Abstract
With an ageing population, dementia has become one of the world's primary health challenges. However, existing remedies offer limited benefits with certain side effects, which has prompted researchers to seek complementary and alternative therapies. China has long been known for abundant usage of various herbs. Some of these herbal decoctions are effective in stimulating blood circulation, supplementing vital energy and resisting aging, the lack of which are believed to underlie dementia. These herbs are regarded as new and promising sources of potential anti-dementia drugs. With the rapid evolution of life science and technology, numerous active components have been identified that are highly potent and multi-targeted with low toxicity, and therefore meet the requirements for dementia therapy. This review updates the research progress of Chinese herbs in the treatment of dementia, focusing on their effective principles.
Keywords: dementia, traditional Chinese medicine (TCM), active principle, neuroprotection
Introduction
Dementia, mainly characterized by learning and memory loss, mood changes and communication problems, has been increasingly recognized as an important component of the disease spectrum in most neurodegenerative diseases including Alzheimer's disease (AD) and Parkinson's disease (PD). Dementia has drawn extensive attention due to its high prevalence in older people, underscored in a rapidly ageing world population.
Currently, there are no effective neurotherapeutic interventions. The widely used anti-dementia strategies are those claimed to be effective in treating AD, the most commonly encountered type of dementia. Effective drugs approved by the Food and Drug Administration of the United States include donepezil, galanthamine, rivastigmine and memantine, which have modest effects in modifying the symptoms of AD for a relatively short period of time in subsets of patients. But none of these therapies has any effect on halting the progression of AD. This inadequate effect could be due to the complicated pathological changes encountered with dementia, which include oxidative damages, mitochondrial dysfunction, inflammatory responses and apoptosis, although the underlying molecular mechanisms have not been fully elucidated. As a consequence it is very important to further investigate novel, multi-targeted and low toxicity anti-dementia drugs.
China, with an area of 9 600 000 km2, is renowned for its abundant categories of plants. Persistent attempts and practices made in disease prevention, diagnosis and treatment have allowed the formation of an integrated theoretical system of Chinese medicine. Many herbal decoctions have claimed, in the Chinese literature (such as A Chinese Bestiary, The Medical Classic of the Yellow Emperor and Compendium of Materia Medica), to be effective for specific symptoms through stimulating blood circulation, nourishing Yin, supplementing vital energy and suppressing excess Yang. Such abundant natural resources could provide additional natural compounds for treating neurodegenerative disorders including AD. It is estimated that 25% of modern drugs directly or indirectly originated from plants. Yet most active principles from plants are not known. In recent years, scientists have isolated many novel compounds from herbs, some of which alleviate dementia with fewer side effects than conventional drugs and thus are regarded as promising anti-dementia drugs.
The current review summarizes the latest research progress on Chinese herbal medicines that show benefical effects in dementia therapy with a particular focus on active extracts from Chinese herbs and their possible pharmacological mechanisms.
Chinese herbs and active principles in the treatment of AD
Huperzia serrata (Thunb) Trev
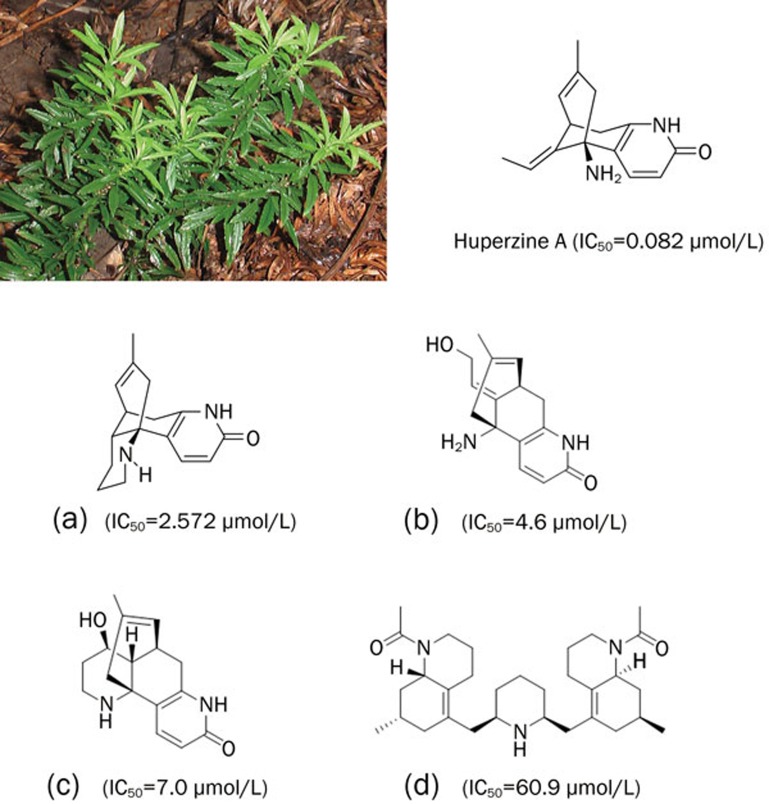
Huperzia serrata (Thunb) Trev (Qian Ceng Ta) is mainly distributed in northeastern, southern and southwestern China along the Changjiang River zone. It has been traditionally used to relieve pain and as an antidote for poison, as well as for the alleviation of swelling. In the 1970s, schizophrenic patients using the extract from this herb showed signs of cholinergic stimulation, such as dizziness, nausea and vomiting, suggesting that this herb might be effective for treating cholinergic dysfunction. On the basis of the above clinical findings, novel Lycopodium alkaloids were isolated from Huperzia serrata (Thunb) Trev, which included (−)-huperzine A (Hup A), (−)-huperzine B (Hup B), N-methyl-Hup B, huperzinine, carinatumin A and carinatumine B. All of the aforementioned Lycopodium alkaloids possess anti-acetylcholinesterase (AChE) acitivity 1 (Figure 1).
Figure 1.
Huperzia serrata (Thunb) Trev and the representative active principles with anti-AChE activity. (a) Huperzine B; (b) Carinatumin A; (c) Carinatumin B; (d) Lycoperine A.
Hup A is the most potent acetylcholinesterase inhibitor (AChEI) among the aforementioned active principles isolated from Huperzia serrata (Thunb) Trev. Hup A was shown to be effective in the treatment of myasthenia gravis in previous studies 1, 2. In the 1990s, Hup A was approved by the State Food and Drug Administration (SFDA) to treat AD. Compared with the US FDA approved AChEIs such as tacrine, donepezil, galanthamine and rivastigmine, Hup A shows the most potent AChE inhibition activity in vivo, better penetration through the blood–brain barrier (BBB), higher oral bioavailability, and a longer duration of anti-AChE action 3.
Hup A is an effective cognition enhancer in a number of different AD/vascular dementia (VaD) animal models and patients 3. Hup A administration enhances learning and memory in intact adult rodents, aged rodents and monkeys, as well as in rodents cognitively impaired by ischemia, scopolamine, AF64A, cycloheximide, D-galactose (D-gal), etc 3. Moreover, in randomized, double-blind, placebo-controlled clinical trials, Hup A significantly improved cognitive functions [measured with the Mini-mental State Examination Scale (MMSE) and the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog)], non-cognitive functions (measured with mood and behavior-ADAS-non-Cog) and activity in daily living [measured with Activity of Daily Living Scale (ADL)] 3, 4, 5. In addition, Hup A is also effective in ameliorating neurocognitive impairments in multi-infarct dementia (MID), brain trauma, schizophrenia, and benign senescent forgetfulness (BSF) 3. However, the precise mechanisms involved in the beneficial effects of Hup A remain unclear. Hup A exerts multiple neuroprotective effects in addition to its anti-AChE activity. Other underlying mechanisms may involve the activation of muscarinic acetylcholine receptor (mAChR)/nicotinic acetylcholine receptor (nAChR), a reduction of inflammation/apoptosis-related gene/protein expression, scavenging of reactive oxygen species (ROS)/regulating activities of antioxidative enzymes, attenuation of mitochondrial dysfunction, blocking over-stimulated N-methyl-D-aspartate acid (NMDA) receptors as well as promoting the production of neurotrophic factors 6. These neuroprotective effects of Hup A appear to be independent of AChE inhibition. (+)-Hup A and (−)-Hup A showed similar potency towards NMDA receptors and in Aβ-induced cytotoxicity but (+)-Hup A is 50-fold less potent than (−)-Hup A in the activity of AChE inhibition in vivo. In the latest research, Hup A protects isolated mitochondria against Aβ-induced mitochondrial swelling, ROS and cytochrome c release 7, further extending the noncholinergic activitiy of Hup A. The most recent data from a clinical trial in China demonstrated that Hup A affords significant improvement in the memory of elderly people and patients with AD and VaD without noticeable side effects 8. Moreover, a Phase II clinical trial completed in the US found dose-related improvements after Hup A administration with higher MMSE scores at higher doses and no serious side effects 4.
Hup B is another notable active principle from Huperzia serrata (Thunb) Trev. It is also a potent, reversible AChEI exhibiting more selective inhibition on AChE activity than galanthamine in vitro 9, 10, 11. In vivo studies suggest improved memory retention and retrieval in both adult and aged mice treated with Hup B. The compound also reverses the disruption of memory retention induced by scopolamine, NaNO2, electroconvulsive shock and cycloheximide in mice while less peripheral side effects were detected compared with galanthamine and physostigmine 11. The antioxidant activity of the compound may partially contribute to its neuroprotective effect 12. Hup B also exhibits an antagonist effect on NMDA receptors in addition to its inhibitory effect on AChE 13. Some derivatives of Hup B are potent multifunctional cholinesterase inhibitors 14. Further clinical investigations will be required to determine the effect of Hup B on dementia.
