Abstract
CD4+CD25+ T regulatory (Treg) cells are critical in inducing and maintaining immunological self-tolerance as well as transplant tolerance. The effect of low doses of whole-body irradiation (WBI) on CD4+CD25+Foxp3+ Treg cells has not been determined. The proportion, phenotypes and function of CD4+CD25+ Treg cells were investigated 0.5, 5 and 15 days after euthymic, thymectomized or allogeneic bone marrow transplanted C57BL/6 mice received 2-Gy γ-rays of WBI. The 2-Gy WBI significantly enhanced the ratios of CD4+CD25+ Treg cells and CD4+CD25+Foxp3+ Treg cells to CD4+ T cells in peripheral blood, lymph nodes, spleens and thymi of mice. The CD4+CD25+ Treg cells of the WBI-treated mice showed immunosuppressive activities on the immune response of CD4+CD25− T effector cells to alloantigens or mitogens as efficiently as the control mice. Furthermore, 2-Gy γ-ray WBI significantly increased the percentage of CD4+CD25+Foxp3+ Treg cells in the periphery of either thymectomized mice or allogeneic bone marrow transplanted mice. The in vitro assay showed that ionizing irradiation induced less cell death in CD4+CD25+Foxp3+ Treg cells than in CD4+CD25− T cells. Thus, a low dose of WBI could significantly enhance the level of functional CD4+CD25+Foxp3+ Treg cells in the periphery of naive or immunized mice. The enhanced proportion of CD4+CD25+Foxp3+ Treg cells in the periphery by a low dose of WBI may make hosts more susceptible to immune tolerance induction.
Keywords: CD4+CD25+Foxp3+ Treg cells, Foxp3, γ-ray irradiation, immune tolerance
Introduction
CD4+CD25+ T regulatory (Treg) cells, which usually constitute approximately 5–10% of peripheral CD4+ T cells in humans and mice, are critically involved in immune homeostasis and maintenance of self-tolerance.1, 2, 3, 4 It has been demonstrated that CD4+CD25+ Treg cells are potent suppressors or regulators in controlling allergic responses, allograft rejection, antitumor immunity and microbial immunity, in addition to keeping self-tolerance.5, 6, 7 It is now clear that forkhead family Foxp3 is a specific transcription factor of CD4+CD25+ Treg cells, at least in mice.8, 9, 10 Mutations in Foxp3 cause a similar X-linked recessive autoimmune and inflammatory disorder in humans and mice.11, 12, 13, 14 T cells that overexpress Foxp3 display the phenotype and immunosuppressive function of CD4+CD25+ Treg cells.8, 9 These findings indicate that Foxp3 represents a key role in the development and function of CD4+CD25+ Treg cells.15, 16, 17, 18
Ionizing irradiation has often been used in bone marrow transplants or to treat cancers with or without chemotherapy in clinics and experimental animal models.19, 20, 21, 22 Whole-body irradiation (WBI) significantly decreases the lymphocyte pool, and the various lymphocyte subsets have different sensitivities to irradiation.23 It has been reported that ionizing radiation changes the Th1/Th2 balance.24, 25 Recently, it was reported by us and others that the newly identified immunosuppressive CD4+CD25+ Treg cells displayed different radiation sensitivities compared with CD4+CD25− T effector cells in vivo.26, 27, 28, 29 However, the peripherally changed levels of CD4+CD25+ Treg cells after WBI was due to the direct effect of ionizing irradiation on peripheral T cells and on the thymus function whether has not been addressed. In the present study, we investigated the sensitivity of mouse CD4+CD25+ Treg cells to γ-rays in naive, immunized and thymectomized mice. Our data showed that 2-Gy γ-ray WBI significantly enhanced the frequency of peripheral CD4+CD25+ Treg cells in both naive mice and mouse recipients of allogeneic bone marrow cells (BMCs). The enhanced percentage of CD4+CD25+ Treg cells in the periphery may make hosts favorable to tolerance induction.
Materials and methods
Mice
C57BL/6 (B6, H-2b) and BALB/c (H-2d) mice were purchased from the Institute of Genetics and Development, Chinese Academy of Sciences (Beijing, China). All mice were maintained in a specific pathogen-free facility and were housed in microisolator cages containing sterilized feed, autoclaved bedding and water. All experimental manipulations were undertaken in accordance with the Institutional Guidelines for the Care and Use of Laboratory Animals.
Monoclonal antibodies (mAbs) and reagents
The following mAbs were purchased from BD Biosciences Pharmingen (San Diego, CA, USA): fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD4 mAb (RM4-5; rat IgG2a), phycoerythrin (PE)-labeled rat anti-mouse CD4 mAb, Cy-chrome-labeled anti-mouse CD4 mAb, PE-labeled anti-mouse CD8α mAb (53-6.7; rat IgG2a), FITC-labeled rat anti-mouse CD25 mAb (7D4; IgM) and FITC-labeled hamster anti-mouse TCR β-chain mAb (H57–597). In addition, PE-labeled anti-mouse Foxp3 mAb (FJK-16s) and its staining kit were obtained from eBioscience (San Diego, CA, USA). Rat anti-mouse FcR mAb (2.4G2, IgG2b) was produced by 2.4G2 hybridoma (ATCC, Rockville, MD, USA) in our laboratory.
The culture medium used in the present study was RPMI 1640 (Hyclone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate and 50 µM 2-ME (Sigma, St Louis, MO, USA).
WBI
Five- to seven-week-old C57BL/6 mice received a single dose of 2-Gy γ-ray irradiation from a 60Co source (Peking University Health Science Center, Beijing, China). The irradiation dose rate is 2.01 Gy/min.
Immune cell preparation
Mouse peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient (Sigma) as reported previously.30 At 0.5, 5 and 15 days after γ-ray irradiation, mouse thymi, spleens and lymph nodes (LNs, including cervical, inguinal and axillary LNs) were harvested. Single cell suspensions were prepared as reported previously.31 Splenocytes were treated with a hemolysis buffer (17 mM Tris-HCl and 140 mM NH4Cl, pH 7.2) to remove red blood cells as described previously.32
Immunofluorescence staining and flow cytometry (FCM)
PBMCs, LNs, splenocytes or thymocytes (5×105) were incubated with 2.4G2 to block non-specific staining by FcRs and then incubated with an optimal concentration of fluorochrome-labeled mAbs for 30 min at 4 °C in dark. Cells were washed three times and resuspended in FCM buffer (phosphate buffered saline with 0.1% bovine serum albumin and 0.1% NaN3). At least 10 000 cells were assayed using FASCalibur FCM (Becton Dickinson, San Jose, CA, USA), and data were analyzed with CellQuest software. In some experiments, nonviable cells were excluded using the vital nucleic acid stain propidium iodide. The percentage of cells stained with a particular reagent or reagents was determined by subtracting the percentage of cells stained nonspecifically with the negative control mAb from staining in the same dot-plot region with the indicated mAbs.
