Abstract
Aim:
To investigate the protective effect of tribulosin, a monomer of the gross saponins from Tribulus terrestris, against cardiac ischemia/reperfusion injury and the underlying mechanism in rats.
Methods:
Isolated rat hearts were subjected to 30 min of ischemia followed by 120 min of reperfusion using Langendorff's technique. The hearts were assigned to seven groups: control, ischemia/reperfusion (I/R), treatment with gross saponins from Tribulus terrestris (GSTT) 100 mg/L, treatment with tribulosin (100, 10, and 1 nmol/L) and treatment with a PKC inhibitor (chelerythrine) (1 μmol/L). Infarct size was assessed by triphenyltetrazolium chloride staining. Malondialdehyde (MDA), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) contents as well as superoxide dismutase (SOD) and creatine kinase (CK) activities were determined after the treatment. Histopathological changes in the myocardium were observed using hematoxylin-eosin (H&E) staining. Apoptosis was detected with terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) assay. Bcl-2, Bax, caspase-3, and PKCɛ protein expression were examined using Western blotting.
Results:
Tribulosin treatment significantly reduced MDA, AST, CK and LDH contents, and increased the activity of SOD. The infarct size of I/R group was 40.21% of the total area. GSTT and various concentrations of tribulosin treatment decreased the infarct size to 24.33%, 20.24%, 23.19%, and 30.32% (P<0.01). Tribulosin treatment reduced the myocardial apoptosis rate in a concentration-dependent manner. Bcl-2 and PKCɛ protein expression was increased after tribulosin preconditioning, whereas Bax and caspase-3 expression was decreased. In the chelerythrine group, Bcl-2 and PKCɛ expression was decreased, whereas Bax and caspase-3 expression was increased.
Conclusion:
Tribulosin protects myocardium against ischemia/reperfusion injury through PKCɛ activation.
Keywords: tribulosin, ischemia/reperfusion injury, oxygen free radicals, apoptosis, protein kinase Cɛ, Bcl-2 proteins, caspases
Introduction
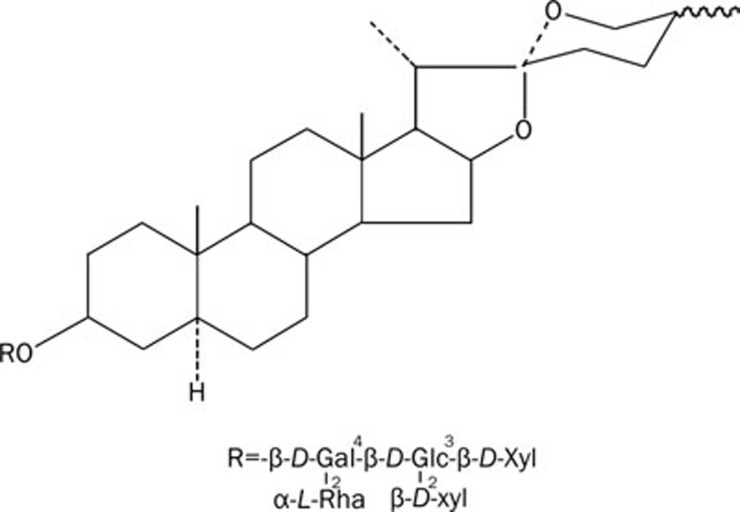
Gross saponins from Tribulus terrestris (GSTT) including spiral vagina steroid and snail steroid are the major derivatives of Tribulus terrestris. GSTT is a well-known Chinese medicine used for the treatment of various diseases including hypertension, hyperlipidemia, platelet aggregation, and aging. Moreover, some studies have proven that GSTT has a significant protective effect against ischemia/reperfusion injury in rat hearts and brains in vitro and in vivo1, 2, 3, 4. However, until now, the specific active component in GSTT was unknown. We have identified eight saponin monomers and named them A, B, C, D, E, F, I, and J. Each of them is a kind of spiral vagina steroid. After preliminary screening, we determined that saponin monomer B is bioactive. Tribulus terrestris saponin B is a component of spiral vagina steroid whose chemical name is tigogenin 3-O-β-D-xylopyranosyl(1-2)-[β-D-xylopyranosyl(1-3)]-β-D-glucopyranosyl(1-4)-[a-L-rhamnopyranosyl(1-2)]-β-D-galactopyranoside. It is also called tribulosin. Its constitutional formula is as follows (Scheme 1).
Scheme 1.
Chemical structure of tribulosin.
To investigate whether tribulosin is the main active component of GSTT, we designed an ischemia/reperfusion injury model in isolated rat hearts and investigated the protective effect and underlying mechanism of tribulosin.
Materials and methods
Drugs and reagents
Tribulosin and GSTT were dissolved in Krebs-Henseleits solution (KH) containing (in mmol/L): glucose 11.1, NaCl 118.5, NaHCO3 25, KCl 4.7, KH2PO4 1.2, CaCl2 22.5, and MgSO4 41.2, and the whole solution with a pH of 7.4. All chemicals were of analytical grade. Creatine kinase (CK), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), superoxide dismutase (SOD), malondialdehyde (MDA), and Coomassie were purchased from Jiancheng Biotechnology. The chelerythrine and anti-β-tubulin were from Sigma Chemicals (St Louis, USA). The anti-PKCɛ antibody was from Santa Cruz Biotechnology. The rabbit polyclonal antibody and anti-rat caspase-3 were from Wuhan BOSTER Bio-Technology Co Ltd. Anti-rat Bcl-2, Bax and horseradish peroxidase-conjugated secondary rabbit antibodies were obtained from Beijing Biosynthesis Biotechnology Co, Ltd. Terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) kit was from Maxim BIO (China).
Animals
Laboratory-bred Wistar rats of either sex (280–300 g) were used in this study. The animals' certificate number was SCXK(JI)2007–0003. All animals were allowed at least 3 d of in-house acclimatization to standard laboratory food and water.
