Abstract
Aim:
To explore whether intestinal microflora plays a role in anti-pruritic activity of baicalin, a main constituent of the rhizome of Scutellaria baicalensis (SB).
Methods:
Baicalin was anaerobically incubated with human fecal microflora, and its metabolites, baicalein and oroxylin A, were isolated. The inhibitory effect of baicalin and its metabolites was accessed in histamine- or compound 48/80-induced scratching behavior in mice.
Results:
Baicalin was metabolized to baicalein and oroxylin A, with metabolic activities of 40.2±26.2 and 1.2±1.1 nmol·h−1·mg−1 wet weight of human fecal microflora, respectively. Baicalin (20, 50 mg/kg) showed more potent inhibitory effect on histamine-induced scratching behavior when orally administered than intraperitoneally. In contrast, baicalein and oroxylin A had more potent inhibitory effect when the intraperitoneally administered. The anti-scratching behavior activity of oral baicalin and its metabolites was in proportion to their inhibition on histamine-induced increase of vascular permeability with oroxylin A more potent than baicalein and baicalin. In Magnus test using guinea pig ileum, oroxylin A is more potent than baicalein and baicalin in inhibition of histamine-induced contraction. The anti-scratching behavioral effect of oral baicalin was significantly reduced when oral antibiotics were simultaneously administered, whereas the effect of baicalein and oroxylin A were not affected.
Conclusion:
Oral baicalin may be metabolized by intestinal microflora into baicalein and oroxylin A, which ameliorate pruritic reactions through anti-histamine action.
Keywords: Scutellaria baicalensis, baicalin, baicalein, oroxylin A, scratching behavior, metabolism, histamine
Introduction
Pruritus, an unpleasant cutaneous sensation which provokes the desire to scratch, can be local or widespread and is associated with atopic dermatitis, uriticaria or systemic disorders (cholestasis, uraemia). Many endogenous chemical agents, like amines, proteases, growth factors, neuropeptides, opioids, ecosanoids, and cytokines can act as pruritogens1, 2. Itching can cause skin lesions and contribute to severe psychological disturbances3. Therefore, inhibition of this response is beneficial for improving the quality of life. To evaluate the anti-pruritic effect of agents, histamine or substance P-induced scratching behavioral mouse models were used1, 2, 3, 4, 5.
The rhizome of Scutellaria baicalensis (SB), which contains baicalin as a main constituent, has long been used in China, Japan, and Korea as a traditional medicine and functional food for inflammation, fever, hepatitis, allergic disease, hypertension, etc6, 7. Baicalin exhibits anti-inflammatory, anti-allergic, anti-oxidant, hepatoprotective, and anti-tumor effects8, 9, 10. It is generally assumed that baicalin is poorly absorbed from the gastrointestinal tract in its native form and must be hydrolyzed by intestinal microflora in the intestine to their aglycones in human and rats11, 12. The metabolite, baicalein, is subsequently conjugated to baicalin, baicalein 6-O-β-glucuronide-7-O-sulfate and baicalein 6,7-diglucuronide in the gut mucosae, liver, and blood13, 14, 15, 16, 17. Akao et al suggested that baicalin might be hydrolyzed to baicalein by intestinal bacteria, absorbed into the blood and conjugated with glucuronic acid and/or sulfuric acid11. However, Abe et al found two kinds of metabolites, two baicalein conjugates and one oroxylin A conjugate, as main metabolites in the bile of rats orally treated with baicalin13. Nevertheless, it has not been clarified where baicalein is transformed to oroxylin A.
Therefore, to investigate the relationship between the metabolites of baicalin and their pharmacological effects, we isolated baicalin from SB, indentified its metabolites by human fecal microflora, and investigated their inhibitory effect in experimental scratching behavior induced by histamine or compound 48/80 in mice. To understand the role of intestinal microflora in their anti-scratching behavioral effect, we also investigated their inhibitory effects in antibiotics-treated mice.
Materials and methods
Drugs and chemicals
Azelastine, histamine, and compound 48/80 were purchased from Sigma Chemical Co (St Louis, MO, USA). Baicalin (purity, >95%) was purified from the rhizome of Scutellaria baicalensis according to the previously reported methods of Takido et al18.
Metabolism of baicalin by human intestinal microflora
Five fecal specimens (each, about 30 g) from human males in their twenties were collected, and then mixed and suspended with cold 270 mL saline according to a previous method19. The fecal suspension was centrifuged at 100×g for 5 min. The supernatant was then centrifuged at 10 000×g for 20 min. The resulting precipitates (about 3 g) were used as a metabolic enzyme source for the assay of enzyme activity. The preparation and assay of the enzyme source were performed within 24 h at 4 °C.