For the intracellular Foxp3 staining, cells were incubated with Cy-chrome-labeled anti-CD4 and FITC-labeled anti-CD25 mAbs first. After being washed, the cells were fixed and permeabilized with Foxp3 staining buffer (eBioscience) and then stained with PE-conjugated anti-mouse Foxp3 mAbs as reported previously.33
Isolation of CD4+CD25+ Treg cells and CD4+CD25− T cells
An enriched mouse CD4+CD25+ Treg cell population was isolated from a mouse splenocyte suspension using a CD4+CD25+ Treg isolation kit with the MidiMACSTM separator according to the manufacturer's protocols (Miltenyi, Bergisch Gladbach, Germany).34 Briefly, splenocytes were suspended in phosphate buffered saline containing 0.5% bovine serum albumin and 2 mM ethylenediaminetetraacetic acid (pH 7.2), and then incubated with a biotin-antibody cocktail against CD8α (Ly2), CD11b (Mac-1), CD45R (B220), CD49B (DX5) and Ter-119 for 20 min at 4 °C and then with a microbead-conjugated anti-biotin mAb (Bio318E7.2) and a PE-labeled anti-CD25 mAb. The cell suspension was loaded onto an LD column, which was placed in the magnetic field of an MACS separator. The remaining fraction in the column consisted of the enriched CD4+ T cells. For the isolation of CD4+CD25+ T cells, the PE-labeled CD25+ cells in the enriched CD4+ T cells fraction were magnetically labeled with anti-PE microbeads. The magnetically labeled CD4+CD25+ T cells were enriched from the CD4+ T-cell fraction by MACS sorting. The purities of the sorted CD4+CD25+ or CD4+CD25− T-cell population were always >90% as confirmed by FCM.
Mixed lymphocyte reaction
C57BL/6 splenic CD4+CD25+ Treg cells were isolated from either control or γ-ray-irradiated mice as described above. Naive C57BL/6 CD4+CD25− T cells were used as responder T cells. BALB/c splenocytes, which were pre-treated with mitomycin C at the concentration of 30 µg/ml at 37 °C for 30 min, were used as allogeneic stimulator cells. In general, 1×105 responder cells (naive C57BL/6 CD4+CD25− T cells) and 1×105 stimulator cells (BALB/c splenocytes) per well in RPMI 1640 medium supplemented with 10% fetal calf serum were added in 96-well round-bottom plates.35 Enriched CD4+CD25+ Treg cells were subsequently added to each well in different ratios to CD4+CD25− T cells. Cells were cultured at 37 °C and 5% CO2 for 5 days. A total of 0.5 µCi [3H]thymidine (185 GBq/mmol; Atomic Energy Research Establishment, Beijing, China) was added for the last 16 h. Cells were harvested with an automatic cell harvester (Tomtec, Toku, Finland). The radioactivity of each sample was assayed in a Liquid Scintillation Analyzer (Beckman Instruments, Fullerton, CA, USA). Values are expressed as counts per minute of triplicate wells.
The proliferation of T cells to concanavalin A (ConA)
Enriched C57BL/6 CD4+CD25− T cells (5×104 cells/well) were cultured in U-bottom, 96-well plates with syngenic accessory cells (5×104 splenocytes/well, pre-treated with 30 µg/ml mitomycin C at 37 °C for 30 min),35 3 µg/ml ConA and the indicated numbers of C57BL/6 CD4+CD25+ Treg cells isolated from either control or irradiated mice for 72 h at 37 °C in 5% CO2. A total of 0.5 µCi [3H] thymidine was added to each well for the last 12 h. Cells were harvested on glass fiber filters with an automatic cell harvester (Tomtec). Samples were assayed in a liquid scintillation analyzer (Beckman Instruments). Values are presented as counts per minute of triplicate wells.
Thymectomy and BMC transplantation procedures
Thymectomies were performed on the 5-week-old mice as described previously.36, 37 The survival rate of operated mice was above 85%.
For allogeneic BMC transplantation, C57BL/6 recipients at 6 weeks of age were irradiated by 2-Gy γ-rays (60Co source, dose rate, 2.01 Gy/min; Peking University Health Science Center). BMCs of allogeneic BALB/c mice obtained by flushing femora and tibiae were resuspended in RPMI 1640 medium and incubated with anti-CD4 mAb (GK1.5) and anti-CD8 mAb (2.43) at 4 °C for 30 min. The cells were then washed and incubated with complements (rabbit sera, 1∶14) at 37 °C for 45 min to deplete the T cells. BMCs were then washed three times in RPMI 1640 medium. A total of 2×107 T cell-depleted allogeneic BMCs was intravenously injected into C57BL/6 mice within 8 h after WBI.
Statistical analysis
All data are presented as mean±SD. Student's unpaired t-test for comparison of means was used to compare groups. A P value less than 0.05 was considered statistically significant.
Results
2-Gy γ-ray WBI significantly increased the proportion of CD4+CD25+ Treg cells in the periphery of C57BL/6 mice
To evaluate the effects of 2-Gy γ-ray irradiation on CD4+CD25+ Treg cells in mice, we first compared the levels of CD4+CD25+ Treg cells in irradiation-treated and control mice. As shown in Figure 1, the percentage of CD4+ T cells in the peripheral blood was increased at day 5 after 2-Gy γ-ray irradiation (P<0.05; Figure 1a), while the levels of CD8+ T cells in the peripheral blood of mice did not show significant alteration after WBI (data not shown). These data indicate that T cells, especially CD4+ T cells, are more resistant to ionizing irradiation compared to other immune cell subsets such as B cells, though innate immune cells are much more resistant to irradiation, as previously reported.23, 38 Importantly, the percentage of CD4+CD25+ Treg cells in the peripheral blood was significantly higher in irradiated mice than in control mice on day 5 (P<0.01; Figure 1b and c).
Figure 1.