Amaryllidaceae family
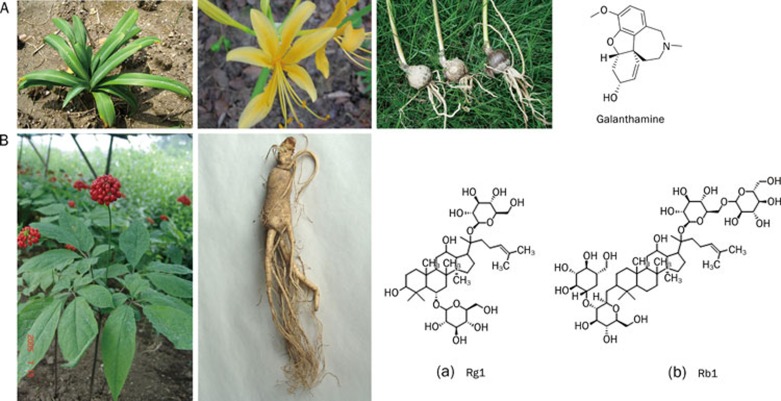
Plants of the Amaryllidaceae family are largely distributed in China, especially in Yunnan province. The plants have been traditionally used in China for the treatment of poisonous snake bites, acute laryngeal infection, rheumatoid arthritis, paralysis and muscle diseases brought on by infantile paralytic sequela 15. One of the widely studied alkaloids from Amaryllidaceae is galanthamine, which is a tertiary alkaloid originally extracted in 1952 by a Russian scientist from the bulbs of Galanthus woronowii, a plant of the Amaryllidaceae family (Figure 2A). Since 1960, researchers in China have been isolating this compound from Lycoris squamigera maxim. Later, it was also isolated from many other plants of the Amaryllidaceae family, such as L radiata Herb, L aruea Herb, and L squamigeric Herb.
Figure 2.
Amaryllidaceae and its extract galanthamine (Panel A); Panax ginseng CA Meyer and its saponins: (a) Rg1; (b) Rb1 (Panel B).
Considerable studies indicate that galantamine may potentiate the central cholinergic system and improve memory deficits 16, 17. However, because the natural content of the compound in plants is low and the extraction procedure is expensive, synthetic sources are currently under development 18. Galanthamine is a selective, reversible and competitive inhibitor of AChE; less potent than Hup A and donepezil but more potent than rivastigmine 3. In addition, the compound is recognized as an allosteric modulator of nAChRs. Selective stimulation or modulation of nAChR subtypes improves learning and memory. Such an agonist for nAChRs could increase the synthesis of neurotrophic factors and protect neuronal cells against toxic effects of glutamate, trophic factor deprivation, hypoxia, Aβ and ischemic injury 19, 20. Moreover, the combination of galanthamine and nicotine has a synergistic effect on the inhibition of microglia activation 21. In addition to its actions in the regulation of cholinergic transmission, galanthamine alleviates oxidative stress 22, potentiates the NMDA response 23, 24, 25 and up-regulates anti-apoptotic protein expression 26, 27, 28. In summary, galanthamine is a natural product with multiple molecular targets. However, the precise mechanism of its action needs further investigation.
The co-administration of galanthamine and piracetam is more effective in alleviating scopolamine-induced memory deficiency compared to treatment with either of these compounds alone, as demonstrated by our previous studies on rats subject to passive avoidance and radial maze training (unpublished data). The use of galanthamine for the treatment of mild to moderate AD has been approved by the SFDA in China and the FDA in the US. A Phase III clinical trial has been completed and has confirmed the efficacy and safety of galanthamine in the treatment of AD (http://clinicaltrials.gov/ct2/show/NCT00338117). A recent investigation indicated that the compound may also improve the cognition of patients with severe AD but it failed to improve the co-primary parameter of overall activities of daily living 29. Patients with AD combined with cerebral vascular disease (CVD) experienced significant cognitive and functional improvement when treated with galanthamine for six months. Collectively, these findings support the effectiveness of this compound in slowing the progression of dementia.
Panax ginseng CA Meyer
Panax ginseng CA Meyer, one of the most commonly used and highly researched species of ginseng, is largely distributed in northeast China. It has been used in Chinese traditional medicine for over 2000 years as a tonic remedy for weakness, fatigue and ageing. The main active components of Panax ginseng CA Meyer are ginsenosides, which are triterpene saponins enriched in the stalks and leaves of the herb. Among the ginsenosides, Rb1 and Rg1 are regarded as the main compounds responsible for many of the pharmacological actions of ginseng (Figure 2B). Today, extraction, purification and analyzation procedures are optimized for large-scale production of ginsenosides 30.
Rg1 improves learning and memory in normal, aged animals and in various model animals with impaired memory induced by anisodine, cycloheximide, alcohol, Aβ, scopolamine and ischemia 31. Besides the significant enhancement of cholinergic function through an increase in acetylcholine (ACh) level and density of muscarinic receptors 31, the mechanism of the protective effect of Rg1 involves blockade of the inflammation cascade 32, calcium channels and cerebral nerve cell apoptosis, and regulation of neurite lengths and numbers of dopaminergic cell cultures 33, nitric oxide and its synthase, together with effects on antiperoxidation, estrogen-like action, as well as the amelioration of mitochondrial dysfunction.
Similar to Rg1, Rb1 may also improve learning and memory. The possible mechanisms could involve an enhancement of cholinergic metabolism, an increase in hippocampal synaptic density, and the maintanence of intracellular calcium homeostasis and microtubule integrity 34, 35, 36. However, Rb1 has significant effects on both repeated and chronic stress-related changes, while Rg1 does not appear to possess an anti-stress property. Moreover, Rb1 has no effect on the decline of immune function in aged rats, which is an additional difference between Rg1 and Rb1 31. Taken together, these studies suggest that Rb1 may rescue or protect neurons from insult and may be a potential candidate to improve the cognitive deficit of AD.
Clinical trials in China report that ginseng tonic decoction significantly improves MMSE and reduces clock drawing (CD) scores in AD patients 37. Phase II clinical trials in China found another ginsenoside, Rd, to be of some benefit in acute ischemic stroke 38. All the above suggest Panax ginsengs CA Meyer will be a promising source of principles for the treatment of dementia.
With the application of modern scientific technology, new ginsenosides such as Rg3, Rh1, Rh2, Rb3, and Re have been isolated and purified 30, 39, 40. Many of these compounds possess bioactive effects against dementia. For example, Rg1's primary metabolite ginsenoside, Rh1, exihibted more potent actions than Rg1 in improving memory and hippocampal excitability 41. However, more investigations are needed to determine the efficacy of these novel active principles in the therapy for dementia.
Ginkgo biloba Linn
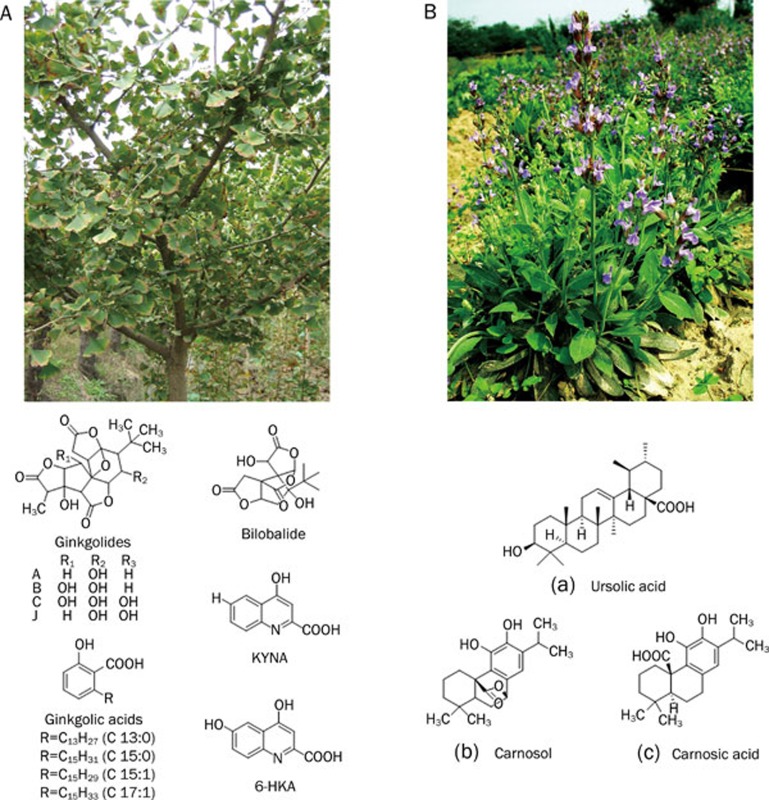
Ginkgo biloba Linn, more than 200 million years old, is one of the best-known examples of a living fossil. It requires acidic soil with proper drainage and full sunlight at planting site. For centuries it was thought to be extinct in the wild, but it is now known to be distributed mainly in the Jiangsu, Hubei, Shandong, and Zhejiang provinces in China due to the pleasant natural conditions in those provinces. The most famous distribution district of Ginkgo biloba Linn might be the Tian Mu Shan Reserve in Zhejiang province, well-known for its scenic beauty and the diversity of its flora. Ginkgo biloba Linn extracts have long been used in China as traditional medicine for various health disorders. EGb761, the standardized extract of its leaves, contains 24% flavonoids and 6% terpene lactones 42, 43. A mixture of many active components including ginkgolides, bilobalide, ginkgolic acids, as well as KYNA and 6-HKA (Figure 3A) may account for its various pharmacological effects, such as the alleviation of VaD, early-stage AD, peripheral claudication and tinnitus of vascular origin 44, 45, 46, 47. Extraction of active principles from Ginkgo biloba Linn has been studied for a long time in China and high production levels and purity have been achieved 48, 49, 50, 51.