To identify the metabolites of baicalin by human intestinal bacteria, the reaction mixture contained 100 mg of baicalin and 2.5 g fresh human feces in a final volume of 100 mL of anaerobic dilution medium. The mixture was incubated at 37 °C for 20 h. An aliquot (1 mL) of the reaction mixture was periodically extracted with ethyl acetate, concentrated, dissolved in MeOH, and their metabolites were analyzed by LC-MS/MS [Agilent 1200 series LC-MS/MS system consisting of a quaternary pump, a vacuum degasser, an autosampler, a thermostat column compartment, a diode array detector, and an Agilent G6410 triple quadrupole mass spectrometer with an electro spray ionization (ESI) source]: column, ZORBAX Extend C18 (100 mm×2.1 mm id, 1.8 μm, Agilent); elution solvent, a linear-gradient applied by 5% solvent A to 95% B for 13 min (solvent A-0.1% aqueous formic acid and solvent B-acetonitrile); and elution rate, 0.3 mL/min. Mass spectra was acquired in ESI mode using nitrogen gas at a temperature of 350 °C, flow rate of 10 L/min, nebulizer pressure of 45 psi, quadrupole temperature of 30 °C, and capillary voltage of 4000 V. The mass spectrometer was operated in positive mode with multiple reaction monitoring (MRM). The instrument was controlled and data were processed by Agilent MassHunter workstation software (Rev B.01.00).
Each odd reaction mixture was evaporated to afford an EtOAc fraction (0.1 mg). The ethyl acetate fraction was subjected to a mediurn pressure liquid chromatography (MPLC) equipped with a slica gel 100 C18 column (3 cm×50 cm) - reversed phase eluted with 30% MeOH (5 L). The EtOAc fraction of baicalin afforded two metabolites, baicalein (21 mg) and oroxylin A (11 mg). These metabolites were verified by comparison of their NMR data with those of the literatures18, 20.
Baicalein
Pale yellow needles. mp 258–260 °C, FABMS (m/z): 271 [M+1]+.
Oroxylin A
Pale yellow needles. mp 203–204 °C, FABMS (m/z): 285.5 [M+1]+.
Animals
The male ICR and BALB/c mice (18–22 g) were supplied from Charles River Orient Experimental Animal Breeding Center (Seoul, Korea). All animals were housed in wire cages at 20–22 °C, a relative humidity of 50%±10%, a frequency of air ventilation of 15–20 times/h, and 12 h illumination (0.7:00–19:00; intensity, 150–300 Lux), fed standard laboratory chow (Charles River Orient Experimental Animal Breeding Center, Seoul Korea) and allowed water ad libitum. All procedures relating to the animals and their care conformed to the international guidelines 'Principles of Laboratory Animals Care' (NIH publication No 85–23, revised 1985; Kyung Hee University, Animal Experiment Guideline 2006).
Scratching behavioral experiment
Before the experiment, ICR and BALB/c mice were put into acrylic cages (22 cm×22 cm×24 cm) for about 10 min for acclimation. The scratching behavioral experiments were performed according to the method of Sugimoto et al21. The rostral part of the skin on the backs of mice was clipped, and 300 μg/50 μL of histamine (in ICR mice) or 50 μg/50 μL of compound 48/80 (in BALB/c mice) for each mouse was intradermally injected with the use of a 29 gauge needle. The scratching agents were dissolved in saline prior to injection. Control mice received a saline injection in place of the scratching agent. Immediately after the intradermal injection, the mice (one animal/cage) were put back into the same cage for the observation of scratching behavior; their behaviors were recorded using an 8-mm video camera (SV-K80, Samsung, Seoul, Korea) under unmanned conditions. The scratching behavioral frequency of the injected site by the hind paws was counted and compared with that of other sites, such as the ears. Each mouse was used for only one experiment. The mice generally showed a scratching behavioral frequency of several scratching behaviors per second, and a series of these behaviors were counted as one incident of the scratching behaviors for 60 min.
Test agents were orally or intraperitoneally administered either 1 or 5 h before treatment with the scratching agent, histamine, in mice treated with or without antibiotics. The antibiotics-treated groups were treated with streptomycin (100 mg/kg) and tetracycline (50 mg/kg) orally 24 h before the injection of histamine, a scratching inducer.
Measurement of vascular permeability
The increase in vascular permeability caused by histamine was assessed as reported previously22. After the intradermal injection of 300 μg/50 μL of histamine into the rostral part of the back of each mouse, 0.2 mL of 1% Evans blue solution in saline was injected intravenously. Baicalin and baicalein were orally administered 1 h before treatment with histamine. Mice were sacrificed 60 min later by ether and the scratching agent-injected site excised. The skin specimen was dissolved in 1 mL of 1 mol/L KOH solution by overnight incubation, and 4 mL of a mixture of 0.2 mol/L phosphoric acid solution-acetone (5:13) was added. After vigorous shaking, the precipitates were filtered off and the amount of dye was measured colorimetrically at 620 nm.
Histopathologic examination
The skin specimen injected with histamine, was postfixed in 50 mmol/L phosphate buffer (pH 7.4) containing 4% paraformaldehyde overnight and then immersed in 30% sucrose solution (in 50 mmol/L phosphate buffered saline). Frozen specimen was sectioned in a cryostat at 30 μm and stained with hematoxylin-eosin, and then assessed under light microscopy.
Anti-histamine action assay
Male Hartley guinea pigs (300±30 g) were sacrificed by exsanguination and the ilea were prepared in cold Tyrode's solution. The prepared ileal strip was then suspended in a 10 mL Magnus tube (32 °C, 95 % O2+5% CO2) containing Tyrode's solution. Each test agent was added to the preparation 30 s before treatment with histamine (1×10−6mol/L). The percentage contraction is shown as a percentage of the maximal response to histamine.
Statistical analysis
All the data were expressed as the mean±standard deviation, and statistical significance was analyzed by one way ANOVA followed by Student-Newman-Keuls test.