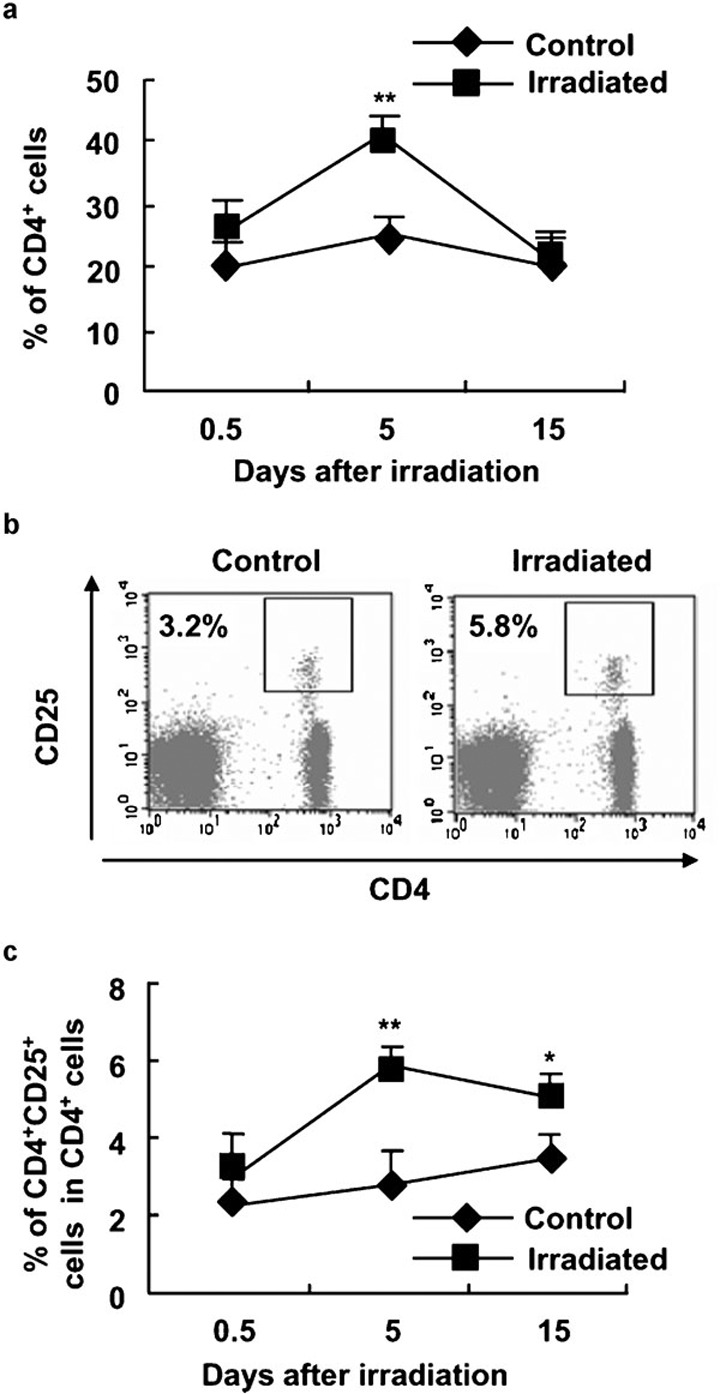
The significantly enhanced percentage of CD4+CD25+ Treg cells in PBMCs of mice after 2-Gy WBI. C57BL/6 mice were irradiated with 2-Gy γ-rays as described in the section on ‘Materials and methods'. At different time points after irradiation, PBMCs were stained with FITC-labeled anti-CD4 mAb and PE-labeled anti-CD25 mAb or PE-labeled anti-CD8 mAb and assayed by FCM. (a) The percentages of CD4+ T cells in PBMCs of control and 2-Gy γ-ray-irradiated mice. (b) One representative of PBMC staining with anti-CD4 and anti-CD25 mAbs. Numbers in the dot plot indicate the percentages of CD4+CD25+ Treg cells. (c) The percentages of CD4+CD25+ T cells in CD4+ PBMCs of control and 2-Gy γ-ray-irradiated mice. *P<0.05, **P<0.01, compared with the identical controls. The data are shown as mean±SD (n=6). One representative of three separate experiments with similar results is shown. FITC, fluorescein isothiocyanate; FCM, flow cytometry; mAb, monoclonal antibody; PBMC, peripheral blood mononuclear cells; PE, phycoerythrin; Treg, T regulatory.
The total cell numbers in spleens and LNs were markedly decreased after WBI, as reported previously (Figure 2a and b).38 The proportions of CD4+ T cells in the spleens and LNs of irradiated C57BL/6 mice were somewhat higher than those of control mice at day 5 after 2-Gy γ-ray irradiation (P<0.01; Figure 2c and d). However, the proportion of CD8+ T cells in the spleens of irradiated C57BL/6 mice was less than that of control mice at different time points (data not shown).
Figure 2.
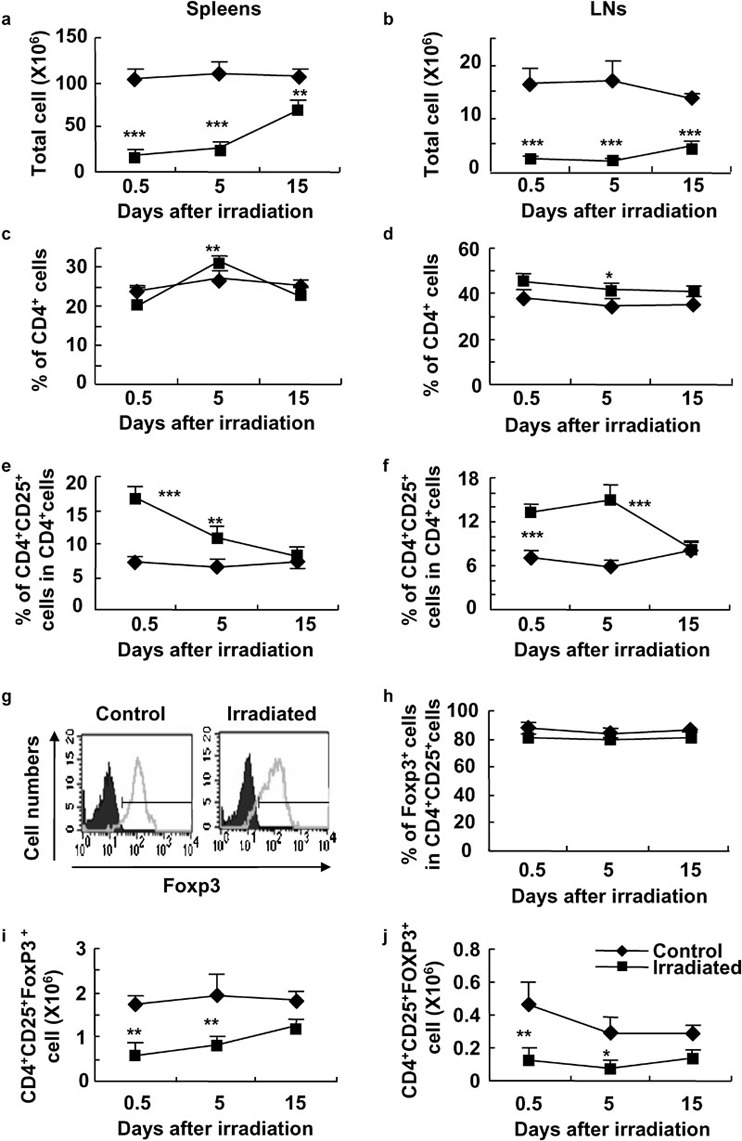
The enhanced ratios of CD4+CD25+ Treg cells to CD4+ T cells in spleens and LNs of mice treated with a low dose of WBI. Cells from spleens and LNs were analyzed for the expression of CD4, CD25, CD8, Foxp3 and PI by FCM. The total cell numbers of splenocytes (a) and cells in LNs (b) in control and WBI mice are shown. The percentages of CD4+ T cells in spleens (c) and LNs (d) in control and WBI mice are shown. The percentages of CD4+CD25+ Treg cells in spleens (e) and LNs (f) in control and irradiated mice are summarized. (g) One representative FCM for Foxp3 staining in CD4+CD25+ cells of LNs is shown. The black part is the isotype control and the gray curve indicates Foxp3 staining. (h) The mean percentages of CD4+CD25+Foxp3+ T cells in CD4+CD25+ T cells of control and WBI mice. The total cell numbers of CD4+CD25+Foxp3+ cells in spleens (i) and LNs (j) in control and WBI mice are shown. The results are shown as mean±SD (n=5 in each group). One representative of three independent experiments is shown. *P<0.05, **P<0.01, ***P<0.001, compared with the indicated groups. FCM, flow cytometry; LN, lymph node; PI, propidium iodide; Treg, T regulatory; WBI, whole-body irradiation.