Treatment protocols
The ischemic/reperfusion (I/R) injury model in isolated rat hearts proceeded as follows. Wistar rats were anesthetized with 20% urethane at 1.5 g/kg via intraperitoneal injection. The hearts were rapidly excised and washed with KH, then perfused by the non-recirculating Langendorff's technique, in constant pressure mode with a modified KH solution. The buffer solution, equilibrated with 95% O2+5% CO2, was delivered to the aortic cannula with 37 °C under a preload of 1.17 kPa. The rat hearts were subjected to perfusion with KH stabilization, followed by 30 min of zero-flow (ischemia) and 120 min of reflow (reperfusion) with KH.
Groups studied
The rats were assigned to seven groups, as follows.
Group A (Control)
Rat hearts were subjected to perfusion with KH only and did not undergo ischemia/reperfusion.
Group B (I/R)
Rat hearts were subjected to perfusion with KH stabilization, followed by 30 min of ischemia and 120 min of reperfusion with KH.
Group C
After stabilizing with KH, the rat hearts were subjected to 10 min of perfusion with GSTT 100 mg/L, followed by 30 min of ischemia and 120 min of reperfusion with KH.
Group D
After stabilizing with KH, the rat hearts were subjected to 10 min of perfusion with tribulosin 100 nmol/L, followed by 30 min of ischemia and 120 min of reperfusion with KH.
Group E
After stabilizing with KH, the rat hearts were subjected to 10 min of perfusion with tribulosin 10 nmol/L, followed by 30 min of ischemia and 120 min of reperfusion with KH.
Group F
After stabilizing with KH, the rat hearts were subjected to 10 min of perfusion with tribulosin 1 nmol/L, followed by 30 min of ischemia and 120 min of reperfusion with KH.
Group G
After stabilizing with KH, the rat hearts were subjected to 10 min of perfusion with chelerythrine 1 μmol/L, followed by 10 min of perfusion with tribulosin 100 nmol/L, followed by 30 min of ischemia and 120 min of reperfusion with KH.
All groups had the same experiment time. At the end of each experiment, myocardial tissue was stored in liquid nitrogen for biochemical estimations and in 10% buffered formalin for histopathological studies. All assays were done in triplicate.
Preparation of rat heart tissue homogenate
Tissue homogenate was prepared in a ratio of 1 g of wet tissue to 10 times (w/v) ice-cold phosphate buffer (pH 7.4) and homogenized with a homogenizer. After the preparation was centrifuged for 10 min at 3000 round per minute with 4 °C, SOD and MDA were estimated using 0.2 mL of supernatant. Protein levels were determined with Coomassie Brilliant Blue.
Biochemical parameters
At the end of each experiment, the perfusate was used to measure CK, AST, and LDH in all the groups. SOD and MDA levels in the tissue homogenate were measured.
Infarct size
Hearts were cut into 2-mm transverse slices. The slices were incubated in 1% 2,3,5-triphenyltetrazolium chloride (TTC) in a 7.4 pH buffer for 10 min at 37 °C to allow the demarcation of the infarcted region. Because TTC stains viable tissue a deep red color, unstained tissue was presumed to be infarcted. The infarct areas in each slice were measured with computer morphometry using the Image-Pro Plus software, and the percentage of infarcted area was calculated.
Histopathology
Myocardial tissue was fixed in 10% neutral buffered formalin, routinely processed, and embedded in paraffin. Paraffin sections were cut, placed on slides, and stained with hematoxylin and eosin (H&E).
Apoptosis measurement
Terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) assay was carried out as directed by the kit's operating manual. The percentage of cell death was calculated from the number of TUNEL-positive cells divided by the total number of cells.
Immunohistochemical analysis
Myocardial tissue was fixed in 10% buffered formalin, routinely processed, and embedded in paraffin. Paraffin sections were cut and placed on slides to determine the protein expression of PKCɛ. The mean density was measured with computer morphometry using the Image-Pro Plus software.
Protein extract preparation and Western blotting analysis of Bax, Bcl-2, caspase, and PKCɛ
Frozen myocardial tissue samples were ground to powder with a prechilled stainless steel mortar and pestle. Cardiac proteins were extracted using glass–glass homogenization in a buffer containing 10 mmol/L NaCl, 10 mmol/L Tris-HCl (pH 7.5), 1 mmol/L EDTA, 10 mmol/L SDS, sucrose 0.25 mol/L, 1 mg pepstatin A, 1 mg Aprotin, 1 mg leupeptin, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 1 μmol/L Microcystin LR. The homogenate was centrifuged at 14 000×g for 10 min and the supernatant was collected.
After quantification with Coomassie Brilliant Blue, 20 μg of protein was separated with 12% denaturing polyacrylamide gel electrophoresis and blotted onto a nitrocellulose membrane. Adequate background blocking was accomplished by incubating the nitrocellulose membranes with 5% nonfat dry milk in phosphate-buffered saline with Tween for 2 h and protein was detected with antibodies.
Statistical analysis
All values were expressed as mean±SEM. ANOVA was applied to test the significance of the biochemical data of different groups. The results were considered significant at a value of P<0.05.
Results
Biochemical parameters
The levels of CK, AST, LDH, MDA, and SOD in the study groups are shown in Tables 1 and 2.