Results
Metabolism of baicalin by human intestinal microflora
To investigate the metabolites of baicalin produced by human intestinal microflora, it was anaerobically incubated with human fecal microflora for 20 h and analyzed by LC-MS/MS (Figure 1A). In the reaction mixture of baicalin, two metabolites, one major and one minor, were observed. A main metabolite possessed an MS peak at m/z=271 [M+1]+, and the minor metabolite produced an MS peak at m/z=285.5 [M+1]+ (Figure 1A). In order to identify these metabolites, we anaerobically incubated baicalin with human fecal microflora, isolated its metabolites and analyzed them by 1H and 13C NMR. When their NMR data were compared with those of the literatures18, 20, the metabolites having (m/z): 271 [M+1]+ and 285.5 [M+1]+ ion peaks in FAB-MS were baicalein and oroxylin A, respectively.
Figure 1.
Metabolism of baicalin by human fecal microflora. (A) Identification of baicalin isolated (a) and baicalin metabolites with LC-MS/MS; (i) baicalin, (ii) baicalein, and (iii) oroxylin A. (B) Activity of baicalin metabolism by human fecal microflora. (n=5). (C) Proposed pathway of baicalin metabolism by human intestinal microflora. →, main pathway;  , minor pathway.
, minor pathway.
When the baicalin-metabolizing activity of five human fecal specimens was preliminarily assayed, the average of the hydrolyzing activity of baicalin to its aglycone was 40.2±26.2 nmol·h−1·mg−1 wet weight of fecal bacteria (Figure 1B). The transforming activity of baicalin to oroxylin A was 1.2±1.1 nmol·h−1·mg−1 wet weight of fecal bacteria.
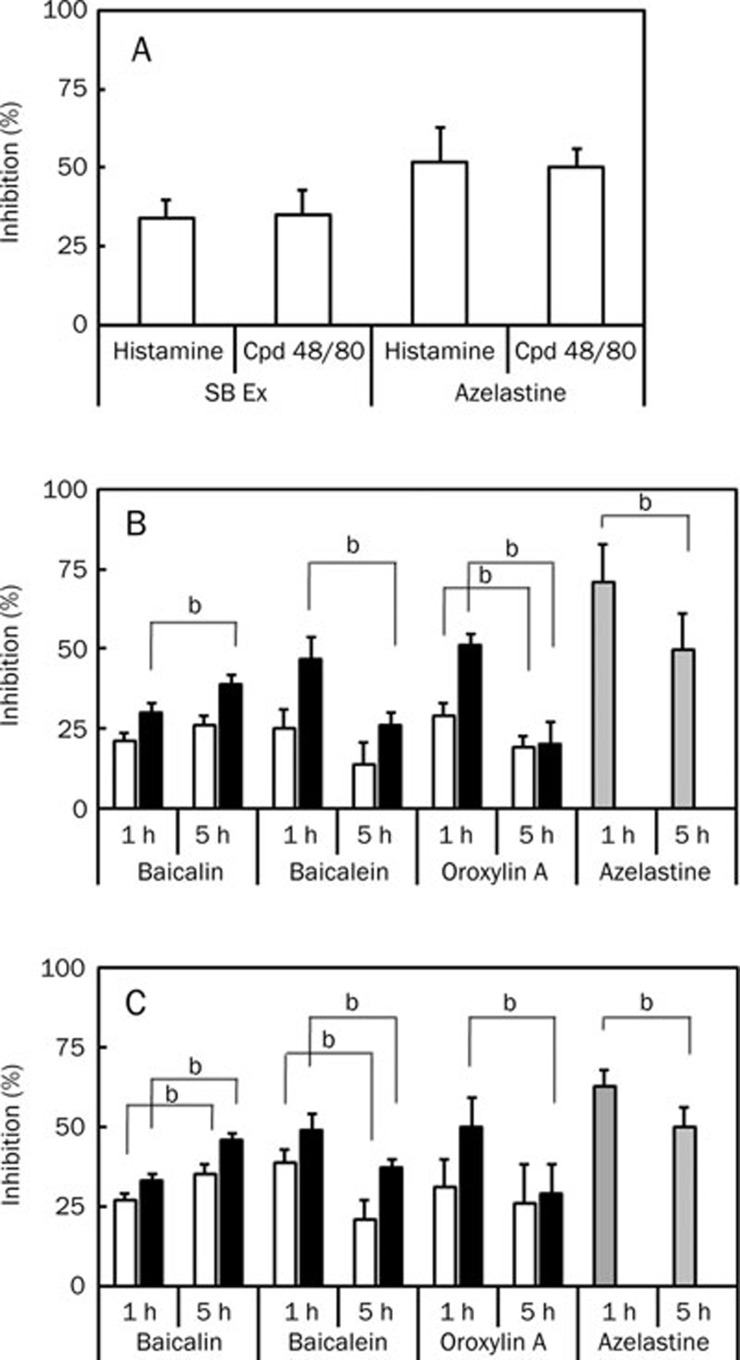
Effect of orally administered baicalin and its metabolites on scratching behavior induced histamine or compound 48/80 in mice
SB reduced scratching behavior induced by histamine or compound 48/80 (Figure 2A). Therefore, we isolated baicalin, a main constituent of SB, and its metabolites, baicalein and oroxylin A, and their inhibitory effects were investigated in scratching behavior induced by histamine (Figure 2B). When baicalin at doses of 20 and 50 mg/kg was administered orally 1 or 5 h before treatment with histamine, it inhibited the scratching behavior. When baicalin (50 mg/kg) was administered orally 5 h before treatment with histamine, it had a more potent inhibitory effect on the scratching behavior than when administered 1 h before treatment with histamine (P<0.05). When its metabolites, baicalein and oroxylin A, at doses of 20 and 50 mg/kg were administered orally 1 or 5 h before treatment with histamine, these metabolites 1 h before treatment with histamine had a more potent inhibitory effect on the scratching behavior than when administered 5 h before treatment with histamine.