The percentage of CD4+CD25+ Treg cells in the spleens and LNs of irradiated mice was markedly increased on days 0.5 and 5 after 2-Gy γ-ray irradiation, respectively (P<0.01; Figure 2e and f). As forkhead winged-helix protein Foxp3 is a unique transcription factor in CD4+CD25+ Treg cells and plays a critical role in the development and function of Treg cells,8, 9, 39 we thus evaluated the expression of Foxp3 in CD4+CD25+ Treg cells in mice treated with or without irradiation. The ratio of CD4+CD25+Foxp3+ Treg cells to CD4+CD25+ Treg cells in LNs did not show significant change after WBI (Figure 2g and h). The mean fluorescence intensities of Foxp3 in splenic CD4+CD25+ Treg cells of mice that received WBI were identical to those of control mice (data not shown). Furthermore, the expressions of glucocorticoid-induced tumor necrosis factor receptor, CD44, CD45RB, CD62L, CD69 and CTLA-4 molecules were identical in CD4+CD25+ Treg cells in the spleens of mice treated with or without WBI, as detected by FCM (data not shown). The total cell numbers of CD4+CD25+Foxp3+ Treg cells in spleens and LNs were significantly decreased on day 0.5 and 5 after WBI (P<0.05 or P<0.01; Figure 2i and j). These data suggest that a low dose of WBI could relatively enhance the frequency of CD4+CD25+ Treg cells in the peripheral immune tissues, including blood, spleen and LNs.
CD4+CD25+ Treg cells are functional in mice treated with 2-Gy WBI
It has been demonstrated that CD4+CD25+ Treg cells have an immunosuppressive effect on CD4+CD25− T effector cells.9 To evaluate the effect of WBI on the function of CD4+CD25+ Treg cells, we compared the immunosuppressive effects of CD4+CD25+ Treg cells separated from WBI-treated and control mice using a standard immunoassay. As shown in Figure 3, CD4+CD25+ Treg cells were able to remarkably suppress the ConA-induced proliferation of CD4+CD25− T cells in a dose-dependent manner, regardless of whether the CD4+CD25+ Treg cells were from control or irradiated mice (Figure 3a). Furthermore, CD4+CD25+ Treg cells of WBI-treated and control mice showed identical and significant inhibition on the alloantigen-induced proliferation of CD4+CD25− T cells in a dose-dependent manner (Figure 3b). Thus, 2-Gy WBI did not cause detectable functional changes in CD4+CD25+ Treg cells in mice.
Figure 3.
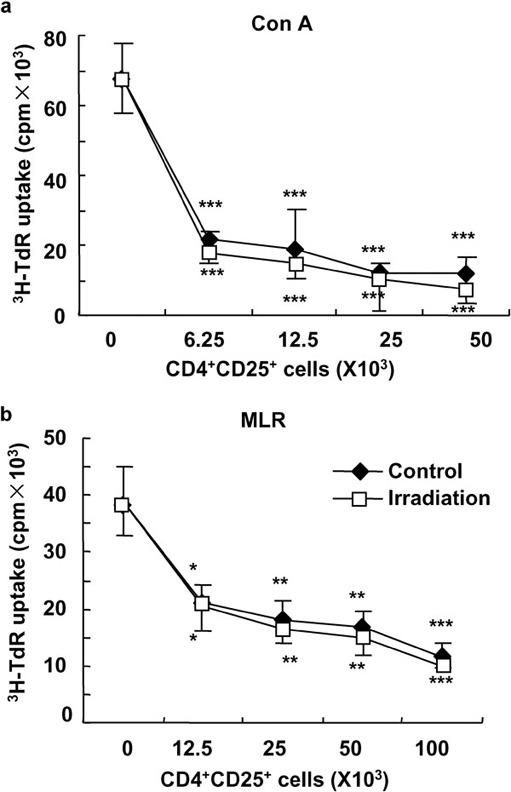
The unimpacted function of CD4+CD25+ Treg cells in mice that received a low dose of γ-ray irradiation. The immunosuppressive function of enriched CD4+CD25+ Treg cells was determined using an in vitro standard assay as described in the section on ‘Materials and methods'. Enriched CD4+CD25+ Treg cells from control and low dose-irradiated mice significantly inhibited the proliferation of CD4+CD25− T cells in response to mitogen ConA (a) or alloantigens (b). *P<0.05, **P<0.01, ***P<0.001, compared with the indicated groups. Data are shown as mean±SD (n=4). One representative of two independent experiments is shown. ConA, concanavalin A; MLR, mixed lymphocyte reaction; Treg, T regulatory.
The percentage of thymic CD4+CD8−CD25+ Treg cells was markedly enhanced in WBI-treated mice
The naturally arising CD4+CD25+ Treg cells are produced in the thymus and play key roles in the maintenance of immunological self-tolerance and negative control of a variety of physiological and pathological immune responses.4 To see whether a low dose of WBI has effects on the development of CD4+CD25+ Treg cells in the thymus, we detected the thymocyte subsets including CD4 and CD8 single-positive (SP), CD4/CD8 double-positive, CD4/CD8 double-negative and CD25+CD4SP cells using multi-color FCM after WBI. As shown in Figure 4, the percentage of double-positive cells in the thymi was remarkably decreased and the percentages of other subsets, including CD4SP, CD8SP and double-negative thymocytes, in the thymi, were significantly increased at day 5 after a low dose of WBI (P<0.05 or P<0.01; Figure 4a, b and c). Interestingly, the percentages of CD25+CD4SP cells or CD4+CD25+Foxp3+ Treg cells in CD4SP cells of irradiated mice were significantly higher than those of the control mice on days 0.5 and 15 after WBI (Figure 4d and data not shown). The total cell numbers of CD4+CD8−CD25+cells in the thymus was significantly decreased on day 0.5 and 5 after WBI (P<0.01; Figure 4e). These data suggest that a low dose of WBI does have a different impact on CD25+CD4SP and CD25−CD4SP thymocyte subsets in mice.
Figure 4.