Figure 3.
Active principles (EGb761), ginkgolides A, B, C, and J, bilobalide, ginkgolic acids, KYNA, and 6-HKA isolated from the Chinese herb Gingko biloba Linn (Panel A); Salvia officinalis Linn and its extracts: (a) Ursolic acid; (b) Carnosol; (c) Carnosic acid (Panel B).
Ginkgo biloba Linn leaf preparations were marketed in France and Germany 30 years ago for the treatment of cardiovascular disease, cerebrovascular diseases and dementia. They are now classified as a food supplement in the United States and widely used in China to alleviate dementia. The protective effects of EGb761 were shown in in vitro injury models induced by hypoxia, hydrogen peroxide, glutamate, verapamil, Aβ and nitric oxide (NO) 52, as well as in an in vivo model induced by ischemia 53, 54. The purported biological effects of EGb761 include amelioration of mitochondrial dysfunction 55, blockade of the NMDA receptor 56, 57, scavenging of free radicals, lowering of oxidative stress, reduction of neural damages, reduction of platelet aggregation, anti-inflammation 58 and anti-aging 59. Clinically, EGb761 has been prescribed to treat central nervous system (CNS) disorders such as AD and cognitive deficits, which may be attributed to the aforementioned effects. EGb761 treatment is beneficial in patients with mild to moderate dementia compared with placebo treatment 60. Also, several studies confirmed the equal efficacy and tolerability of EGb761 in comparison to commonly prescribed AChEIs 61, 62. However, analytical results derived from several recent clinical trials found the predictable and clinically significant beneficial effects of Ginkgo biloba extracts to be inconsistent and unreliable in people with dementia or cognitive impairment 47, 63. Further studies are needed to better understand this herbal extract.
Salvia officinalis Linn
Salvia officinalis Linn (sage) (Figure 3B), native to the Mediterranean region, grows in the Zhejiang, Anhui, Jiangsu, Jiangxi, Hubei, Fujian, Guangdong, Guangxi, and Taiwan provinces of China. It has a long-standing application in medicinal herbalism as an antibiotic, antihydrotic, astringent and antifungal. The leaves of the plant are well known for their antioxidative properties 64. A recent clinical trial showed that extracts of the plant are effective in treating mild to moderate AD 65. Ursolic acid (UA), the main constituent in chloroform extracts, is a natural pentacyclic triterpenoid carboxylic acid, which could markedly reverse D-gal-induced learning and memory impairment. Increased activity of antioxidant defense enzymes together with a reduced level of lipid peroxidation were found to underlie the neuroprotective effect of UA 66. UA is also the major component involved in anti-inflammatory activity of sage, which is two fold more potent than that of indomethacin, a reference non-steroidal anti-inflammatory drug (NSAID) 67. Moreover, UA also effectively inhibits AChE actitivity in vitro 68. Carnosol and carnosic acid, which are ethanol extracts from dried leaves of the plant, inhibit binding of t-butylbicyclophosphoro[35S] thionate (TBPS) to the chloride channels of rat cerebrocortical membranes 69. Ethanol extracts could also potentiate memory retention in rats, possibly through an interaction with the muscarinic and nicotinic cholinergic systems that are involved in the memory retention process 70. Activation of PPAR-γ may underlie the anti-inflammatory effect of carnosol and carnosic acid 71, 72, 73. Rosmarinic acid (RA), a naturally occurring hydroxylated compound, is present in water extracts of Salvia officinalis Linn. RA dose dependently inhibits the formation of Aβ fibrils from Aβ1-40 and Aβ1-42, as well as their extension. In addition, RA dose dependently destabilizes preformed Aβ fibrils 74, 75. The aforementioned activities of the Salvia officinalis Linn extracts predict the herb to be a promising drug for dementia.
Camellia sinensis (L) O Ktze
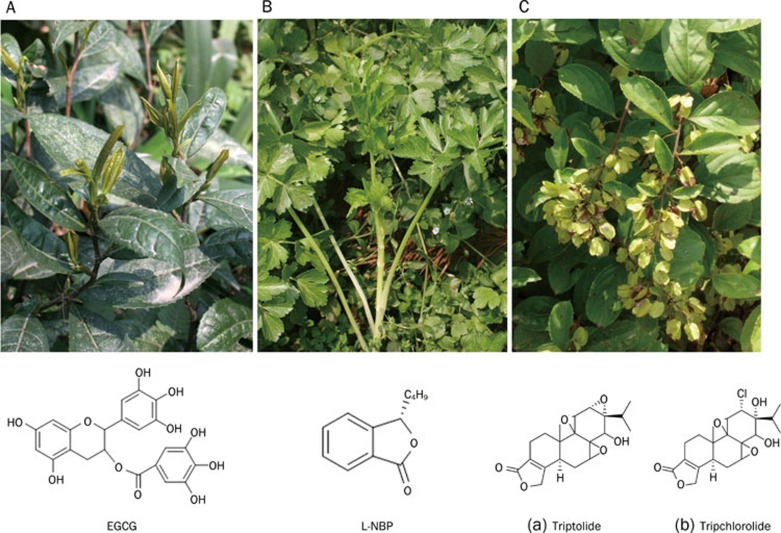
If there were a prize for achievement in liquid nutrition, it would have to go to green tea, which is popular among Chinese people and widely grown in China. Green tea is made from the dried leaves of Camellia sinensis (L) O Ktze. It is well known that green tea allows people to keep healthy, delay the onset of cardiovascular diseases, obesity and cancer, as well as slow down the process of ageing. Therefore, the effect of green tea in the prevention and alleviation of dementia has been of particular interest in recent years. As one of the oldest and healthiest beverages in the world, green tea enables people to think clearly and coherently, as indicated in a recent study on more than 1000 subjects carried out in Japan 76. Human epidemiological and new animal data suggest that drinking green tea may decrease the incidence of dementia. The most active component of green tea involved in its therapeutic effects is (−)-epigallocatechin-3-gallate (EGCG) (Figure 4A), whose antioxidant activity is significantly more potent than vitamin C, vitamin E, resveratrol and its counterparts (−)-epicatechin (EC), (−)-epigallocatechin (EGC) and (−)-epicatechin-3-gallate (ECG) 77, 78, 79.
Figure 4.
Camellia sinensis (L) O Ktze and its extract EGCG (Panel A); Apium graveolens Linn and its extract l-3-n-butylphthalide (Panel B); Tripterygium wilfordii Hook F and its extracts: (a) Triptolide; (b) Tripchlorolide (Panel C).
EGCG exerts neuroprotective activities in a wide array of cellular and animal models of neurological disorders 80, 81. The neuroprotective effect of EGCG could be achieved through complementary mechanisms involving the down-regulation of pro-apoptotic genes 82, 83, the influence of amyloid precursor protein (APP) processing by the elevation of α-secretase activity 84/inhibition of β-secretase activity 85, the promotion of cell survival, defense against oxidative stress 86, anti-inflammation 87, the scavenging of ROS 88, 89 and iron chelating, as well as the stabilization of mitochondrial function 90. The antioxidative activity of green tea has also been verified in human clinical trials 91.
Recently, numerous studies proved the beneficial effects of EGCG on learning and memory performance in various animal models, including the APP transgenic mouse model 92 and mouse models of cognitive impairment induced by Aβ 93, D-gal 94, and 3-NP 95. The abovementioned data suggest that green tea, and in particular EGCG, could be a promising drug candidate for dementia.
Apium graveolens Linn
Apium graveolens Linn, a herb from Europe and temperate parts of Asia, is a species in the family of Apiaceae. It was believed in Chinese folklore that Apium graveolens Linn alleviates hypertension, vertigo, headache, redness of the face and eyes, swelling and pain. L-3-n-butylphthalide (L-NBP) (Figure 4B) is the active component of the essential oil extracted from the seeds of Apium graveolens 96.
L-NBP is a potential new agent for the treatment of neurodegenerative diseases through multiple targets. L-NBP treatment could significantly reverse Aβ-induced cytotoxicity, probably through an inhibition of tau protein hyperphosphorylation 97. L-NBP significantly attenuates cerebral hypoperfusion-induced learning dysfunction and brain damage in rats 98. Moreover, recent results indicate that long-term treatment with L-NBP may prevent age-associated cognitive dysfunction in rats 99. In addition to the inhibition of β-amyloid cleaving enzyme (BACE) expression and tau hyperphosphorylation reported in the present research, increase in cerebral blood flow, enhancement of energy metabolism, improvement of mitochondrial function, inhibition of neuronal apoptosis and reduction of oxidative stress may be related to the neuroprotective effects of L-NBP. Therefore, the compound may emerge as a new drug candidate for the long-term treatment of dementia 99. To this end, the compound has been recently approved for Phase I clinic trials by the SFDA.