Table 1. Effects of tribulosin on CK, AST and LDH levels in reperfused ischemic rat hearts. n=8. Mean±SEM. bP<0.05, cP<0.01 vs control group. eP<0.05, fP<0.01 vs I/R group.
| Dose | CK (U/mL) | LDH (U/L) | AST (IU/L) | |
|---|---|---|---|---|
| Control | 1.68±0.33 | 1224.16±430.04 | 35.75±7.96 | |
| I/R | 2.80±0.80b | 2163.03±281.36c | 78.97±15.53c | |
| GSTT | 100 mg/L | 1.80±0.54e | 1470.16±295.74f | 41.21±12.33f |
| Tribulosin | 100 nmol/L | 1.90±0.57e | 1304.22±234.13f | 52.54±10.01f |
| 10 nmol/L | 1.94±0.35e | 1387.19±332.81e | 56.07±14.64e | |
| 1 nmol/L | 2.03±0.38e | 1455.60±235.37e | 59.37±11.83e | |
| Chelerythrine | 1 μmol/L | 2.55±0.73b | 1882.10±358.68b | 64.91±21.26b |
Table 2. Effects of tribulosin on SOD activity and MDA concentration in reperfused ischemic rat hearts. n=6. Mean±SEM. cP<0.01 vs control group. eP<0.05, fP<0.01 vs I/R group.
| Dose | SOD (U·mg−1 prot) | MDA (μmol·g−1 prot) | |
|---|---|---|---|
| Control | 105.99±6.99 | 17.18±3.26 | |
| I/R | 72.08±15.34c | 23.05±1.94c | |
| GSTT | 100 mg/L | 94.11±8.93e | 18.66±3.55e |
| Tribulosin | 100 nmol/L | 92.59±14.88e | 17.84±3.93e |
| 10 nmol/L | 98.12±11.86f | 19.10±2.92e | |
| 1 nmol/L | 93.33±13.16e | 19.35±2.49e | |
| Chelerythrine | 1 μmol/L | 84.80±10.96c | 20.15±3.80 |
There were significant (P<0.01) increases in CK, AST, LDH, and MDA in group B (I/R) compared to group A, while these parameters decreased significantly (P<0.01 and P<0.05) in those groups treated with GSTT and tribulosin in comparison with group B (I/R). There was a significant (P<0.01) reduction in myocardial SOD activity in group B when compared with group A, but SOD levels increased significantly (P<0.01, P<0.05) in groups C, D, E, and F compared with group B (I/R). However, preconditioning with chelerythrine, a PKC inhibitor, offset the inhibitory action.
Infarct size
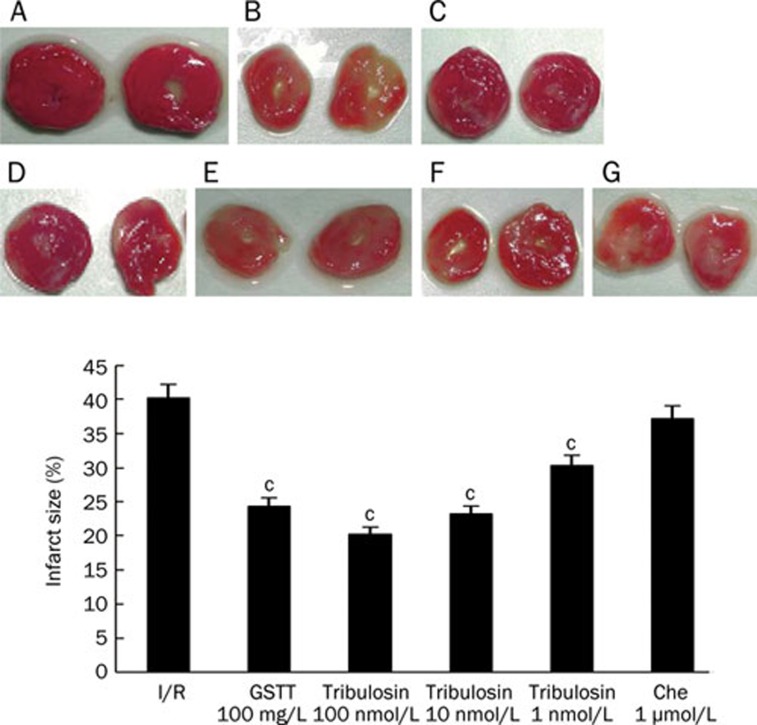
In the I/R group, the infarct size was 40.21% of the total area; after treatment with GSTT and various concentrations of tribulosin the infarct size was reduced to 24.33%, 20.24%, 23.19%, and 30.32% (P<0.01 compared with the I/R group). Administration of the PKC inhibitor chelerythrine before myocardial ischemia/reperfusion did not affect infarct size (37.19%). These results indicate that tribulosin's protection against I/R injury is associated with the PKC pathway (Figure 1).
Figure 1.
Effect of tribulosin on myocardial infarct of rat hearts after ischemia/reperfusion. Rat hearts were subjected to 30 min ischemia and 120 min reperfusion. Myocardial infarct size was determined by TTC staining, and the percentage of infarct volumes to the left ventricle volumes was calculated. A) Control; B) I/R; C) GSTT 100 mg/L; D) tribulosin 100 nmol/L; E) tribulosin 10 nmol/L; F) tribulosin 1 nmol/L; G) Chelerythrine 1 μmol/L+tribulosin 100 nmol/L. n=6. Mean±SD. cP<0.01 vs I/R group.