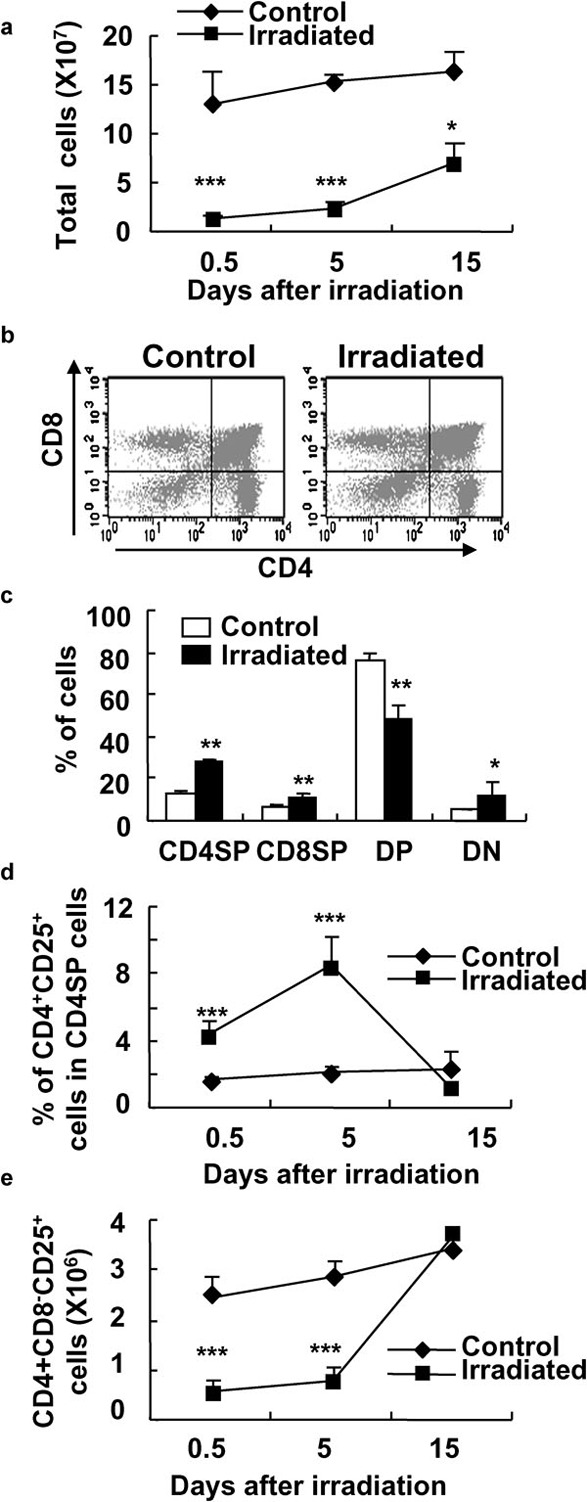
Significantly enhanced frequency of CD4+CD8−CD25+ Treg cells in the thymi of mice after WBI. At different time points after the treatment with 2-Gy γ-ray irradiation, mouse thymocytes were stained with Cy-chrome-labeled anti-CD4 mAb, FITC-labeled anti-CD8 mAb and PE-labeled anti-CD25 mAb and analyzed by FCM. (a) The total cell numbers of thymocytes in control and irradiated mice. (b) One representative of thymocytes of control and WBI mice stained with anti-CD4 and anti-CD8 mAbs and assayed by FCM. Study was done at 5 days after irradiation. (c) The mean percentages of CD4SP, CD8SP, DP and DN thymocytes in control and irradiated mice at 4 days after WBI. (d) The mean percentages of CD4+CD25+ T cells in CD4+CD8− T cells in the thymi of control and irradiated mice. (e) The cell numbers of CD4+CD8−CD25+ T cells in the thymi of control and irradiated mice. *P<0.05, **P<0.01, ***P<0.001, compared with the control mice. The results are shown as mean±SD (n=6). One representative of three independent experiments is shown. DN, double-negative; DP, double-positive; FCM, flow cytometry; FITC, fluorescein isothiocyanate; mAb, monoclonal antibody; PE, phycoerythrin; SP, single-positive; Treg, T regulatory; WBI, whole-body irradiation.
Preferential survival of CD4+CD25+ Treg cells after 2-Gy γ-ray irradiation in vitro
To further determine the sensitivity of CD4+CD25+ Treg cells and CD4+CD25− T cells to a low dose of irradiation, splenocytes and thymocytes were irradiated in vitro. As shown in Figure 5, the percentage of CD4+CD25+ Treg cells in splenic CD4+ T cells was significantly increased after the treatment with 2-Gy irradiation in vitro (P<0.01; Figure 5A), whereas the percentage of Foxp3+ T cells in CD4+CD25+ Treg cells did not show any detectable change after a low dose of irradiation (Figure 5b). We then detected the dead cells by propidium iodide staining with gating of splenic CD4+CD25+ Treg cells and CD4+CD25− T cells. Irradiation caused significant cell death of both CD4+CD25+ Treg cells and CD4+CD25− T cells, as expected (Figure 5c). However, irradiation induced significantly more cell death in CD4+CD25− T cells than that in CD4+CD25+ Treg cells (P<0.01; Figure 5c). Consistent with splenocytes, significantly higher percentages of CD25+CD4SP cells in CD4SP thymocytes were observed after isolated thymocytes received a low dose of irradiation in vitro (P<0.01; Figure 5d). The level of Foxp3 expression in CD4+CD25+ Treg thymocytes did not show any detectable changes after irradiation in vitro (P>0.05; Figure 5e). These results indicate that CD4+CD25+ Treg cells may be more resistant to irradiation than CD4+CD25− T effector cells.
Figure 5.
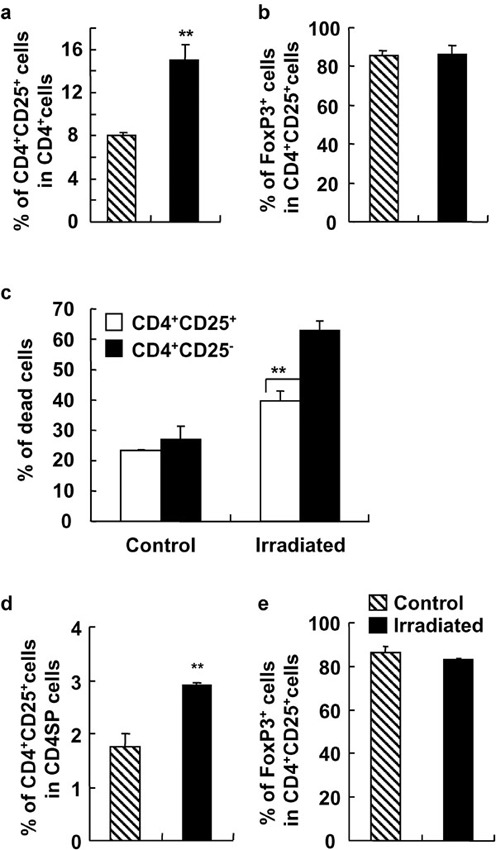
Different responses to γ-ray irradiation of splenic and thymic CD4+CD25+ T cells and CD4+CD25− T cells in vitro. Splenocytes and thymocytes of C57BL/6 mice were irradiated by 2-Gy γ-ray and cultured for 12 h. The cells were analyzed for the expressions of CD4, CD25, CD8 and Foxp3 by FCM. The ratio of CD4+CD25+ T cells to CD4+ T cells (a) and the percentage of CD4+CD25+Foxp3+ cells in CD4+CD25+ T cells (b) of control and irradiated splenocytes are shown. (c) The percentages of dead cells in splenic CD4+CD25+ T cells and CD4+CD25− T cells after irradiation. The ratio of CD4+CD25+ T cells to CD4SP thymocytes (d) and the percentage of Foxp3+ cells in CD25+CD4SP thymocytes (e) after 2-Gy γ-ray irradiation were determined. The results are shown as mean±SD (n=4). One representative of three independent experiments is shown. **P<0.01, compared with the indicated groups. FCM, flow cytometry; SP, single-positive.
Significantly increased percentage of CD4+CD25+ Treg cells in the periphery of thymectomized mice after WBI
To examine whether the enhanced percentage of CD4+CD25+ Treg cells in the periphery of WBI-treated mice is caused by the direct effect of irradiation on the peripheral T-cell subsets or by the high output of the thymus in mice, we used thymectomized mice as recipients to study the level of CD4+CD25+ Treg cells in the periphery after a low dose of WBI. As shown in Figure 6, though CD4+ T cells in PBMCs, spleens and LNs in thymectomized mice were decreased after WBI (P<0.05 or P<0.01; Figure 6a–c), the percentages of CD4+CD25+Foxp3+ Treg cells in PBMCs and spleens but not in LNs were significantly enhanced when thymectomized mice received 2-Gy WBI (P<0.05 or P<0.01; Figure 6d–f). The intercellular Foxp3 expressions in CD4+CD25+ Treg cells in both groups were identical (data not shown). Thus, a low dose of WBI can directly cause the increased frequency of peripheral CD4+CD25+ Treg cells without the contribution of the thymus in mice.