DL-3-n-butylphthalide (DL-NBP) has been synthesized and shown to be effective in multiple ischemia models. The underlying mechanism may involve improvement of microvessel potency and increasing of newly developed microvessels 100, 101, enhancing the activity of antioxidative enzymes, improving mitochondrial function, inhibiting lipid peroxidation 102, blocking platelet aggregation 103, increasing regional cerebral blood flow 104, as well as reducing ischemia ipsilateral cortical calcineurin and calpain activities 105. These would collectively result in an elevated survival rate of neurons and a smaller infarct volume of the brain, thus protecting rats from severe ischemic injury 106. The compound was approved by the SFDA of China for clinical use in stroke patients in 2002.
Tripterygium wilfordii Hook F
Tripterygium wilfordii Hook F (Figure 4C), widely known as Lei Gong Teng in China, is mainly distributed in the Zhejiang, Fujian, Jiangxi, Hunan, Hubei, Guangdong, and Taiwan provinces of China. It has long been used for the treatment of rheumatoid arthritis due to its anti-inflammatory and immunosuppressive properties. With the progress of neuroimmunological research on neurodegenerative diseases such as AD and PD, the neuroprotective capabilities of Tripterygium wilfordii Hook F to rescue neurons from immunological injury are currently being explored.
The most abundant and active component is triptolide (Figure 4C), which was shown to be promising for the treatment of PD. Accumulating evidence has shown that triptolide can promote neuronal survival and neurite outgrowth, facilitate brain functional recovery through an inhibition of neuroinflammatory toxicity of activated microglia, reduce oxidative stress, stimulate the expression and release of nerve growth factor (NGF) and inhibit nuclear factor kappa B (NF-κB) activation 107. An in vivo study demonstrated that triptolide significantly inhibited microglial activation, partially prevented dopaminergic cells from death and improved behavioral performances in MPP+-induced hemiparkinsonian rats 108. The beneficial activities of triptolide on dopaminergic neurons were further comfirmed in an inflammatory PD model by injection of LPS into the substantia nigra 109. In vivo administration of the compound significantly attenuated the rotational behavior challenged by D-amphetamine in rats subject to transection of the medial forebrain bundle. Increased survival rate of dopaminergic neurons in the substantia nigra pars compacta (SNpc) area together with elevated levels of dopamine in the striatum were found in rats treated with tripchlorolide after axotomy 110.
Although research regarding the action of Tripterygium wilfordii Hook F extracts on CNS dysfunction were mainly carried out in PD models 111, the abovementioned neuroprotective effects predict that these extracts may be promising anti-dementia drugs, because neuronal death in AD models is related to the overwhelming inflammatory response.
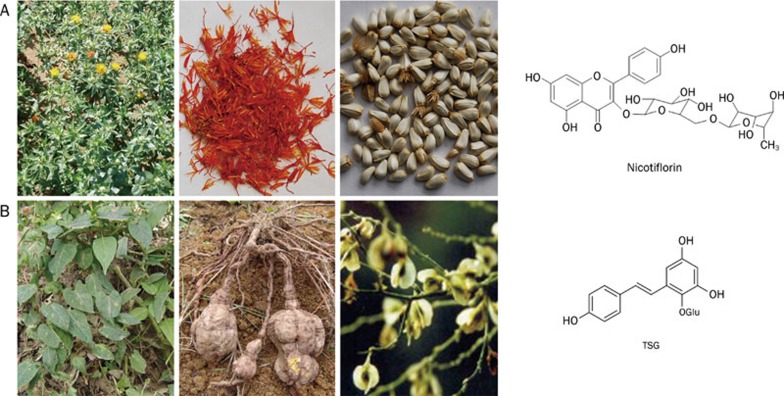
Carthamus tinctorius Linn
Carthamus tinctorius Linn (safflower) (Figure 5A), originating in Egypt, is widely cultivated in the Henan, Hebei, Zhejiang, and Sichuan provinces of China. Safflower is an important crude drug in traditional Chinese medicine for promoting blood circulation, restoring menstrual flow and removing obstruction in the channels and collaterals. It has long been used in the prevention and treatment of cardiovascular diseases and thrombosis in Chinese people.
Figure 5.
Carthamus tinctorius Linn (safflower) and its extract nicotiflorin (Panel A); Polygonum multiflorum Thunb and its extract TSG (Panel B).
The major component of the safflower are flavonoids, which have been intensively studied in China 112. Nicotiflorin (Figure 5A), a flavonoid extracted from the dried and powdered corona of the safflower, appears to be a kind of yellow amorphous powder soluble in acetoacetate, ethyl alcohol and methyl alcohol. In a permanent model of focal brain ischemia, nicotiflorin administration reduced the size of the infarction and ameliorated the neurological deficits 113. Moreover, nicotiflorin also enhances learning and memory performance in a multi-infarct ischemia rat model. Such improvement could be attributed to its potency in protecting energy metabolism and alleviating oxidative stress 114. Furthermore, safflower oil extracts, N-(p-coumaroyl)serotonin and N-feruroylserotonin, which are two serotonin derivatives, could prevent the guinea pig Langendorff heart from injury caused by ischemia-reperfusion, probably through direct quenching of the activity of active radicals 115.
Recent clinical experiences from many hospitals in China have shown that safflower extracts have good efficacy in the treatment of stroke-induced brain damage and diabetic peripheral nervous system lesions. Accumulating evidence suggests that stroke and diabetes both have a close relationship with the incidence of dementia, especially VaD, which is thought to be caused by blood stasis and phlegm deposits according to Chinese traditional medicine. Interestingly, as demonstrated by Liu Junfeng, decoction of safflower with herbals like Angelica sinesis, Rhizoma Chuanxiong, Semen Persicae, Salvia Miltrorrhiza, Radix Platycodi, and others available to specific patients could significantly improve cognition, memory and behavior in more than 90% of the patients in clinical trials by promoting circulation, expelling phlegm, and cultivating healthy mentality. Based on the promising effect of safflower on the cardiovascular system, it is worthwhile to put more effort into investigating its novel active principles in the treatment of dementia, especially VaD.
Polygonum multiflorum Thunb
Polygonum multiflorum (PM) (Figure 5B), the dry root of Polygonum multiflorum Thunb, rejuvenates the body and has been used in China since ancient times to prevent ageing. Wild PM mainly grows in the Guangxi, Henan, and Guizhou provinces, with some growth scattered throughout the Yunnan, Guangdong, Hubei, Henan, and Jiangsu provinces. PM is traditionally used for the treatment of hyperlipemia, neurasthenia, premature white hair, nerve injury and constipation. Recent pharmacological studies suggest that PM has various functions, which include anti-ageing, improvement of the immune system, lowering of serum cholesterol, reversal of hardening of the arteries, improvement of bowel movements, stimulation of the adrenal cortex, regulation of epinephrine, norepenephrine and blood sugar, and others 116. A great deal of effort has gone into clarifying the nature of the active principles in PM. PM contains a number of active principles such as stilbene glycosides, anthraquinone and phospholipids. One of the consitutents effective in dementia is 2,3,5,4′-tetrahydroxy stilbene-2-O-β-D-glucoside (TSG), which could be obtained through heating and reflux in 50% alcohol 117.
TSG significantly decreases amyloid plaque formation and improves learning and memory abilities in APP transgenic mice 118 and rats subject to chronic ischemia 119, ibotenic acid or Aβ injection 120, 121. Multi-target pharmacological mechanisms contribute to the cognitive enhancement of TSG, which may involve calcium channel blockade, antioxidation, anti-cholinesterase activity and anti-apoptotic activity.
VaD patients administered PM extractum tablets showed a dramatic improvement in scores of MMSE, ADL, Blessed–Roth Scale, and Clinical Global Impression (CGI) without their vital signs being affected and laboratory indices influenced 122. These effects allow the active components of PM such as TSG to be of considerable value in treating dementia.
