that could be used to treat myeloid leukemia with allogeneic infusion. The infusion of perfectly mismatched donor NK cells has been shown to induce a strong graft-versus-leukemia response but a lower graft-versus-host disease response.14, 15 Furthermore, there are two additional advantages for NK cell-based infusion: the cytolytic subset of NK cells (CD56dimCD16bright) was preferentially expanded in culture, and CXCR3 was more highly expressed in this population of expanded NK cells.
Our results indicate that large numbers of cytotoxic NK cells can be produced by combined stimulation of PBMCs with iMICA, IL-15 and s4-1BBL. Such expanded NK cells can be used in the setting of adoptive immunotherapy against refractory relapses of leukemia and other tumors. However, further studies are required to evaluate the recruitment and killing activity of these in vitro expanded and activated NK cells in vivo.
Acknowledgments
This work was supported by the National Natural Science Foundation (30400399 and 30671917), the Natural Science Fund of Jiangsu Province (BK2008215 and BK2004404) and the Natural Science Fund of the Educational Committee of Jiangsu Province (04KJB320162) in China.
References
- Bahram S, Bresnahan M, Geraghty DE, Spies T. A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci USA. 1994;91:6259–6263. doi: 10.1073/pnas.91.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nausch N, A Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. . Proc Natl Acad Sci USA. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O'Reilly RJ, Dupont B, et al. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol. 2003;171:6891–6899. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- Cheuk AT, Mufti GJ, Guinn B. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Ther. 2004;11:215–226. doi: 10.1038/sj.cgt.7700670. [DOI] [PubMed] [Google Scholar]
- Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AI, Lee L, Schwarz E, Groh V, Spies T, Ebert EC, et al. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J Immunol. 2001;167:5527–5530. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- Horng T, Bezbradica JS, Medzhitov R. NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat Immunol. 2007;8:1345–1352. doi: 10.1038/ni1524. [DOI] [PubMed] [Google Scholar]
- Colucci F. Unexpected partnership between IL-15 and DAP10. Nat Immunol. 2007;8:1289–1291. doi: 10.1038/ni1207-1289. [DOI] [PubMed] [Google Scholar]
- Sutherland CL, Rabinovich B, Jan Chalupny N, Brawand P, Miller R, Cosman D. ULBPs, human ligands of the NKG2D receptor, stimulate tumor immunity with enhancement by IL-15. Blood. 2006;108:1313–1319. doi: 10.1182/blood-2005-11-011320. [DOI] [PubMed] [Google Scholar]
- Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- Farag SS, Fehniger TA, Becknell B, Blaser BW, Caligiuri MA. New directions in natural killer cell-based immunotherapy of human cancer. Expert Opin Biol Ther. 2003;3:237–250. doi: 10.1517/14712598.3.2.237. [DOI] [PubMed] [Google Scholar]
- Carlens S, Gilljam M, Chambers BJ, Aschan J, Guven H, Ljunggren HG, et al. A new method for in vitro expansion of cytotoxic human CD3−CD56− natural killer cells. Human Immunol. 2001;62:1092–1098. doi: 10.1016/s0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- Andre P, Castriconi R, Espeli M, Anfossi N, Juarez T, Hue S, et al. Comparative analysis of human NK cell activation induced by NKG2D and natural cytotoxicity receptors. Eur J Immunol. 2004;34:961–971. doi: 10.1002/eji.200324705. [DOI] [PubMed] [Google Scholar]
- Gong WJ, Ji MC, Cao ZF, Wang LH, Qian YY, Hu MZ, et al. Establishment and characterization of a cell based artificial antigen-presenting cell for expansion and activation of CD8+ T cells ex vivo. . Cell Mol Immunol. 2008;5:47–53. doi: 10.1038/cmi.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, et al. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106:1711–1717. doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Contini P, Carosio R, Arvigo M, Ghio M, Oddone D, et al. Soluble HLA class I molecules induce natural killer cell apoptosis through the engagement of CD8: evidence for a negative regulation exerted by members of the inhibitory receptor superfamily. Blood. 2002;99:1706–1714. doi: 10.1182/blood.v99.5.1706. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Contini P, Dondero A, Carosio R, Puppo F, Indiveri F, et al. Soluble HLA class I induces NK cell apoptosis upon the engagement of killer-activating HLA class I receptors through FasL–Fas interaction. Blood. 2002;100:4098–4107. doi: 10.1182/blood-2002-04-1284. [DOI] [PubMed] [Google Scholar]
- Marcenaro E, Cantoni C, Pesce S, Prato C, Pende D, Agaugue S, et al. Uptake of CCR7 and acquisition of migratory properties by human KIR+ NK cells interacting with monocyte-derived DC or EBV cell lines: regulation by KIR/HLA-class I interaction. Blood. 2009;114:4108–4116. doi: 10.1182/blood-2009-05-222265. [DOI] [PubMed] [Google Scholar]
- Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008;68:8437–8445. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- Lopez-Larrea C, Suarez-Alvarez B, Lopez-Soto A, Lopez-Vazquez A, Gonzalez S. The NKG2D receptor: sensing stressed cells. Trend Mol Med. 2008;14:179–189. doi: 10.1016/j.molmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Burgess SJ, Maasho K, Masilamani M, Narayanan S, Borrego F, Coligan JE. The NKG2D receptor: immunobiology and clinical implications. Immunol Res. 2008;40:18–34. doi: 10.1007/s12026-007-0060-9. [DOI] [PubMed] [Google Scholar]