Figure 6.
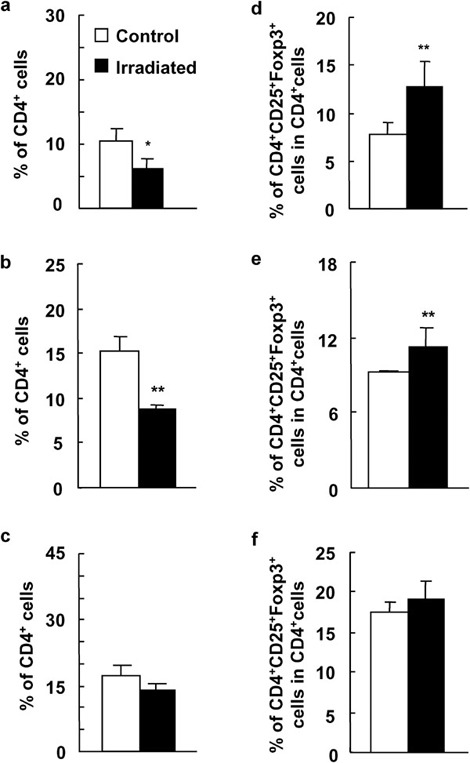
Significantly increased percentage of CD4+CD25+ T cells in thymectomized mice after a low dose of WBI. Fifteen days after thymectomized C57BL/6 mice received 2-Gy γ-ray irradiation, PBMCs, LNs and splenocytes were analyzed for expression of CD4 and CD25 by two-color FCM. The percentages of CD4+ T cells (a) and CD4+CD25+ Treg cells (d) in PBMCs of thymectomized mice that did or did not receive WBI. The percentages of CD4+ T cells (b) and CD4+CD25+ Treg cells (e) in spleens of thymectomized mice that did or did not receive WBI. The percentages of CD4+ T cells (c) and CD4+CD25+ Treg cells (f) in LNs of thymectomized mice that did or did not receive WBI. The results are shown as mean±SD (n=6). One representative of two independent experiments is shown. *P<0.05, **P<0.01, ***P<0.001, compared with the corresponding groups. FCM, flow cytometry; LN, lymph node; PBMC, peripheral blood mononuclear cell; Treg, T regulatory; WBI, whole-body irradiation.
The effect of WBI on the proportion of CD4+CD25+Foxp3+ Treg cells in mouse recipients of allogeneic BMCs
To investigate the effect of a low dose of WBI on CD4+CD25+ Treg cells in hosts with an ongoing immune response and to make the present study more clinically relevant, we observed the impact of 2-Gy WBI on the peripheral level of CD4+CD25+ Treg cells in mice that received allogeneic BMCs on day 15 after WBI as described in the the section on ‘Materials and methods'. First, the donor cells in PBMCs, spleens and LNs were determined by FCM after cells were stained with anti-H-2Dd-FITC and anti-H-2Db-PE mAbs; no donor cells were detected in the samples (data now shown), indicating that donor cells were rejected by host immunity. However, as shown in Figure 7, the frequency of CD4+CD25+ Treg cells or CD4+CD25+Foxp3+ Treg cells in PBMCs, spleens and LNs in mouse recipients of allogeneic BMCs was significantly enhanced after WBI compared with mice that received allogeneic BMCs alone (P<0.05 or P<0.01; Figure 7 and data not shown). These data suggest that a low dose of WBI can significantly increases the frequency of CD4+CD25+ Treg cells in the periphery of immunized mouse hosts.
Figure 7.
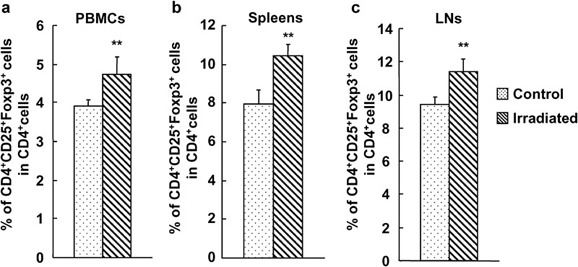
Significantly increased percentage of CD4+CD25+ T cells in mouse recipients of allogeneic BMCs after WBI. Fifteen days after C57BL/6 mice received 2-Gy γ-ray irradiation and BALB/c mice received T cell-depleted BMCs, the cells of PBMCs, LNs and splenocytes were analyzed for the expression of CD4, CD25 and Foxp3 by FCM. The percentages of CD4+CD25+Foxp3+ T cells in CD4+ T cells in PBMCs (a), spleens (b) and LNs (c) in mouse recipients of allogeneic BMCs after 2-Gy γ-ray WBI. The results are shown as mean±SD (n=4). One representative of two independent experiments is shown. *P<0.05, **P<0.01, compared with the control group. BMC, bone marrow cell; FCM, flow cytometry; LN, lymph node; PBMC, peripheral blood mononuclear cell; WBI, whole-body irradiation.
Discussion
In the present study, 2-Gy γ-ray WBI significantly enhanced the frequency of CD4+CD25+ Treg cells and CD4+CD25+Foxp3+ Treg cells not only in the peripheral blood, LNs and spleens, but also in the central immune organ, the thymus. Importantly, 2-Gy WBI also increased the frequency of peripheral CD4+CD25+Foxp3+ Treg cells in thymectomized mice or recipients of allogeneic BMCs. The in vitro study showed that CD4+CD25+Foxp3+ Treg cells are more resistant to ionizing radiation than CD4+CD25− T effector cells. These data indicate that a low dose of WBI can enhance the frequency of peripheral CD4+CD25+ Treg cells in naive and immunized recipients, which may potentially make hosts more susceptible to immune tolerance induction or immunodeficiency in some cases.
Ionizing irradiation has been widely used to induce immunosuppression as it causes injuries in tissue and cells or DNA damage.40, 41 Evidence has shown that various subsets of immune cells have variable sensitivities to ionizing irradiation.42, 43 For example, B cells are sensitive to irradiation, while the T and natural killer cells appear resistant to irradiation-induced cell death, such that these cells are observed at a higher level in the irradiated mice than control mice.24, 44 Our data showed that CD4+CD25+Foxp3+ Treg cells are more resistant to radiation than CD4+CD25− T effector cells. This observation is consistent with the increased resistance of CD4+CD25+Foxp3+ Treg cells to other cell death-inducing reagents such as dexamethasone and anti-CD4-depleting mAb relative to CD4+CD25− T effector cells, as recently reported.33, 45 The molecular mechanisms for the differential responses of murine CD4+CD25+Foxp3+ Treg and CD4+CD25− T cells to cell death may be related to the distinguished expression levels of anti-apoptosis molecules (such as Bcl-2), cell death-relevant molecules or relevant receptors.33, 45, 46, 47
CD4+CD25+Foxp3+ Treg cells keep the immune system intact via preventing excessive inflammation and/or autoimmunity.48, 49 CD4+CD25+Foxp3+ Treg cells in mice that received a low dose of WBI displayed unaltered immunosuppressive function as determined by a standard assay. The transcription factor Foxp3 is recognized to play an essential role in the development and function of CD4+CD25+ Treg cells.8, 9, 10 No detectable alteration of Foxp3 expression or comolecules in/on CD4+CD25+ T cells in spleens, LNs and thymi of 2-Gy γ-ray-irradiated mice may explain why a low dose of irradiation treatment does not affect the immunosuppressive ability of CD4+CD25+ Treg cells. The enhanced ratio of functional CD4+CD25+Foxp3+ Treg cells to CD4+CD25− T cells will drive host cellular immunity favorably to a tolerant or even immunodeficient state.