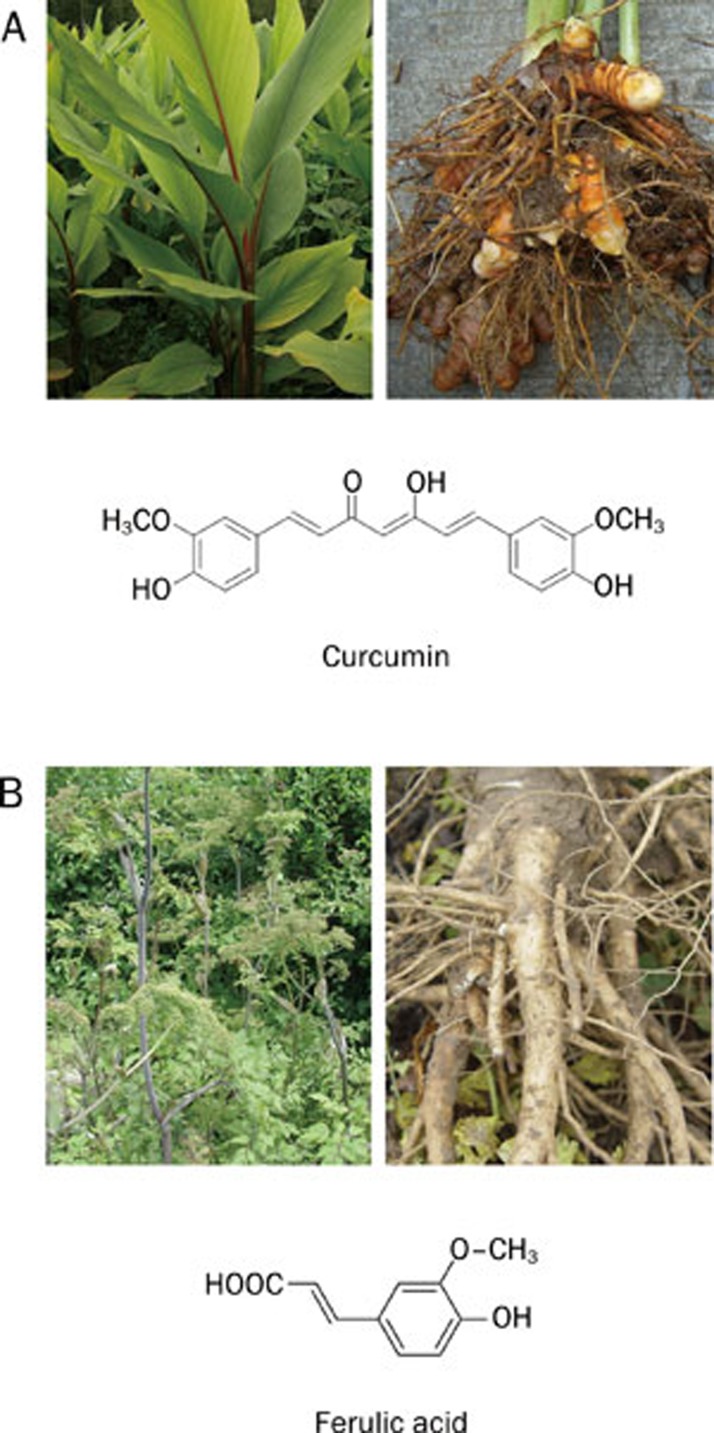
Curcuma longa Linn
There are about 16 different Curcuma L plants growing mainly in the southeastern and the western parts of China. One of the most widely used herbs in traditional Chinese medicine is Curcuma longa Linn (Figure 6A), which is mainly distributed in the Guangdong, Guangxi, and Taiwan provinces. It was traditionally used to relieve pain, pressure, edema and hematoma and to improve the symptoms of depression.
Figure 6.
Curcuma Longa Linn and its extract curcumin (Panel A); Angelica sinensis (Oliv) Diels and its extract ferulic acid (Panel B).
Curcumin, a potent polyphenol extract of Curcuma longa root, is the major component of this herb (Figure 6A). The extraction of the compound has been widely studied in China 123, 124. Recently, its effect in the prevention and alleviation of dementia has been of particular interest. A large number of studies indicate that curcumin is effective on multiple pathological targets, such as inhibiting lipid peroxidation, scavenging ROS and reactive nitrogen species (RNS) 125, elevating γ-glutamyl-cysteinal synthetase and glutathione (GSH)-linked detoxifying enzymes 126, inhibiting NF-κB activation 127, and suppressing inflammation 128. The activity of curcumin to increase heme oxygenase-1 (HO-1) in astrocytes and vascular endothelial cells may partially explain its antioxidant property 129, 130. Interestingly, curcumin could also significantly interfere with Aβ-associated pathogenesis through decreasing cholesterol level, inhibiting aggregation of proteins in non-native conformation via binding to α-helical intermediate and amyloid forms of prion protein 131, together with increasing microglia adjacent to plaques, which may stimulate microglial phagocytosis of amyloid 125, 128.
Studies in animal models suggested that curcumin is beneficial to improving learning and memory deficiency caused by AlCl3, D-gal 132, and streptozotocin 133. Human clinical trials in patients with mild to moderate AD were performed at the University of California, Los Angeles (UCLA) 134.
Angelica sinensis (Oliv) Diels
Angelica sinensis (Oliv) Diels (AS) (Figure 6B) is a kind of herb from the Apiaceae family indigenous to China. It is distributed in provinces like Gansu, Yunnan, Sichuan, Qianghai, Shaanxi, Hunan, Hubei, and Guizhou. Its dry root, commonly known as Radix Angelicae Sinensis, or Chinese angelica, is widely used in China to treat amnesia, mild anemia, gynecological ailments and hypertension. Non-aromatic fractions, such as ferulic acid (FA) (Figure 6B), and polysaccharides are found in the aqueous phase of AS extracts. Although the traditional water extraction procedure has been optimized to allow for a high yield of FA, the semi-bionic extraction is a more suitable procedure 135.
FA has various pharmacological activities involving anti-tumor, anti-virus and anti-bacteria actions, as well as heart protection and inhibition of cholesterol synthesis and the release of TXA2. A considerable number of studies has demonstrated that FA can significantly reduce inflammation, oxidative stress and apoptosis in vivo 136, 137. FA treatment significantly alleviated middle cerebral artery occlusion (MCAO)-induced injury, which is mediated through the down-regulation of ICAM-1 transcription, reducing the number of activated microglia 137. FA prevents cognitive dysfunction caused by trimethyltinas 138 and buthionine-sulfoximine 139. Although clinical trials in China have found that Danggui Shaoyao San, a mixture made from the decoction of AS with other herbs, could improve the MMSE and AIM scores for AD patients while reducing the level of blood superoxide dismutase (SOD) and lipid peroxidase (LPO) 140, clinical trials will be needed for the evaluation of the anti-dementia effect of FA.
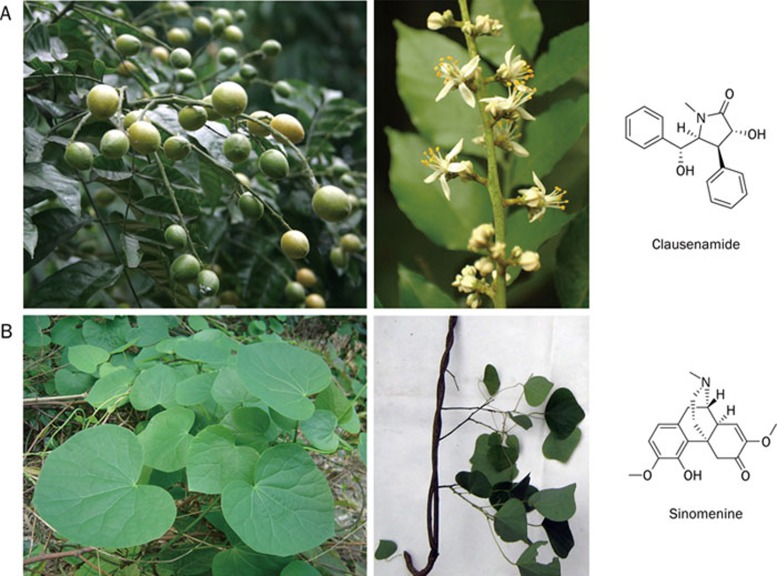
Clausena lansium (Lour) Skeels
Clausena lansium (Lour) Skeels (Figure 7A), native to southern China, is in the family Rutaceae. It was mainly used for cough and indigestion in ancient Chinese times. Recent studies indicate that clausenamide, one of the major components isolated from the aqueous extract of the leaves of Clausena lansium (Lour) Skeels, may be a promising candidate for the treatment of dementia.
Figure 7.
Clausena lansium (Lour) Skeels and its extract clausenamide (Panel A); Sinomenium acutum (Thunb) Rehd EtWils and its principle extract sinomenine (Panel B).
Compared with piracetam, a nootropic drug developed in Europe, clausenamide is more favorable in learning and memory improvement 141. Its neuroprotective effect has been recognized in multiple models. In addition to prolonging survival time of mice subject to acute brain ischemia, clausenamide inhibits lipid peroxidation caused by alcohol, probably by increasing GSH peroxidase activity. It also increases blood flow in the brain by inhibiting basilar artery contraction induced by 5-HT, PGF2α, and arachidonic acid 142. Clausenamide could also protect nerve cells against apoptosis induced by ischemia-reperfusion in renovascular hypertensive rats by elevating Bcl-2 expression 143.
(−)-Clausenamide, the first chiral nootropic agent developed in China, increased the magnitude of long-term potentiation (LTP) and potentiated synaptic transmission in rats by interfering with calcineurin, calpain and voltage-dependent calcium channels 144. (−)-Clausenamide was again confirmed to protect hippocampus neurons from apoptosis caused by sodium nitroprusside through the up-regulation of anti-apoptotic proteins and the down-regulation of pro-apoptotic proteins. In okadaic acid-treated in vitro models and Aβ25–35-injected ovariectomized (OVX) rats, (−)-clausenamide promoted cell survival and ameliorated learning and memory in a step-through test 142. (−)-Clausenamide was approved by the SFDA in Jan 2007 and a Phase I clinical trial is underway.