Histopathological results
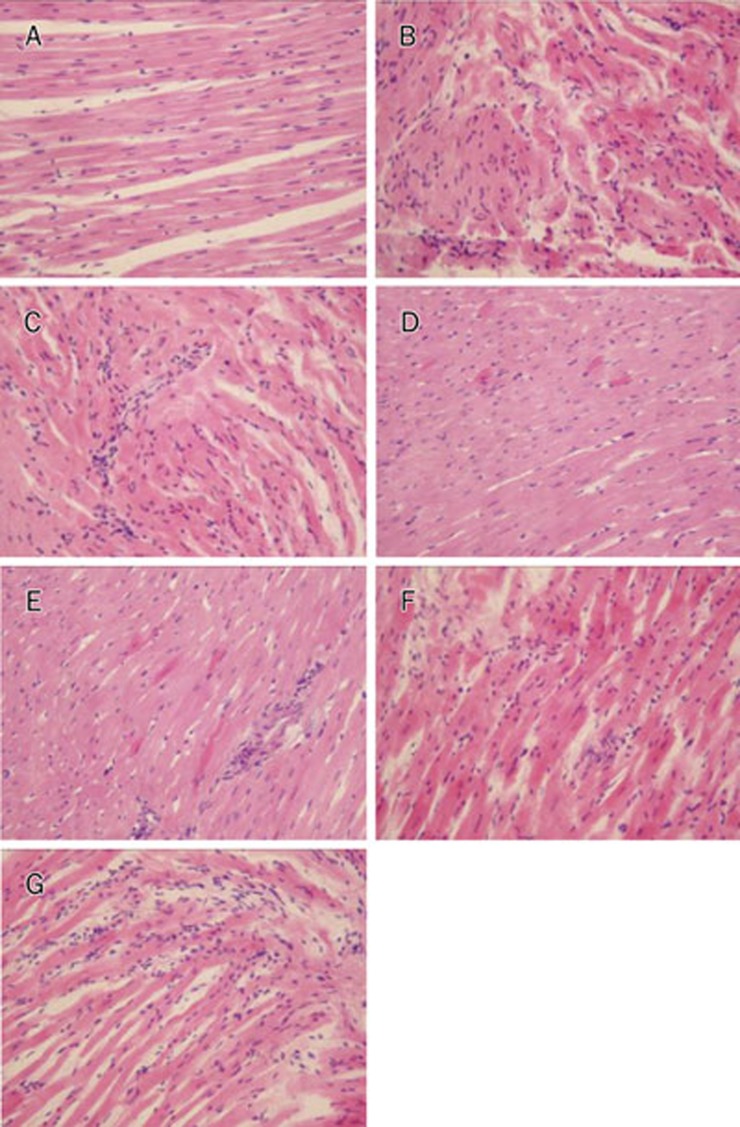
H&E-stained heart sections revealed that the cardiac muscle fibers lined up in order, and transverse striation was clear and well-distributed in the control group. No edema or necrosis was observed in the hearts in the control group. Cardiac muscle fibers in the I/R and chelerythrine groups were irregular, and transverse striation was unclear or absent. The I/R group exhibited large areas of necrosis, interstitial edema with neutrophil infiltration, and sarcoplasm agglomeration. Groups C, D, E, and F exhibited gentle widening of the muscle fibers with focal loss of myofibers, inflammation, and trivial sarcoplasm agglomeration. No myonecrosis was seen (Figure 2).
Figure 2.
Effects of tribulosin on pathomorphology of myocardium in ischemia/reperfusion rat hearts. Light micrograph of heart tissue (H&E staining, ×200). A) Control: No focal separation of myocardial fibre; B) I/R: 30-min ischemia and 120-min reperfusion with KH solution. Focal destruction of myocardial fibres with neutrophil infiltration and sarcoplasm agglomeration; C) GSTT 100 mg/L; D) tribulosin 100 nmol/L; E) tribulosin 10 nmol/L; F) tribulosin 1 nmol/L; G) Chelerythrine 1 μmol/L+tribulosin 100 nmol/L.
TUNEL staining for apoptosis
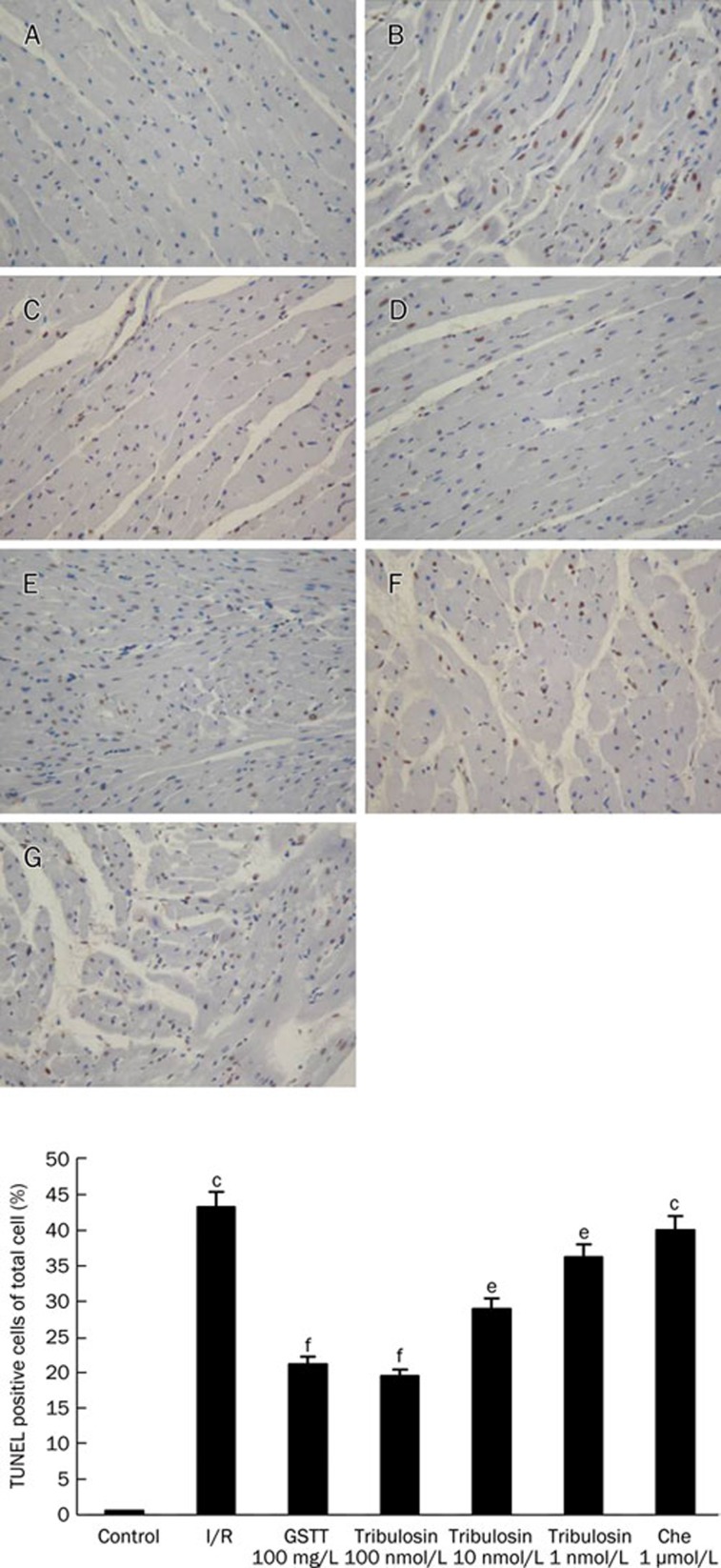
To examine the extent of apoptotic cell death, TUNEL staining was performed. A count of TUNEL-positive nucleoli showed that the apoptosis rate was higher after myocardial ischemia-reperfusion (Figure 3). The apoptosis rate decreased significantly with tribulosin pretreatment. Administering chelerythrine before myocardial ischemia/reperfusion made no difference to the apoptosis rate compared to that of the I/R group.