Figure 2.
Inhibitory effects of orally administered Scutellariae rhizome extract (SB ex), baicalin, its metabolites, and azelastine, on scratching behavior induced by histamine or compound 48/80 in mice. The scratching behavior was induced by histamine and compound 48/80 in ICR and BALB/c mice, respectively. (A) Effect of SB ex. Treatment with SB ex (100 mg/kg) or azelastine (10 mg/kg) orally 5 h before the intradermal injection of 300 μg/50 μL of histamine or 50 μg/50 μL of compound (Cpd) 48/80. (B) Effect of baicalin, baicalein, oroxylin A and azelastine in histamine-induced scratching behavior in ICR mice. (C) Effect of baicalin, baicalein, oroxylin A and azelastine on compound 48/80-induced scratching behavior in BALB/c mice. These agents [10 (gray bar), 20 (white bar) and 50 mg/kg (black bar)] were administered orally 1 or 5 h before the intradermal injection of histamine or compound 48/80. The frequency of scratching behavior in the normal group treated with saline alone for 1 h were 2±1, respectively, and the frequency of scratching behavior in the control group treated with histamine in ICR mice and compound 48/80 in BALB/c mice was 89±5 and 168±18, respectively. The values indicate the mean±SD (n=6). bP<0.05.
We also investigated the inhibitory effects of baicalin, baicalein, and oroxylin A on compound 48/80-induced scratching behavior (Figure 2C). The oral administration of baicalin at doses of 20 and 50 mg/kg 5 h before treatment with compound 48/80 had a more potent inhibitory effect on scratching behavior than baicalin 20 and 50 mg/kg administered 1 h before treatment with compound 48/80, which was similar to histamine. The metabolites, baicalein (20 and 50 mg/kg) and oroxylin A (50 mg/kg), when administered 1 h before treatment with histamine, inhibited the scratching behavior compared to administration 5 h before treatment with histamine.
Effects of intraperitoneally administered baicalin and its metabolites on histamine-induced scratching behavior in mice
The inhibitory effects of intraperitoneally administered baicalin and its metabolites, baicalein and oroxylin A, (10 and 20 mg/kg) on histamine-induced scratching behavior were measured (Figure 3). Although all these agents, baicalin and its metabolites, inhibited the scratching behavior, baicalein and oroxylin A were more effective than baicalin. The anti-scratching behavior activity of oral baicalin and its metabolites was in proportion to their inhibition on histamine-induced increase of vascular permeability with oroxylin A more potent than baicalein and baicalin.
Figure 3.
Inhibitory effects of baicalin, its metabolites, and azelastine administered intraperitonelly on histamine-induced scratching behavior in mice. The scratching behavior was induced by histamine in ICR mice. Mice were treated with or without intraperioneal administration of the test agents [5 (gray bar), 10 (white bar) and 20 mg/kg (black bar)] 1 h before the intradermal injection of 300 μg/50 μL of histamine into the skin on the backs of mice. The frequency of scratching behavior in the normal (treated with saline alone) and control groups (treated with histamine) for 1 h was 2±1 and 85±4, respectively. The values indicate the mean±SD. (n=6). bP<0.05.