Sinomenium acutum (Thunb) Rehd EtWils
Sinomenium acutum (Thunb) Rehd EtWils (Figure 7B) is mainly distributed in the Anhui, Jiangsu, Zhejiang, Fujian, Hubei, Sichuan and Guizhou provinces of China. It is clinically used for the treatment of rheumatoid arthritis due to its anti-inflammatory and analgesic activities. One of the active components of Sinomenium acutum (Thunb) Rehd EtWils is sinomenine, which is a kind of NSAID with multiple neuroprotective effects and few side effects.
A number of studies demonstrated that sinomenine could protect aganist cerebral ischemia/reperfusion injury through resisting inflammatory responses 145 and regulating apoptotic gene expression 146. Based on the promising effects of NSAIDs in the treatment of dementia, sinomenine may also be beneficial for alleviating pathological changes that occur in AD and VaD, although further studies are needed to test this hypothesis. Interestingly, our studies showed that S52, a derivative of sinomenine 147 isolated from Sinomenium acutum (Thunb) Rehd EtWils, exerted neuroprotective effects in multiple in vitro and in vivo models (unpublished data). This synthetic alkaloid could elevate cortical ACh levels with no anti-AChE activity. S52 was effective in various cytotoxicities induced by H2O2, glutamate, rotenone or Aβ25–35. In vivo studies also showed that S52 ameliorated scopolamine- or ischemia-induced deficiency in spatial performance and cognition in mice. The underlying mechanisms may involve resisting oxidative stress, anti-apoptosis, and attenuating mitochondrial dysfunction.
Other Chinese herbs potential for anti-dementia drug development
In addition to the aforementioned herbs, there are others shown to be active in alleviating dementia. Some of them have been extensively studied with the chemical structures and pharmacology of the active principles elucidated and the extraction procedures optimized. Yet there are many other plants which have only their crude extractions studied and deserve further research, such as Radix puerariae, Rhizoma Chuanxiong, Salvia miltiorrhiza, and others (Table 1).
Table 1. Potential herbal extracts for dementia therapy.
| Active components (Herb) | Molecular structure | Improvement on learning and memory impairment induced by | |||
|---|---|---|---|---|---|
| Aβ | Scopolamine | Ischemia | Others | ||
| Puerarin [Pueraria lobata (Willd) Ohwi] | 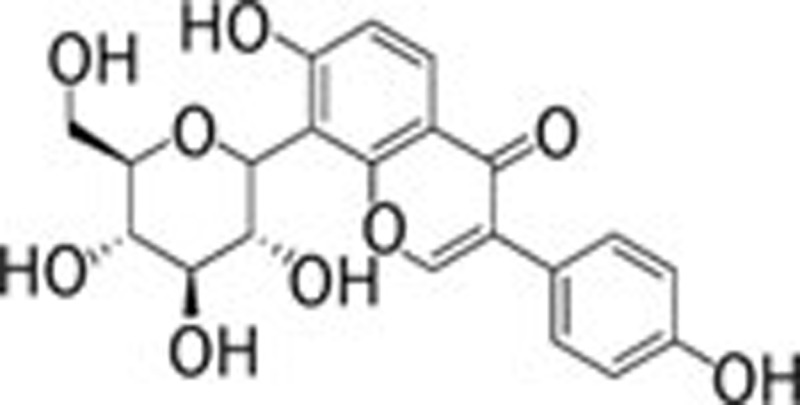 |
148, 149 | 150 | SAMP8 151 | |
| Ligustrazine (Ligusticum wallichii Franch) | 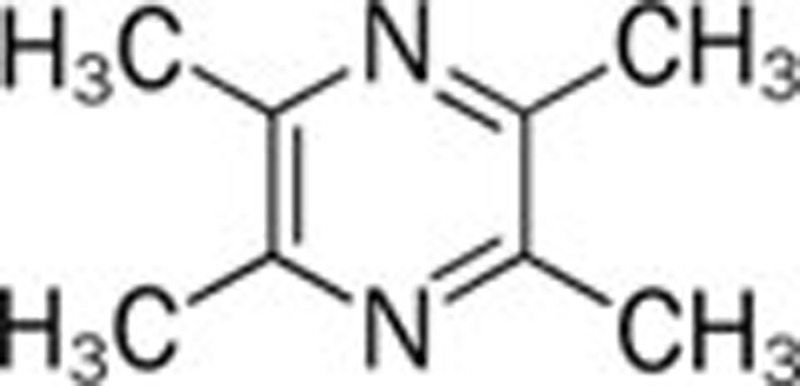 |
152 | NaNO2/AlCl3 153 | ||
| Resveratrol (Polygonum cuspidatum sieb et zucc) | 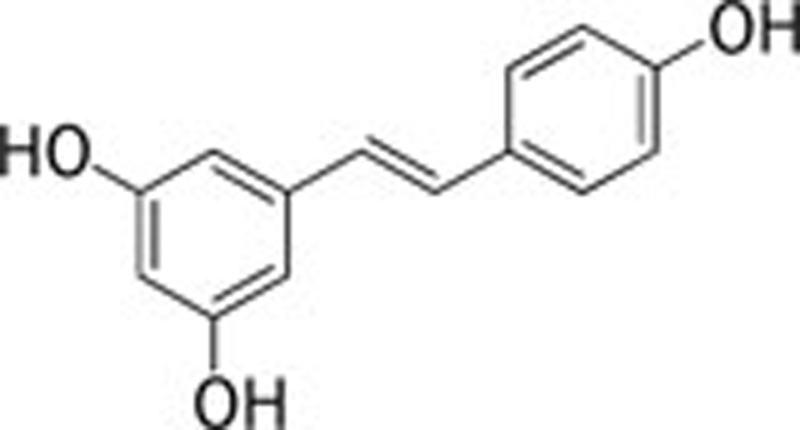 |
Colchicine 154; STZ 155; TBI 156 | |||
| Rhodosin (Rhodiola Rosae Linn) | 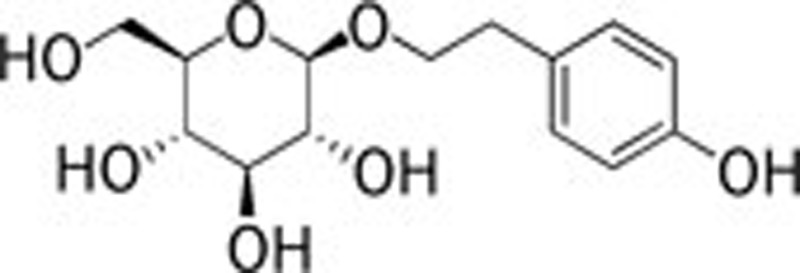 |
157 | 158 | ||
| Paeoniflorin (Paconia lactiflora Pall) | 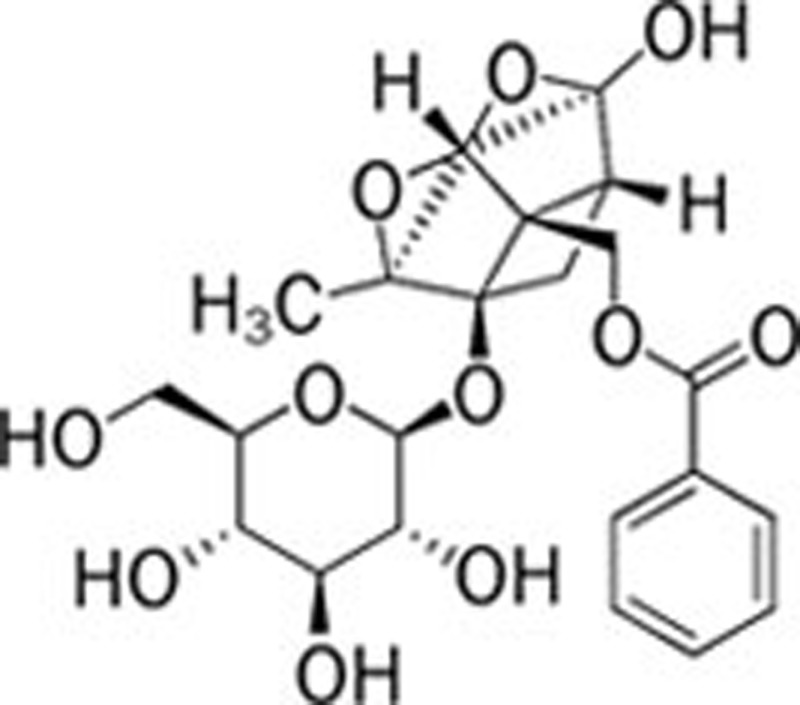 |
159 | 160 | NaNO2/Ethanol 161 | |
| Asarone (Acorus gramineus Soland) | 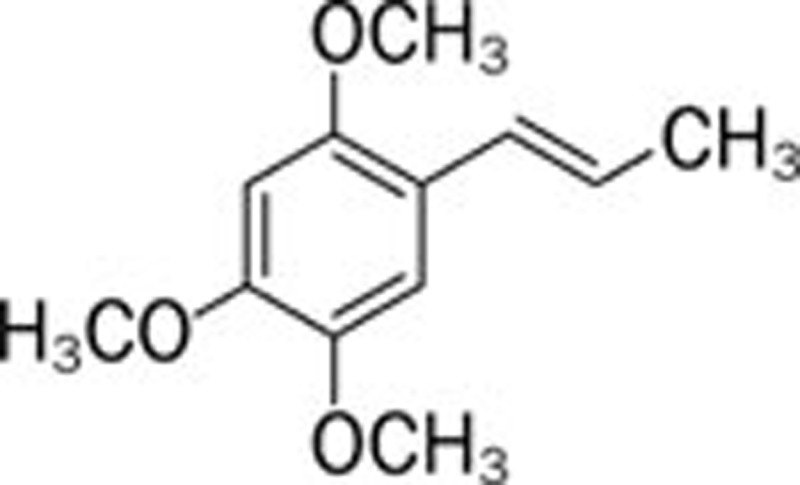 |
AlCl3 162 | |||
| Gastrodine (Gastrodia elata BI) | 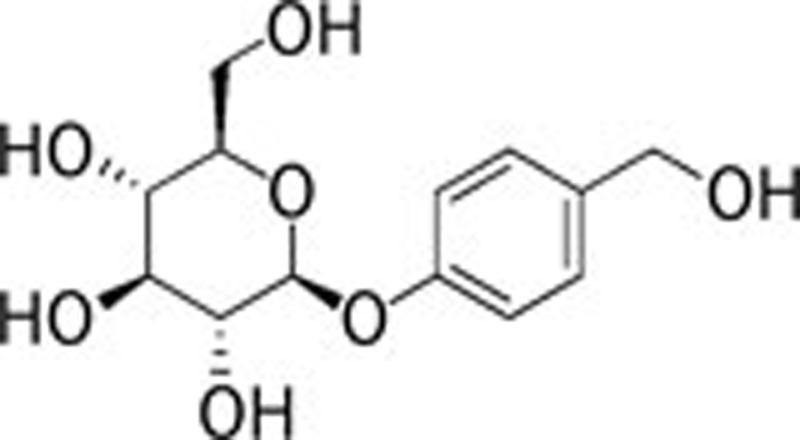 |
163, 164 | |||
| Icariin (Epimedium brevicornum Maxim) | 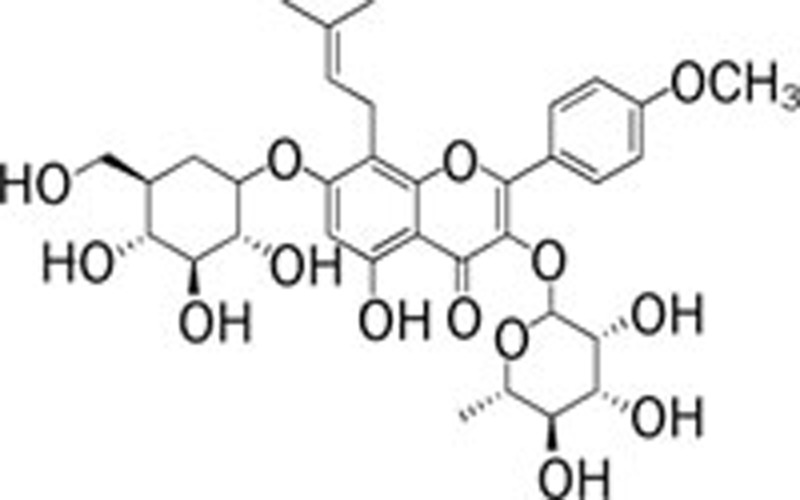 |
165 | AlCl3 166 | ||
| Osthol (C monnieri (L) Cusson) | 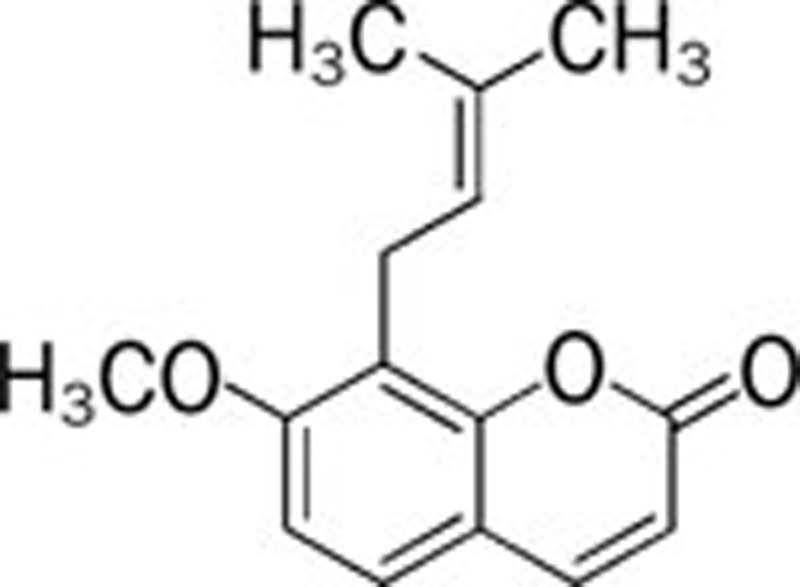 |
167 | AlCl3 168; NaNO2 169 | ||
| Schisandrone (S sphenanthera Rehd et Wils) |  |
170 | |||
Summary
Elderly people are particularly prone to affliction by certain debilitating diseases such as dementia, which bring endless anguish to the patients and their families. The pathology of dementia is such a complex process that for years the mechanisms have not been fully elucidated. Over-production of ROS, decreased levels of antioxidants and neurotrophic factors, glutamate toxicity, activation of apoptosis signaling pathways, aggregation of misfolded proteins and dysfunction of blood circulation are believed to speed up the progression of the disease. Today, the majority of approved drugs for dementia are