Figure 3.
Effects of tribulosin on apoptosis in ischemia/reperfusion rat hearts by TUNEL staining (×200). A) Control; B) I/R; C) GSTT 100 mg/L; D) tribulosin (100 nmol/L); E) tribulosin (10 nmol/L); F) tribulosin (1 nmol/L); G) Chelerythrine (1 μmol/L)+tribulosin (100 nmol/L). Upper panels display representative histologic images. Lower panels display the percentage of TUNEL-positive cells per high power field. Values are mean±SEM for five individual experiments randomly chosen in each group. cP<0.01 vs control group; eP<0.05, fP<0.01 vs I/R group.
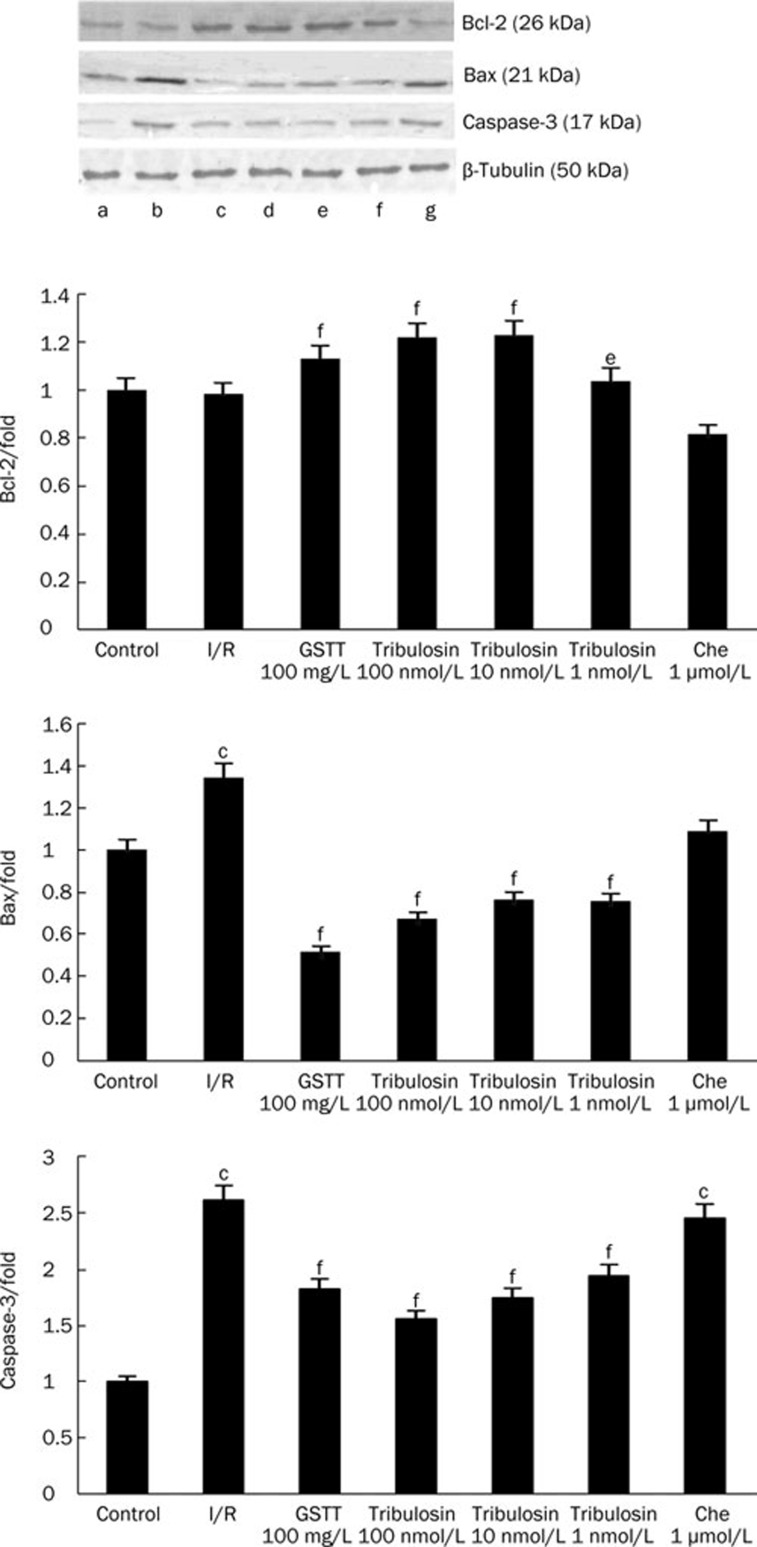
Bax, Bcl-2, and caspase-3 protein expression
To define the mechanisms by which tribulosin inhibits I/R-induced cardiocyte apoptosis, protein expression in the tested groups was quantified with Western blotting analysis (Figure 4). Bcl-2 expression was significantly reduced in the I/R group compared with expression in the control group. Tribulosin preconditioning increased Bcl-2 expression (P< 0.05, P<0.01 versus I/R). The expression of the pro-apoptotic proteins Bax and caspase-3 was significantly increased in the I/R group. Tribulosin preconditioning inhibited the increase of Bax and caspase-3 (P<0.01 versus I/R). The PKC inhibitor chelerythrine blocked the effects of tribulosin.