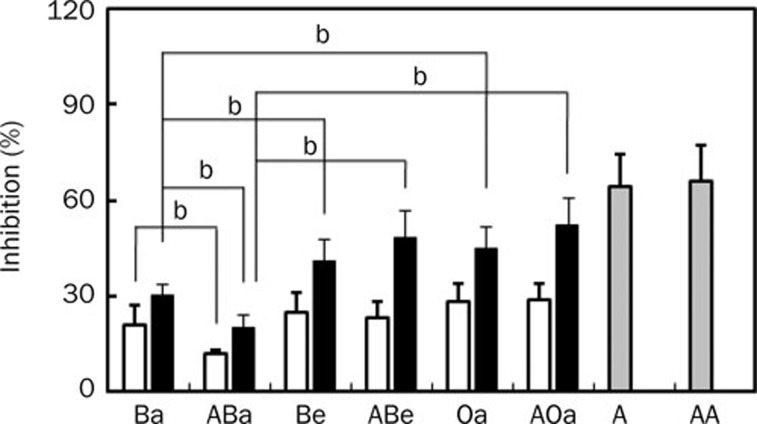
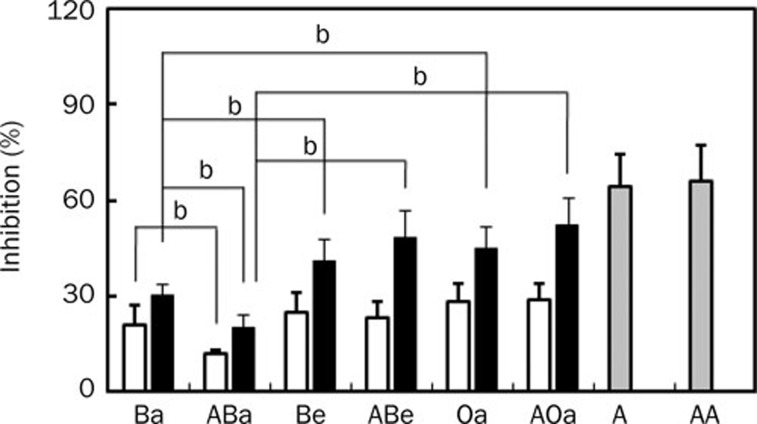
Effects of orally administered baicalin, baicalein, and oroxylin A on histamine-induced scratching behavior in antibiotics-treated mice
To understand the role of intestinal microflora in the anti-scratching behavioral effect of baicalin, the inhibitory effects of baicalin and its metabolites, baicalein and oroxylin A, in mice treated with and without antibiotics were investigated (Figure 4). Baicalin inhibited histamine-induced scratching behavior in mice treated with and without antibiotics. Of them, oral administration of baicalin in antibiotics-untreated mice showed a more potent inhibition than in antibiotics-treated mice. However, the anti-scratching behavioral effects of baicalein and oroxylin A on mice treated with and without antibiotics were not different. Their anti-scratching behavioral effects were more potent than that of baicalin.
Figure 4.
Inhibitory effects of baicalin, baicalein, and azelastine administered orally on histamine-induced scratching behavior in mice treated with or without antibiotics. The scratching behavior was induced by histamine in ICR mice treated with or without antibiotics. The antibiotics were administered orally 1 d before histamine treatment. Oral administration of test agents [10 (gray bar), 20 (white bar), and/or 50 mg/kg (black bar)] was given 1 h before the intradermal injection of 300 μg/50 μL of histamine into the skin on the backs of mice. The frequency of scratching behavior in the normal group treated with saline alone in normal and antibiotics-treated mice for 1 h were 2±2 and 2±1, respectively, and the frequency in the control group treated with histamine in normal and antibiotics-treated mice was 84±4 and 87±5, respectively. Ba, baicalin; ABa, baicalin with antibiotics; Be, baicalein; ABe, baicalein with antibiotics; Oa, oroxylin A; AOa, oroxylin A with antibiotics; A, azelastine; AA azelastine with antibiotics. The values indicate the mean±SD. n=6. bP<0.05.
Anti-histamine effect of orally administered baicalin, baicalein and oroxylin A
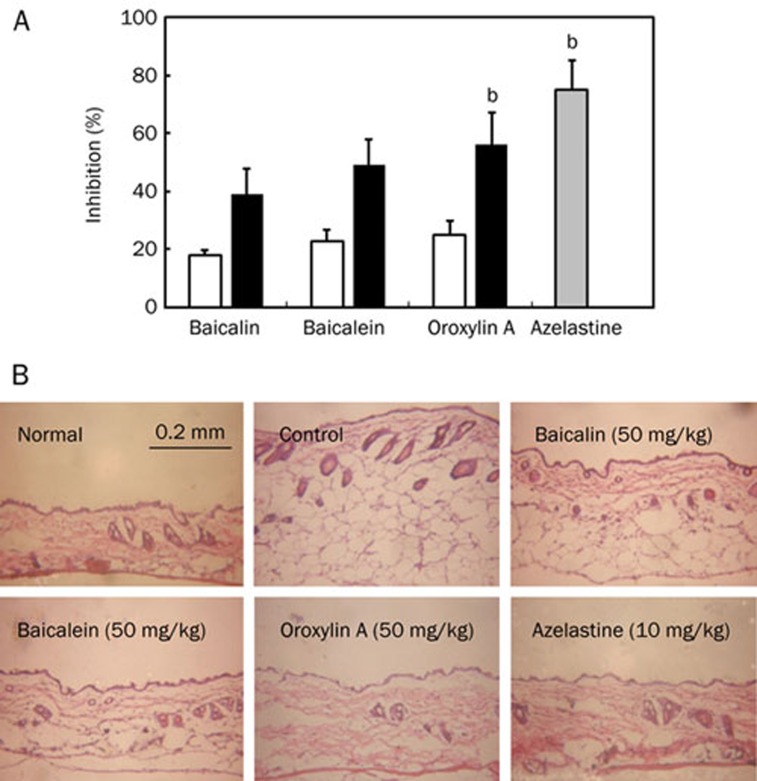
When histamine was used as an inducer for scratching, the histamine not only induced scratching behaviors, but also increased vascular permeability. Baicalin, baicalein and oroxylin A at doses of 20 and 50 mg/kg inhibited vascular permeability induced by histamine (Figure 5A). The inhibitory activities of these compounds against vascular permeability were in proportion to the inhibition against scratching behavior. The histopathologic examination of skin specimens showed that histamine caused massive edema of epithelial cells, but baicalin, baicalein or oroxylin A inhibited histamine-induced edema (Figure 5B). Among the test agents, oroxylin A had the most potently inhibitory effect.
Figure 5.