WBI significantly increased the frequency of CD4+CD25+Foxp3+ Treg cells in the peripheral immune tissues, including blood, spleens and LNs, of thymectomized mice. The different effects of ionizing irradiation on CD4+CD25+Foxp3+ Treg and CD4+CD25− T cells were also observed in an in vitro assay, showing that splenic CD4+CD25+Foxp3+ Treg cells are more resistant to radiation-induced cell death than CD4+CD25− T cells. These data indicate that ionizing irradiation can directly impact the peripheral balance between CD4+CD25+Foxp3+ Treg and CD4+CD25− T cells via the direct effect on the peripheral immune cells, possibly through cell death or apoptosis pathways. However, 2-Gy WBI also significantly increased the frequency of CD4+CD25+Foxp3+ Treg cells in the thymus. Thus, the peripheral enhanced frequency of CD4+CD25+Foxp3+ Treg cells by irradiation may be due to the resistance to cell death of peripheral CD4+CD25+Foxp3+ Treg and/or the increased output of CD4+CD25+Foxp3+ Treg cells from the thymus. The resistance to irradiation of thymic CD4+CD25+Foxp3+ Treg cells was determined by an in vitro assay. These data suggest that the resistance to irradiation of thymic CD4+CD25+Foxp3+ Treg cells may contribute to the enhanced frequency of CD4+CD25+Foxp3+ Treg cells in the thymus.
Consistent with the observation in naive mice, 2-Gy WBI significantly increased the proportion of CD4+CD25+Foxp3+ Treg cells in the periphery of mouse recipients of allogeneic BMCs. WBI is widely used for mixed chimera establishment and other transplant settings to deplete immune and hematopoietic cells and make space.22, 50, 51, 52, 53, 54 The present study suggests that in addition to these direct effects, 2-Gy WBI may enhance the peripheral frequency of CD4+CD25+Foxp3+ Treg cells during bone marrow transplant to avoid or decrease the rejection of allogeneic donor BMCs by host immunity.
It should be noted that reports on the effects of ionizing radiation on different subsets of T cells have been controversial.26, 27, 28, 55, 56 The inconsistencies may be due to differences in animal models, cell samples, radiation ratios and observation time points. Understanding the reasons for the inconsistent data in the future is essential.
In summary, CD4+CD25+Foxp3+ Treg cells are more resistant to ionizing irradiation-induced cell death than CD4+CD25− T cells. WBI significantly enhanced the percentages of peripheral CD4+CD25+Foxp3+ Treg cells in naive or BMCs-transplanted mice without altering their immunosuppressive function. Though further studies are required to better define mechanisms of this process, the present data suggest that WBI may make hosts more susceptible to tolerance induction.
Acknowledgments
The authors wish to thank Dr Yiying Luo for his expertise and assistance on the thymectomy experiments, Ms Jianxia Peng and Ms Jing Wang for their expertise and technical assistance, and Ms Qinghuan Li for her excellent laboratory management. This work was supported by grants from the Chinese Academy of Sciences (KJCX2-YW-L08, YZ) and the National Natural Science Foundation, China (30630060, YZ).
References
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, et al. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Toda A, Piccirillo CA. Development and function of naturally occurring CD4+CD25+ regulatory T cells. J Leukoc Biol. 2006;80:458–470. doi: 10.1189/jlb.0206095. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Hiura T, Kagamu H, Miura S, Ishida A, Tanaka H, Tanaka J, et al. Both regulatory T cells and antitumor effector T cells are primed in the same draining lymph nodes during tumor progression. J Immunol. 2005;175:5058–5066. doi: 10.4049/jimmunol.175.8.5058. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- d'Hennezel E, Ben-Shoshan M, Ochs HD, Torgerson TR, Russell LJ, Lejtenyi C, et al. FOXP3 forkhead domain mutation and regulatory T cells in the IPEX syndrome. N Engl J Med. 2009;361:1710–1713. doi: 10.1056/NEJMc0907093. [DOI] [PubMed] [Google Scholar]
- Le Bras S, Geha RS. IPEX and the role of Foxp3 in the development and function of human Tregs. J Clin Invest. 2006;116:1473–1475. doi: 10.1172/JCI28880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage. Immunity. 2003;19:165–168. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Zhang C, Todorov I, Lin CL, Atkinson M, Kandeel F, Forman S, et al. Elimination of insulitis and augmentation of islet beta cell regeneration via induction of chimerism in overtly diabetic NOD mice. Proc Natl Acad Sci USA. 2007;104:2337–2342. doi: 10.1073/pnas.0611101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo-Saito C, Schlom J, Camphausen K, Coleman CN, Hodge JW. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clin Cancer Res. 2005;11:4533–4544. doi: 10.1158/1078-0432.CCR-04-2237. [DOI] [PubMed] [Google Scholar]
- Cunha-Rodrigues M, Portugal S, Febbraio M, Mota MM. Bone marrow chimeric mice reveal a dual role for CD36 in Plasmodium berghei ANKA infection. Malar J. 2007;6:32. doi: 10.1186/1475-2875-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Geddes JW, Owens JL, Holmberg EG. X-irradiation reduces lesion scarring at the contusion site of adult rat spinal cord. Histol Histopathol. 2005;20:519–530. doi: 10.14670/HH-20.519. [DOI] [PubMed] [Google Scholar]
- Kajioka EH, Andres ML, Li J, Mao XW, Moyers MF, Nelson GA, et al. Acute effects of whole-body proton irradiation on the immune system of the mouse. Radiat Res. 2000;153:587–594. doi: 10.1667/0033-7587(2000)153[0587:aeowbp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Park HR, Jo SK, Paik SG. The NK1.1+ T cells alive in irradiated mice play an important role in a Th1/Th2 balance. Int J Radiat Biol. 2006;82:161–170. doi: 10.1080/09553000600632873. [DOI] [PubMed] [Google Scholar]
- Pecaut MJ, Nelson GA, Gridley DS. Dose and dose rate effects of whole-body gamma-irradiation: I. Lymphocytes and lymphoid organs. In Vivo. 2001;15:195–208. [PubMed] [Google Scholar]
- Tsukimoto M, Nakatsukasa H, Sugawara K, Yamashita K, Kojima S. Repeated 0.5-Gy gamma irradiation attenuates experimental autoimmune encephalomyelitis with up-regulation of regulatory T cells and suppression of IL17 production. Radiat Res. 2008;170:429–436. doi: 10.1667/rr1352.1. [DOI] [PubMed] [Google Scholar]
- Qu Y, Jin S, Zhang A, Zhang B, Shi X, Wang J, et al. Gamma-ray resistance of regulatory CD4+CD25+Foxp3+ T cells in mice. Radiat Res. 2010;173:148–157. doi: 10.1667/RR0978.1. [DOI] [PubMed] [Google Scholar]