Figure 4.
Effects of tribulosin on Bcl-2, Bax, and caspase-3 expression in rat hearts after ischemia/reperfusion. Western blotting analysis of Bcl-2, Bax, and caspase-3 in myocardial abundance are shown (top). β-Tubulin (bottom lanes) was used as control. The lower panels were summary data of Bcl-2, Bax, and caspase-3 expression in myocardial tissue in different groups. (a) Control; (b) I/R; (c) GSTT 100mg/L; (d) tribulosin 100 nmol/L; (e) tribulosin 10 nmol/L; (f) tribulosin 1 nmol/L; (g) Chelerythrine 1 μmol/L+tribulosin 100 nmol/L. Values are expressed as fold changes over the control and are presented as mean±SEM representative of 3 independent experiments. bP<0.05, cP<0.01 vs control group; eP<0.05, fP<0.01 vs I/R group.
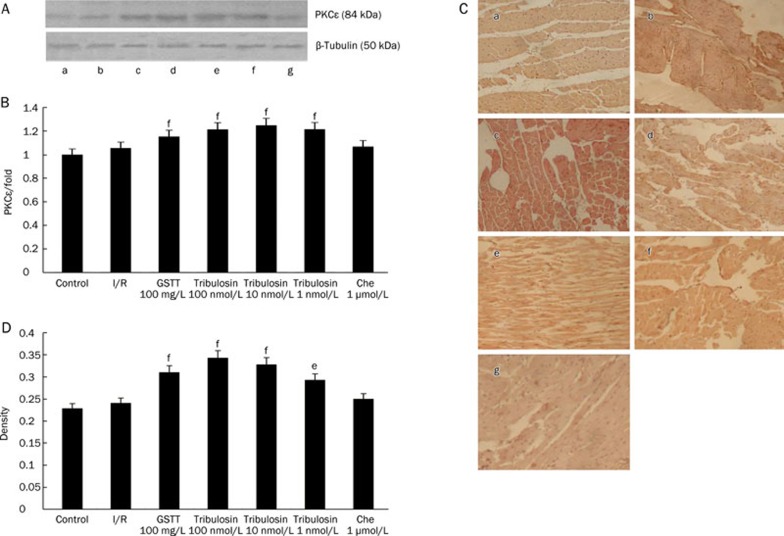
PKCɛ expression
To investigate whether PKCɛ was involved in tribulosin-induced cardioprotection, the expression of PKCɛ and its activity was evaluated(Figure 5). After treatment with tribulosin, total PKCɛ expression in the hearts was obviously augmented, while the PKC inhibitor chelerythrine blocked the effect of tribulosin.
Figure 5.
Effect of tribulosin on PKCɛ of myocardium in ischemia/reperfusion rat hearts (×200). (A) Expression of total-PKCɛ proteins was analyzed by Western blotting. β-Tubulin was immunodetected with a monoclonal antibody as an internal control. The expression levels were measured in the hearts excised from the IR rats at the indicated times. A representative blot of 3 independent experiments is shown. (B) Graphic presentations of PKCɛ expression in myocardial tissue quantified by integrating the volume of image element density. The data presented in each imagine are representative of three separate groups. Values are expressed as fold changes over the control and are presented as mean±SEM. fP<0.01 vs I/R group. (C) Expression of phospho-PKCɛ proteins was analyzed by immunohistochemistry. (a) Control; (b) I/R; (c) GSTT 100mg/L; (d) tribulosin 100 nmol/L; (e) tribulosin 10 nmol/L; (f) tribulosin 1 nmol/L; (g) Chelerythrine 1 μmol/L+tribulosin 100 nmol/L. (D) Mean densities from the signals of phospho-PKCɛ were measured. Values are presented as mean±SEM. eP<0.05, fP<0.01 vsI/R group.
Immunohistochemistry showed that there was no phospho-PKCɛ expression in the control group, while the I/R group exhibited a small amount of phospho-PKCɛ expression. In the tribulosin group, phospho-PKCɛ expression was significantly increased. The expression of phospho-PKCɛ was inhibited in hearts pretreated with chelerythrine.
Discussion
In this study, we demonstrated that tribulosin has an antioxidative effect and attenuates myocardial apoptosis. These effects are mediated by activation of the PKC pathway.
Thrombotic occlusion of coronary arteries often leads to myocardial ischemia. However, reperfusion also appears to activate a series of responses. Myocardial ischemia and the reperfusion that follows can cause injuries similar to or worse than pure ischemia; this phenomenon is called myocardial ischemia/reperfusion injury (I/R). I/R generates oxygen-derived free radicals, which cause lipid peroxidation5, 6, 7 and may result in additional tissue damage, including cardiomyocyte apoptosis. In this study, the increases of LDH, CK, AST, and MDA and the decrease of SOD in the reperfused heart confirmed the damage to cardiocytes during ischemia/reperfusion. After preconditioning with tribulosin, LDH, CK, AST, and MDA levels decreased and SOD activity increased, which revealed that tribulosin protected cardiocytes from ischemia/reperfusion injury. Infarct size was significantly reduced after tribulosin treatment, indicating a protective effect against ischemia/reperfusion injury in cardiocytes as well.