Inhibitory effects of baicalin, its metabolites, and azelastine administered orally on vascular permeability increased by histamine in mice. (A) Effect in vascular permeability. The vascular permeability was increased by histamine in ICR mice. Mice were treated with or without the oral administration of test agents [10 (gray bar), 20 (white bar), and/or 50 mg/kg (black bar)] 1 h before the intradermal injection of 300 μg/50 μL of histamine into the skin on the backs of mice. In the vascular permeability assay, the amount of Evan blue extravasated from the dorsal skin (1 cm×1 cm) of the control group stimulated with histamine and the vehicle-treated group was 12±3 μg and 4±2 μg, respectively. The values indicate the mean±SD. (n=6). bP<0.05 vs baicalin (50 mg/kg) treated group. (B) Histologic photograph. The skin tissues were stained with hematoxylin-eosin and assessed by light microscopy.
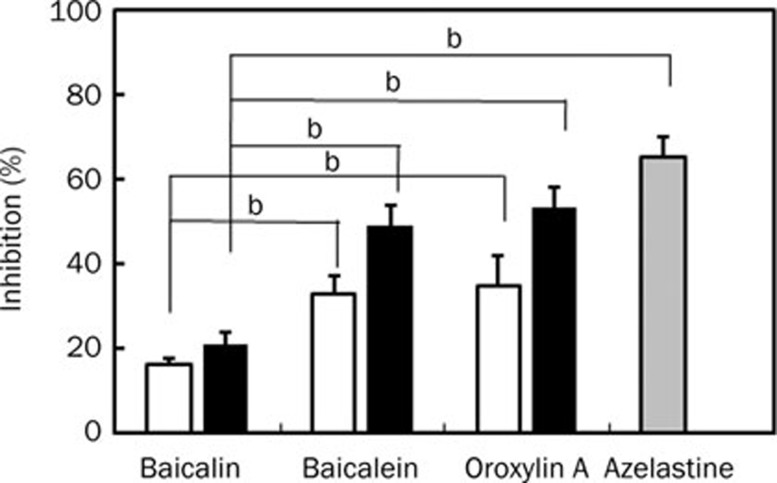
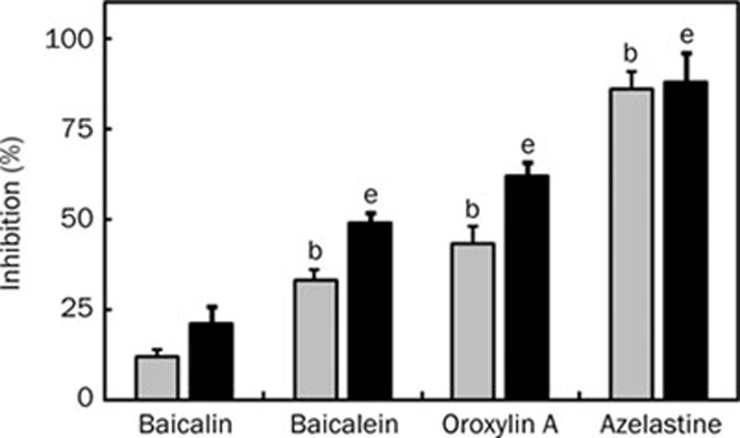
These compounds also significantly inhibited the contraction of guinea pig ileum induced by histamine (Figure 6). Of them, oroxylin A most potently inhibited it, with 50% inhibitory concentration of 0.28 mmol/L, although its inhibitory potency was slightly weak, compared to those of azelastine, a commercially available anti-histamine drug.
Figure 6.
Anti-contraction effect of baicalin, its metabolites, and azelastine in Magnus test using guinea pig ileum. The ileal strip was set in a 10 mL Magnus tube (32 °C, 95% O2+5% CO2) containing Tyrode's solution. Each test agent [0.1 mmol/L (gray bar) and 0.4 mmol/L (black bar), dissolved in 2% trition X-100] was added to the preparation 30 s before treatment with histamine (1×10−6mol/L). The values indicate mean±SD. (n=3). bP<0.05 vs 0.1 mmol/L baicalin treated group. eP<0.05 vs 0.4 mmol/L baicalin treated group.
Discussion
The inhibitory effects of SB and baicalin against allergic disease, asthma and inflammation have been reported23, 24, 25. However, its anti-scratching behavioral effect has not been thoroughly examined. During the screening program to discover anti-scratching behavioral compounds from natural products, SB was found to show inhibitory activity on histamine or compound 48/80-induced experimental scratching behavior. To understand its anti-scratching behavioral effect, we first isolated its main constituent, baicalin, and identified its metabolites produced by fecal microflora. Because, among the components of orally administered herbal medicines including SB, hydrophilic constituents, such as baicalin, are inevitably brought into contact with intestinal microflora in the alimentary tract, metabolized by intestinal microflora and may be absorbed from the intestine to the blood. Therefore, studies on the metabolism of the constituents by human intestinal microflora are of a great importance to an understanding of their biological effects26, 27. When baicalin was anaerobically incubated with human fecal suspension, it was metabolized to its aglycone baicalein, and then produced oroxylin A. These metabolites may be absorbed from the intestine into the blood. This suggestion is supported by the previous reports that baicalein 6-O-glucuronide-7-O-sulfate, baicalein 6,7-diglucuronide and oroxylin A 7-O-β-glucuronide were detected in the bile of rats orally treated with baicalin13 and that baicalein conjugates was detected in the blood of rats orally treated with baicalin16, 17.