single-targeted, whose therapeutic effects are not ideal. In recent years, a multi-target strategy has been attracting more attention. China has long been known for its numerous species of herbs and the long-standing practice of traditional Chinese medicine. It has a long history and rich experience in treating amnesia, tremor and rigidity, most of which are symptoms of progressive CNS debilitation. Although the philosophy of traditional Chinese medicine differs from that of occidental medicine, sound therapeutic effects promote more scientists, domestic and abroad, to study extracts from herbal medicines. Today, a great number of compounds from herb extracts have proven to be multi-targeted, low toxicity and potent in alleviating dementia. In light of the complex organization of the CNS, currently available Western remedies may not be suitable for the treatment of dementia because of their side effects. The wide distribution of herbs in China, their multiple active principles and low toxicity, together with progress in isolation procedures would render traditional Chinese medicine particularly suitable for the long-term treatment of dementia.
Acknowledgments
The work was supported in part by the Ministry of Science and Technology of China (G199805110, G1998051115, No 2004CB518907), National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” China (Number: 2009ZX09103-063), and the National Natural Science Foundation of China (39170860, 39770846, 3001161954, 30123005, 30271494, 30572169, and 30801402). The authors are grateful to Dr Xiao-qiu XIAO, Dr Jin ZHOU, Dr Han YAN, Dr Zhi-fei WANG, Dr Xin GAO, and others for their contributions to Hup A, ZT-1 or S-52 studies.
The authors are grateful to Prof Nae J DUN (Temple University, USA) for his critical comments and English revision on the manuscript.
References
- Ma X, Tan C, Zhu D, Gang DR, Xiao P. Huperzine A from Huperzia species — an ethnopharmacolgical review. J Ethnopharmacol. 2007;113:15–34. doi: 10.1016/j.jep.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Yan XF, Lu WH, Lou WJ, Tang XC. Effects of huperzine A and B on skeletal muscle and electroencephalogram. Acta Pharmacol Sin. 1987;8:117–23. [PubMed] [Google Scholar]
- Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin. 2006;27:1–26. doi: 10.1111/j.1745-7254.2006.00255.x. [DOI] [PubMed] [Google Scholar]
- Desilets AR, Gickas JJ, Dunican KC. Role of huperzine A in the treatment of Alzheimer's disease. Ann Pharmacother. 2009;43:514–8. doi: 10.1345/aph.1L402. [DOI] [PubMed] [Google Scholar]
- Wang BS, Wang H, Wei ZH, Song YY, Zhang L, Chen HZ. Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis. J Neural Transm. 2009;116:457–65. doi: 10.1007/s00702-009-0189-x. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Tang XC. Neuroprotective effects of huperzine A: new therapeutic targets for neurodegenerative disease. Trends Pharmacol Sci. 2006;27:619–25. doi: 10.1016/j.tips.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Gao X, Zheng CY, Yang L, Tang XC, Zhang HY. Huperzine A protects isolated rat brain mitochondria against beta-amyloid peptide. Free Radic Biol Med. 2009;46:1454–62. doi: 10.1016/j.freeradbiomed.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang X, Chen Q, Shu L, Wang J, Shan G.Clinical efficacy and safety of huperzine Alpha in treatment of mild to moderate Alzheimer disease, a placebo-controlled, double-blind, randomized trial Chin Med J 200282941–4.Chinese [PubMed] [Google Scholar]
- Xu H, Tang XC. Cholinesterase inhibition by huperzine B. Acta Pharm Sin. 1987;8:18–22. [PubMed] [Google Scholar]
- Liu J, Zhang HY, Wang LM, Tang XC. Inhibitory effects of huperzine B on cholinesterase activity in mice. Acta Pharmacol Sin. 1999;20:141–5. [PubMed] [Google Scholar]
- Tang XC, Zhu XD, Lu WH.Studies on the nootropic effects of huperzine A and B: two selective AChE inhibitorsIn: E Giacobini and R Berker, Editors. Current Research in Alzheimer Therapy: Cholinesterase Inhibitors, Taylor & Francis, New York 1988: 289–94.