Myocardial cell death via necrosis and apoptosis is the primary feature of ischemia and reperfusion. Reducing cardiocyte loss through suppression of cell death is a logical strategy to protect cardiomyocytes8. Apoptosis is the predominant form of cell death in ischemia/reperfusion in the heart. Caspases, a family of cysteine proteases that cleave to aspartate residues, are central to the execution of apoptosis9. During the execution phase of apoptosis, initiator caspases activate effector caspases and ultimately cleave a set of proteins, disassembling the cell9, 10. Some studies suggest that caspase-3 may be the main cause of cells apoptosis9, 10. Therefore, we investigated whether caspase-3 is involved in the protective effects of tribulosin against I/R-induced injury in cardiocytes. Our results showed caspase-3 decreased after tribulosin treatment, indicating that tribulosin improved cell viability by suppressing apoptosis. A TUNEL assay also indicated that tribulosin could suppress cardiocyte apoptosis.
Apoptosis via activation of distinct signaling pathways involves mitochondria, mitochondrial regulatory proteins, and caspase activation8, 11. Caspase activation may be regulated directly or indirectly by members of the Bcl-2 family of proteins. Bcl-2 proteins play a critical role in regulating apoptosis by promptly impacting caspase activation on multiple levels. The main site of action of anti-apoptotic Bcl-2 proteins appears to be the mitochondrion, which resides in the outer mitochondrial wall and regulates apoptosis by inhibiting the release of cytochrome c8. Bax, one of the pro-apoptotic proteins, originates in the cytosol but translocates to the mitochondria and forms a pro-apoptotic complex with Bcl-2. In particular, the balance of Bcl-2 and Bax expression has been suggested as an important factor in determining the extent of apoptosis after I/R in hearts9. Furthermore, it has been demonstrated that I/R significantly decreases the expression of Bcl-2 and increases the expression of Bax in the heart12. Indeed, up-regulation of Bcl-2 or down-regulation of Bax has been found to attenuate apoptotic cell death and prevent I/R injury9, 12. Consistent with these studies, we found that anti-apoptotic protein Bcl-2 expression decreased and pro-apoptotic protein Bax increased in cardiocytes subjected to ischemia/reperfusion, and the decreased Bcl-2 was significantly reversed by tribulosin. In the present study, Bcl-2 expression increased after tribulosin treatment and Bax expression decreased, resulting in an increased ratio of Bcl-2 to Bax.
Protein kinase C (PKC) plays a key role in the signaling pathways underlying both phases of ischemia13, 14, 15, 16. It has been demonstrated that PKC-mediated cardioprotection is isoform specific and that the ɛ-isoform of PKC plays an essential role in the development of PC in rabbit myocardium17, 18, 19, 20. PKCɛ is a member of the PKC family that has been studied extensively in preconditioning and tumorigenesis9. PKCɛ activation is necessary for cardioprotection against ischemia and reperfusion injury21. It has been suggested that targeted disruption of the PKCɛ gene leads to loss of the cardioprotective effect of ischemic preconditioning22, 23. A PKCɛ-inhibitory peptide efficiently inhibited Bcl-2 phosphorylation and augmented hydrogen peroxide-induced apoptosis in a concentration-dependent manner in rat cardiocytes24. In our study, PKCɛ expression increased after using tribulosin. To determine whether tribulosin increases expression of Bcl-2 mediated by PKC, the PKC inhibitor chelerythrine was used in the present study. Treatment with chelerythrine before tribulosin preconditioning inhibited the effects of tribulosin on Bcl-2, Bax, and caspase-3 expression, suggesting that the effect of tribulosin is mediated through PKC signaling.
The mechanisms by which GSTT protects the ischemic heart have been extensively investigated and are believed to involve the activation of PKC, the release of reactive oxygen species, and the opening of ATP-regulated potassium channels25, 26. PKCɛ activation attenuated etoposide-induced caspase-3 cleavage. In vivo studies have demonstrated that during ischemic preconditioning, PKCɛ activation in cardiomyocytes protects against apoptosis, whereas targeted disruption of PKCɛ inhibits the beneficial effect of preconditioning21. However, it was unknown which ingredient in GSTT exerted the cytoprotective action. Tribulosin is a simple substance separated from GSTT with a distinct chemical constitution, suggesting that tribulosin is an active component of GSTT.
Author contribution
Shi-jie YANG designed the study; Shuang ZHANG performed research, analyzed data, and wrote the paper; Hong LI performed research.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No 30472020, 30672654). The authors acknowledge Department of Chemistry of Jilin University for contributing drugs for this study.