Next, we evaluated the anti-scratching behavioral effects of baicalin and its metabolites on histamine or compound 48/80-induced scratching behavior in mice. Histamine and compound 48/80 were injected intradermally into the rostral part of the skin on the backs of mice, and the scratching behavior frequencies were evaluated for 60 min. These scratching agents potently induced scratching behaviors in ICR and BALB/c mice, as previously reported28. In particular, compound 48/80 caused vigorous scratching behaviors in ICR mice. Precise quantification of scratching behavior frequencies was not possible; however, BALB/c mice were insensitive for histamine. Therefore, histamine- and compound 48/80-induced scratching behavior was induced in ICR and BALB/c mice, respectively. When baicalin was administered to mice orally 1 or 5 h before treatment with histamine or compound 48/80, baicalin exhibited a potent anti-scratching behavior effect. The oral administration of baicalin 5 h before treatment with these scratching agents potently inhibited the scratching behavior induced by histamine or compound 48/80 more than when administered orally 1 h before. When the metabolites, baicalein and oroxylin A, were administered orally 1 h before treatment with the scratching agent, the metabolites had a potent inhibitory effect on the scratching behavior. These results suggest that, to generate the anti-scratching behavioral effect of baicalin, it may take a time (6 h) for baicalin to be metabolized to baicalein and/or oroxylin A.
Next, to clarify the role of intestinal bacteria in the metabolism of baicalin, the anti-scratching behavioral effect of baicalin and baicalein microflora was investigated in antibiotics-treated mice. The anti-scratching behavioral effect of baicalin was attenuated in antibiotics-treated mice compared to the effect in antibiotics-untreated mice, whereas the intraperitoneal and oral administration of baicalein and oroxylin A did not affect the anti-scratching behavioral effect in mice. The results suggest that the anti-scratching behavioral effect of baicalin may be dependent on its metabolites produced by intestinal microflora. Oral administration of baicalin and its metabolites inhibited the vascular permeability increased by histamine; however, the metabolites, baicalein and oroxylin A, had potent anti-histamine activity in a guinea pig ileum in vitro assay, with more potent inhibition by oroxylin A than baicalein. The inhibitory activity of these metabolites against vascular permeability was in proportion to the inhibition against scratching behavior. Among the test agents, oroxylin A had the most potently inhibitory effect on vascular permeability. These results suggest that the oral administration of baicalin may be metabolized to baicalein and oroxylin A by intestinal microflora, and these metabolites may exert anti-scratching behavioral effects by anti-histamine action.
These agents also inhibited compound 48/80-induced scratching behavior in BALB/c mice, which is insensitive to histamine29. The compound48/80 causes mast cell-independent scratching behavior30.
Based on these findings, baicalin may be metabolized to baicalein and oroxylin A by intestinal microflora and its anti-pruritic effect may be due to the anti-histamine and unknown actions of the metabolites, baicalein and oroxylin A, than the parent constituent.
Author contribution
Hien-trung TRINH and Eun-ha JOH designed and performed the research (isolation of metabolites and investigation of pharmacological effect); Ho-young KWAK and Nam-in BAEK isolated baicalin; and Dong-hyun KIM analyzed the data and wrote the paper.
Acknowledgments
This study was financially supported by the research fund of Korean Food and Drug Administration (2009).
References
- Hagermark O. Itch mediators. Semin Dermatol. 1955;14:271–6. doi: 10.1016/s1085-5629(05)80047-1. [DOI] [PubMed] [Google Scholar]
- Lerner EA.“Itch: mechanisms and management of pruritis,”Berhard J, editor. New York: McGraw-Hill, 1944. p23–5.
- Schmeiz M, Schmidt R, Bickel A, Handerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiford DS. Pruritus of chronic chlestasis. QJM. 1995;88:603–7. [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–33. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Zhu YP.“Chinese Materia Medica.”Australia: Harwood academic publichers; 1998. p127–35.