- Liu R, Xiong S, Zhang L, Chu Y. Enhancement of antitumor immunity by low-dose total body irradiationis associated with selectively decreasing the proportion and number of T regulatory cells. Cell Mol Immunol. 2010;7:157–162. doi: 10.1038/cmi.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Avidan H, Markovich Y, Mizrahi T, Hauben E, Prigozhina TB, et al. Low-dose gamma-irradiation promotes survival of injured neurons in the central nervous system via homeostasis-driven proliferation of T cells. Eur J Neurosci. 2004;19:1191–1198. doi: 10.1111/j.1460-9568.2004.03207.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li H, Langnas AN, Zhao Y. Altered allogeneic immune responses in middle-aged mice. Cell Mol Immunol. 2004;1:440–446. [PubMed] [Google Scholar]
- Sun Z, Zhao L, Wang H, Sun L, Yi H, Zhao Y. Presence of functional mouse regulatory CD4+CD25+ T cells in xenogeneic neonatal porcine thymus-grafted athymic mice. Am J Transplant. 2006;6:2841–2850. doi: 10.1111/j.1600-6143.2006.01549.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ge BS, Kasai M, Diffendaffer C, Parks N, Li H, et al. Induction of regulatory T cells from mature T cells by allogeneic thymic epithelial cells in vitro. . Transpl Int. 2006;19:404–414. doi: 10.1111/j.1432-2277.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- Yi H, Zhen Y, Zeng C, Zhang L, Zhao Y. Depleting anti-CD4 monoclonal antibody (GK1.5) treatment: influence on regulatory CD4+CD25+Foxp3+ T cells in mice. Transplantation. 2008;85:1167–1174. doi: 10.1097/TP.0b013e31816a1242. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhao L, Sun Z, Sun L, Zhang B, Zhao Y. A potential side effect of cyclosporin A: inhibition of CD4+CD25+ regulatory T cells in mice. Transplantation. 2006;82:1484–1492. doi: 10.1097/01.tp.0000246312.89689.17. [DOI] [PubMed] [Google Scholar]
- Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81:1386–1394. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- Jones CM, Callaghan JM, Gleeson PA, Mori Y, Masuda T, Toh BH. The parietal cell autoantigens recognized in neonatal thymectomy-induced murine gastritis are the alpha and beta subunits of the gastric proton pump [corrected] Gastroenterology. 1991;101:287–294. doi: 10.1016/0016-5085(91)90002-3. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Swenson K, Sergio JJ, Arn JS, Sachs DH, Sykes M. Skin graft tolerance across a discordant xenogeneic barrier. Nat Med. 1996;2:1211–1216. doi: 10.1038/nm1196-1211. [DOI] [PubMed] [Google Scholar]
- Yao Z, Liu Y, Jones J, Strober S. Differences in Bcl-2 expression by T-cell subsets alter their balance after in vivo irradiation to favor CD4+Bcl-2hi NKT cells. Eur J Immunol. 2009;39:763–775. doi: 10.1002/eji.200838657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo CA, Thornton AM. Cornerstone of peripheral tolerance: naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol. 2004;25:374–380. doi: 10.1016/j.it.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Urashima T, Nagasawa H, Wang K, Adelstein SJ, Little JB, Kassis AI. Induction of apoptosis in human tumor cells after exposure to Auger electrons: comparison with gamma-ray exposure. Nucl Med Biol. 2006;33:1055–1063. doi: 10.1016/j.nucmedbio.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya N. New concepts in radiation-induced apoptosis: ‘premitotic apoptosis' and ‘postmitotic apoptosis'. J Cell Mol Med. 2001;5:240–253. doi: 10.1111/j.1582-4934.2001.tb00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taibi N, Aka P, Kirsch-Volders M, Bourgeois P, Fruhling J, Szpireer C. Radiobiological effect of 99mTechnetium-MIBI in human peripheral blood lymphocytes: ex vivo study using micronucleus/FISH assay. Cancer Lett. 2006;233:68–78. doi: 10.1016/j.canlet.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Sprung CN, Chao M, Leong T, McKay MJ. Chromosomal radiosensitivity in two cell lineages derived from clinically radiosensitive cancer patients. Clin Cancer Res. 2005;11:6352–6358. doi: 10.1158/1078-0432.CCR-04-1931. [DOI] [PubMed] [Google Scholar]
- Park HR, Jo SK, Paik SG. Factors effecting the Th2-like immune response after gamma-irradiation: low production of IL-12 heterodimer in antigen-presenting cells and small expression of the IL-12 receptor in T cells. Int J Radiat Biol. 2005;81:221–231. doi: 10.1080/09553000500077088. [DOI] [PubMed] [Google Scholar]
- Chen X, Murakami T, Oppenheim JJ, Howard OM. Differential response of murine CD4+CD25+ and CD4+CD25− T cells to dexamethasone-induced cell death. Eur J Immunol. 2004;34:859–869. doi: 10.1002/eji.200324506. [DOI] [PubMed] [Google Scholar]
- Banz A, Pontoux C, Papiernik M. Modulation of Fas-dependent apoptosis: a dynamic process controlling both the persistence and death of CD4 regulatory T cells and effector T cells. J Immunol. 2002;169:750–757. doi: 10.4049/jimmunol.169.2.750. [DOI] [PubMed] [Google Scholar]
- Pati N, Ghosh S, Hessner MJ, Khoo HJ, Wang X. Difference in gene expression profiles between human CD4+CD25+ and CD4+CD25− T cells. Ann NY Acad Sci. 2003;1005:279–283. doi: 10.1196/annals.1288.043. [DOI] [PubMed] [Google Scholar]
- Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol. 2007;7:231–237. doi: 10.1038/nri2037. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417–424. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- Comerci GD, Jr, Williams TM, Kellie S. Immune tolerance after total lymphoid irradiation for heart transplantation: immunosuppressant-free survival for 8 years. J Heart Lung Transplant. 2009;28:743–745. doi: 10.1016/j.healun.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Sharabi Y, Abraham VS, Sykes M, Sachs DH. Mixed allogeneic chimeras prepared by a non-myeloablative regimen: requirement for chimerism to maintain tolerance. Bone Marrow Transplant. 1992;9:191–197. [PubMed] [Google Scholar]
- Tomita Y, Khan A, Sykes M. Mechanism by which additional monoclonal antibody (mAB) injections overcome the requirement for thymic irradiation to achieve mixed chimerism in mice receiving bone marrow transplantation after conditioning with anti-T cell mABs and 3-Gy whole body irradiation. Transplantation. 1996;61:477–485. doi: 10.1097/00007890-199602150-00028. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Zeng D, Shizuru J, Gworek J, Dejbakhsh-Jones S, Taniguchi M, et al. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169:5564–5570. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- Cao M, Cabrera R, Xu Y, Liu C, Nelson D. Gamma irradiation alters the phenotype and function of CD4+CD25+ regulatory T cells. Cell Biol Int. 2009;33:565–571. doi: 10.1016/j.cellbi.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke G, Avsar M, Morancho M, Peters C, Thissen S, Kruse B, et al. Preoperative low-dose irradiation promotes long-term allograft acceptance and induces regulatory T cells in a porcine model of pulmonary transplantation. Transplantation. 2006;82:93–101. doi: 10.1097/01.tp.0000225833.23093.ed. [DOI] [PubMed] [Google Scholar]