References
- Shi CJ, Qu WJ, Wang JS, Deng TT. Effect of Tribu Saponin from Tribulus terrestris on the formation of atherosclerosis in rats. Nat Prod Res Dev. 2009;21:53–7. [Google Scholar]
- Liu XM, Huang QF, Zhang YL, Lou JL, Liu HS, Zheng H. Effects of Tribulus terrestris L saponion on apoptosis of cortical neurons induced by hypoxia-reoxygenation in rats. J Chin Int Med. 2008;6:45–50. doi: 10.3736/jcim20080110. [DOI] [PubMed] [Google Scholar]
- Lu WW, Qu JB, Yang SJ. Influences of GSTT on hemodynamics and oxygen metabolism in anesthetic thoraco-opened dogs. J Jilin Univ (Med Ed) 2006;32:379–82. [Google Scholar]
- Sun W, Li H, Yang SJ. A triterpene saponin from Tribulus terrestris attenuates apoptosis in cardiocyte via activating PKC signalling transduction pathway. J Asian Nat Prod Res. 2008;10:39–48. doi: 10.1080/10286020701275846. [DOI] [PubMed] [Google Scholar]
- Mu YL, Xie YY, Zhou L, Zhong Y, Liu L, Bai H, et al. Cardioprotective effect of methylamine irisolidone, a new compound, in hypoxia/reoxygenation injury in cultured rat cardiac myocyte. Chem Biodivers. 2009;6:1170–7. doi: 10.1002/cbdv.200800314. [DOI] [PubMed] [Google Scholar]
- Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS. Resveratrol prevents oxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med. 2009;46:1589–97. doi: 10.1016/j.freeradbiomed.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–7. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL, Wang CY, Zhang BJ, Zheng ZZ. Shefu injection suppresses apoptosis by regulation of Bcl-2 and caspase-3 during hypoxia/reoxygenation in neonatal rat cardiomyocytes in vitro. Mol Biol Rep. 2009;36:365–70. doi: 10.1007/s11033-007-9188-x. [DOI] [PubMed] [Google Scholar]
- Basu A, Sivaprasad U. Protein kinase Cɛ makes the life and death decision. Cell Signal. 2007;19:1633–42. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Kim SH, Chun YS, Cho YS, Park JW, Kim MS. In vivo hyperoxic preconditioning prevents myocardial infarction by expressing Bcl-2. Exp Biol Med (Maywood) 2006;231:463–72. doi: 10.1177/153537020623100412. [DOI] [PubMed] [Google Scholar]
- Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardio-protection. Circ Res. 2004;94:7–16. doi: 10.1161/01.RES.0000108082.76667.F4. [DOI] [PubMed] [Google Scholar]
- Raphael J, Abedat S, Rivo J, Meir K, Beeri R, Pugatsch T, et al. Volatile anesthetic preconditioning attenuates myocardial apoptosis in rabbits after regional ischemia and reperfusion via Akt signaling and modulation of Bcl-2 family proteins. J Pharmacol Exp Ther. 2006;318:186–94. doi: 10.1124/jpet.105.100537. [DOI] [PubMed] [Google Scholar]
- Robia SL, Ghanta J, Robu VG, Walker JW. Localization and kinetics of protein kinase C-epsilon anchoring in cardiac myocytes. Biophys J. 2001;80:2140–51. doi: 10.1016/S0006-3495(01)76187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping P, Zhang J, Cao X, Li RC, Kong D, Tang XL, Qiu Y, et al. PKC-dependent activation of p44/p42 MAPKs during myocardial ischemia-reperfusion in conscious rabbits. Am J Physiol. 1999;276:H1468–81. doi: 10.1152/ajpheart.1999.276.5.H1468. [DOI] [PubMed] [Google Scholar]
- Steinberg R, Harari OA, Lidington EA, Boyle JJ, Nohadani M, Samarel AM, et al. A protein kinase Cɛ-anti-apoptotic kinase signaling complex protects human vascular endothelial cells against apoptosis through induction of Bcl-2. J Biol Chem. 2007;282:32288–97. doi: 10.1074/jbc.M704001200. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Chang WT, Chan KC, Wojcik KR, Hsu CW, Li CQ, et al. Hypothermia-induced cardioprotection using extended ischemia and early reperfusion cooling. Am J Physiol Heart Circ Physiol. 2007;292:H1995–2003. doi: 10.1152/ajpheart.01312.2005. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Ping P, Tang XL, Manchikalapudi S, Rizvi A, Zhang J, et al. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that ɛ is the isoform involved. J Clin Invest. 1998;101:2182–98. doi: 10.1172/JCI1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Ping P, Zhang J, Wead WB, Cao X, Gao J, et al. PKCɛ modulates NF-κB and AP-1 via mitogen-activatedprotein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;279:H1679–89. doi: 10.1152/ajpheart.2000.279.4.H1679. [DOI] [PubMed] [Google Scholar]
- Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res. 2007;55:523–36. doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Ping P, Song C, Zhang J, Guo Y, Cao X, Li RC, et al. Formation of protein kinase C (epsilon)-Lck signaling modules confers cardioprotection. J Clin Invest. 2002;109:499–507. doi: 10.1172/JCI13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence KM, Kabir AM, Bellahcene M, Davidson S, Cao XB, McCormick J, et al. Cardioprotection mediated by urocortin is dependent upon PKCɛ activation. FASEB J. 2005;19:831–3. doi: 10.1096/fj.04-2506fje. [DOI] [PubMed] [Google Scholar]
- Kabir AM, Clark JE, Tanno M, Cao X, Hothersall JS, Dashnyam S, et al. Cardioprotection initiated by reactive oxygen species is dependent on activation of PKCɛ. Am J Physiol Heart Circ Physiol. 2006;291:H1893–9. doi: 10.1152/ajpheart.00798.2005. [DOI] [PubMed] [Google Scholar]
- Robia SL, Ghanta J, Robu VG, Walker JW. Localization and kinetics of protein kinase C-epsilon anchoring in cardiac myocytes. Biophys J. 2001;80:2140–51. doi: 10.1016/S0006-3495(01)76187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangat R, Singal T, Dhalla NS, Tappia PS. Inhibition of phospholipase Cɛ augments the decrease in cardiomyocyte viability by H2O2. Am J Physiol Heart Circ Physiol. 2006;291:H854–60. doi: 10.1152/ajpheart.01205.2005. [DOI] [PubMed] [Google Scholar]
- Wang SS, Ji YS, Li H, Yang SJ. Mechanisms of gross saponins of Tribulus terrestris via activating PKCɛ against myocardial apoptosis induced by oxidative stress. Yao Xue Xue Bao. 2009;44:134–9. [PubMed] [Google Scholar]
- Sun W, Li H, Yu HM, Yang SJ. Role of protein kinase C-ɛ (PKCɛ) in GSTT-induced cardioprotection. Chin Pharmacol Bull. 2007;23:1193–7. [Google Scholar]