- Wu S, Sun A, Liu R. Separation and purification of baicalin and wogonoside from the Chinese medicinal plant Scutellaria baicalensis Georgi by high-speed counter-current chromatography. J Chromatogr A. 2005;1066:243–7. doi: 10.1016/j.chroma.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Chou TC, Chang LP, Li CY, Wong CS, Yang SP. The antiinflammatory and analgesic effects of baicalin in carrageenan-evoked thermal hyperalgesia. Anesth Analq. 2003;17:1724–9. doi: 10.1213/01.ANE.0000087066.71572.3F. [DOI] [PubMed] [Google Scholar]
- Jang SI, Kim HJ, Hwang KM, Jekal SJ, Pae HO, Choi BM, et al. Hepatoprotective effect of baicalin, a major flavone from Scutellaria radix, on acetaminophen-induced liver injury in mice. Immunopharmacol Immunotoxicol. 2003;25:585–94. doi: 10.1081/iph-120026443. [DOI] [PubMed] [Google Scholar]
- Kim DH, Cho KH, Moon SK, Kim YS, Kim DH, Choi JS, et al. Cytoprotective mechanism of baicalin against endothelial cell damage by peroxynitrite. J Pharm Pharmacol. 2005;57:1581–90. doi: 10.1211/jpp.57.12.0008. [DOI] [PubMed] [Google Scholar]
- Akao T, Kawabata K, Yanagisawa E. Baicalin, the predominant flavone glucuronide of scutellariae radix, is absorbed from the rat gastrointestinal tract as the aglycone and restored to its original form. J Pharm Pharmacol. 2000;52:1563–8. doi: 10.1211/0022357001777621. [DOI] [PubMed] [Google Scholar]
- Yim JS, Kim YS, Moon SK, Cho KH, Bae HS, Kim JJ, et al. Metabolic activities of ginsenoside Rb1, baicalin, glycyrrhizin and geniposide to their bioactive compounds by human intestinal microflora. Biol Pharm Bull. 2004;27:1580–3. doi: 10.1248/bpb.27.1580. [DOI] [PubMed] [Google Scholar]
- Abe K, Inoue O, Yumioka E. Biliary excretion of metabolites of baicalin and baicalein in rats. Chem Pharm Bull. 1990;38:209–11. [PubMed] [Google Scholar]
- Akao T, Dakashita Y, Hanada M, Goto H, Shimada Y, Terasawa K. Enteric excretion of baicalein, a flavone of Scutellariae Radix, via glucuronidation in rat: involvement of multidrug resistance-associated protein 2. Pharm Res. 2004;21:2120–6. doi: 10.1023/b:pham.0000048205.02478.b5. [DOI] [PubMed] [Google Scholar]
- Lu T, Song J, Huang F, Deng Y, Xie L, Wang G, et al. Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, Radix scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. J Ethnopharmacol. 2007;110:412–8. doi: 10.1016/j.jep.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Xing J, Chen XY, Zhong DF. Absorption and enterohepatic circulation of baicalin in rats. Life Sci. 2005;78:140–6. doi: 10.1016/j.lfs.2005.04.072. [DOI] [PubMed] [Google Scholar]
- Xing J, Chen XY, Sun YM, Luan Y, Zhong DF. Interaction of baicalin and baicalein with antibiotics in the gastrointestinal tract. J Pharm Pharmacol. 2005;57:743–50. doi: 10.1211/0022357056244. [DOI] [PubMed] [Google Scholar]
- Takido M, Aimi M, Takahashi S, Yamamouchi S, Torii H, Dohi S. Studies on the constituents in the water extracts of crude drugs. I. On the roots of Scutellaria baicalensis Georgi (Wōgon) (1) Yakugaku Zasshi. 1975;95:108–13. doi: 10.1248/yakushi1947.95.1_108. [DOI] [PubMed] [Google Scholar]
- Tomimori T, Miyaichi Y, Kizu H. On the flavonoid constituents from the roots of Scutellaria baicalensis Georgi. I. Yakugaku Zasshi. 1982;102:388–91. doi: 10.1248/yakushi1947.102.4_388. [DOI] [PubMed] [Google Scholar]
- Lee DK, Kim YS, Ko CN, Cho KH, Bae HS, Lee KS, et al. Fecal metabolic activities of herbal components to bioactive compounds. Arch Pharm Res. 2003;25:165–9. doi: 10.1007/BF02976558. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Umakoshi K, Nojiri N, Kamei C. Effects of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice. Eur J Pharmacol. 1998;351:1–5. doi: 10.1016/s0014-2999(98)00288-x. [DOI] [PubMed] [Google Scholar]
- Choo MK, Park EK, Han MJ, Kim DH. Antiallergic activity of ginseng and its ginsenosides. Planta Med. 2003;69:518–522. doi: 10.1055/s-2003-40653. [DOI] [PubMed] [Google Scholar]
- Liaw J, Gau YY, Chao YC. Effect of baicalin on tracheal permeability in ovalbumin (OA)-sensitized guinea pigs. Pharm Res. 1999;16:1653–7. doi: 10.1023/a:1011985427736. [DOI] [PubMed] [Google Scholar]
- Koda A, Nagai H, Wada H. Pharmacological actions of baicalin and baicalein. 2. On passive anaphylaxis. Nippon Yakurigaku Zasshi. 1970;66:237–47. doi: 10.1254/fpj.66.237. [DOI] [PubMed] [Google Scholar]
- Taniguchi C, Homma M, Takano O, Hirano T, Oka K, Aoyagi Y, et al. Pharmacological effects of urinary products obtained after treatment with saiboku-to, a herbal medicine for bronchial asthma, on type IV allergic reaction. Planta Med. 2000;66:607–611. doi: 10.1055/s-2000-8626. [DOI] [PubMed] [Google Scholar]
- Kobashi K, Akao T. Relation of intestinal bacteria to pharmacological effects of glycosides. Bioscience Microflora. 1997;16:1–7. [Google Scholar]
- Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1155–60. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Nagao M, Kawasaki H, Kim JF, Nagai H. Scratching behavior in various strains of mice. Skin Pharmacol Appl Skin Physiol. 2001;14:87–96. doi: 10.1159/000056338. [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol. 2006;530:172–8. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Igeta K, Kim JF, Nagao M, Shiraishi N, Nakamura N, et al. Involvement of unique mechanisms in the induction of scratching behavior in BALB/c mice by compound 48/80. Eur J Pharmacol. 2002;448:175–83. doi: 10.1016/s0014-2999(02)01933-7. [DOI] [PubMed] [Google Scholar]