- Zhang HY, Tang XC. Huperzine B, a novel acetylcholinesterase inhibitor, attenuates hydrogen peroxide induced injury in PC12 cells. Neurosci Lett. 2000;292:41–4. doi: 10.1016/s0304-3940(00)01433-6. [DOI] [PubMed] [Google Scholar]
- Wang XD, Chen XQ, Yang HH, Hu GY. Comparison of the effects of cholinesterase inhibitors on [3H]MK-801 binding in rat cerebral cortex. Neurosci Lett. 1999;272:21–4. doi: 10.1016/s0304-3940(99)00567-4. [DOI] [PubMed] [Google Scholar]
- Shi YF, Zhang HY, Wang W, Fu Y, Xia Y, Tang XC, et al. Novel 16-substituted bifunctional derivatives of huperzine B: multifunctional cholinesterase inhibitors. Acta Pharmacol Sin. 2009;30:1195–203. doi: 10.1038/aps.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XC, Jin GZ, Xu B.Neuropharmacologic actions of lycoramine and galanthamine Acta Pharm Sin 196310465–73.Chinese [PubMed] [Google Scholar]
- Tang XC, Xu B.Pharmacology and clinical applications of galanthamine, a new compound with cholinesterase inhibition activity Acta Pharm Sin 19661368–76.Chinese [Google Scholar]
- Barnes CA, Meltzer J, Houston F, Orr G, McGann K, Wenk GL. Chronic treatment of old rats with donepezil or galantamine: effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience. 2000;99:17–23. doi: 10.1016/s0306-4522(00)00180-9. [DOI] [PubMed] [Google Scholar]
- Marco-Contelles J, do Carmo Carreiras M, Rodriguez C, Villarroya M, Garcia AG. Synthesis and pharmacology of galantamine. Chem Rev. 2006;106:116–33. doi: 10.1021/cr040415t. [DOI] [PubMed] [Google Scholar]
- Belluardo N, Mudo G, Blum M, Fuxe K. Central nicotinic receptors, neurotrophic factors and neuroprotection. Behav Brain Res. 2000;113:21–34. doi: 10.1016/s0166-4328(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Greenwald DL, Shafron DH, Akaika A, Maeda T, Kaneko S, et al. Nicotinic alpha 7 receptors protect against glutamate neurotoxicity and neuronal ischemic damage. Brain Res. 1998;779:359–63. doi: 10.1016/s0006-8993(97)00194-7. [DOI] [PubMed] [Google Scholar]
- Giunta B, Ehrhart J, Townsend K, Sun N, Vendrame M, Shytle D, et al. Galantamine and nicotine have a synergistic effect on inhibition of microglial activation induced by HIV-1 gp120. Brain Res Bull. 2004;64:165–70. doi: 10.1016/j.brainresbull.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Ezoulin MJ, Ombetta JE, Dutertre-Catella H, Warnet JM, Massicot F. Antioxidative properties of galantamine on neuronal damage induced by hydrogen peroxide in SK-N-SH cells. Neurotoxicology. 2008;29:270–7. doi: 10.1016/j.neuro.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Cao XZ, Zhang LM, Shen YE, Zhou SS, Jiang GH.NMDA receptors mediate the effect of galantamin on the cognitive function of Alzheimer's disease in rats J Apop Nerv Dis 200623678–80.Chinese [Google Scholar]
- Mulder J, Harkany T, Czollner K, Cremers TI, Keijser JN, Nyakas C, et al. Galantamine-induced behavioral recovery after sublethal excitotoxic lesions to the rat medial septum. Behav Brain Res. 2005;163:33–41. doi: 10.1016/j.bbr.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Marszalec W, Zhao X, Yeh JZ, Narahashi T. Mechanism of action of galantamine on N-methyl-D-aspartate receptors in rat cortical neurons. J Pharmacol Exp Ther. 2004;310:933–42. doi: 10.1124/jpet.104.067603. [DOI] [PubMed] [Google Scholar]
- Melo JB, Sousa C, Garcao P, Oliveira CR, Agostinho P. Galantamine protects against oxidative stress induced by amyloid-beta peptide in cortical neurons. Eur J Neurosci. 2009;29:455–64. doi: 10.1111/j.1460-9568.2009.06612.x. [DOI] [PubMed] [Google Scholar]
- Matharu B, Gibson G, Parsons R, Huckerby TN, Moore SA, Cooper LJ, et al. Galantamine inhibits beta-amyloid aggregation and cytotoxicity. J Neurol Sci. 2009;280:49–58. doi: 10.1016/j.jns.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Arias E, Ales E, Gabilan NH, Cano-Abad MF, Villarroya M, Garcia AG, et al. Galantamine prevents apoptosis induced by beta-amyloid and thapsigargin: involvement of nicotinic acetylcholine receptors. Neuropharmacology. 2004;46:103–14. doi: 10.1016/s0028-3908(03)00317-4. [DOI] [PubMed] [Google Scholar]
- Burns A, Bernabei R, Bullock R, Cruz Jentoft AJ, Frolich L, Hock C, et al. Safety and efficacy of galantamine (Reminyl) in severe Alzheimer's disease (the SERAD study): a randomised, placebo-controlled, double-blind trial. Lancet Neurol. 2009;8:39–47. doi: 10.1016/S1474-4422(08)70261-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shan WH, Liang RX, Shi YZ, Shang LX, Chen WH, et al. Study of extraction processes in Panax ginseng Lishizhen Med & Mat Med Res 200011777–9.Chinese [Google Scholar]
- Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–9. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Joo SS, Won TJ, Lee DI. Reciprocal activity of ginsenosides in the production of proinflammatory repertoire, and their potential roles in neuroprotection in vivo. Planta Med. 2005;71:476–81. doi: 10.1055/s-2005-864145. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Moldzio R, Saito H, Rausch WD. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Chen XM, Zhu JB.Effects and mechanisms of ginsenoside Rgl on learning and memory impairment induced by scopolamine hydrobromide Chin J Clin Pharmacol Ther 200510898–902.Chinese [Google Scholar]
- Lou ZY, Wang M, Luo YY, Ruan DY.Effect of ginsenoside Rgl on long-term potentiation impaired by lead in CA1 region of rat hippocampus Chin J Clin Pharmacol Toxicol 20082217–23.Chinese [Google Scholar]
- Li YK, Chen XC, Zhu YG, Peng XS, Zeng YQ, Shen J, et al. Ginsenoside Rb1 attenuates okadaic acid-induced Tau protein hyperphosphorylation in rat hippocampal neurons Acta Physiol Sin 200557154–60.Chinese [PubMed] [Google Scholar]
- Cao L, Hu Z.Clinical analysis on senile dementia treated by Ginseng tonic decoction J Pract Trad Chin Med 200824207Chinese [Google Scholar]
- Liu X, Xia J, Wang L, Song Y, Yang J, Yan Y, et al. Efficacy and safety of ginsenoside-Rd for acute ischaemic stroke: a randomized, double-blind, placebo-controlled, phase II multicenter trial. Eur J Neurol. 2009;16:569–75. doi: 10.1111/j.1468-1331.2009.02534.x. [DOI] [PubMed] [Google Scholar]
- Zeng QR, Liu F, Zhang ZK.Study on extracting the Ginseng saponin from the Ginseng stem and the leaf Chemical Industry Times 20092326–8.Chinese [Google Scholar]
- Zhu JR, Tao YF, Lou S, Wu ZM. Protective effects of ginsenoside Rb(3) on oxygen and glucose deprivation-induced ischemic injury in PC12 cells. Acta Pharmacol Sin. 2010;31:273–80. doi: 10.1038/aps.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YZ, Chen J, Chu SF, Wang YS, Wang XY, Chen NH, et al. Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1's metabolites ginsenoside Rh1 and protopanaxatriol. J Pharmacol Sci. 2009;109:504–10. doi: 10.1254/jphs.08060fp. [DOI] [PubMed] [Google Scholar]
- Chen ZL.Chemical ingrediants and pharmaceutical quantity of Ginkgo biloba extracts Chin Pharm J 199631326–31.Chinese [Google Scholar]
- Luo Y. Alzheimer's disease, the nematode Caenorhabditis elegans, and ginkgo biloba leaf extract. Life Sci. 2006;78:2066–72. doi: 10.1016/j.lfs.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Sierpina VS, Wollschlaeger B, Blumenthal M. Ginkgo biloba. Am Fam Physician. 2003;68:923–6. [PubMed] [Google Scholar]
- Kasper S, Schubert H. Ginkgo biloba extract EGb 761(R) in the treatment of dementia: evidence of efficacy and tolerability. Fortschr Neurol Psychiatr. 2009;77:494–506. doi: 10.1055/s-0028-1109504. [DOI] [PubMed] [Google Scholar]
- Gertz HJ, Kiefer M. Review about Ginkgo biloba special extract EGb 761 (Ginkgo) Curr Pharm Des. 2004;10:261–4. doi: 10.2174/1381612043386437. [DOI] [PubMed] [Google Scholar]
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2009. p. CD003120. [DOI] [PubMed]
- Chen Z.The chemical constituents and quality of Ginkgo preparations Chin Pharm J 199631326–9.Chinese [Google Scholar]
- Chen Z, Yin M.Comment on various manufacturing processes of EGb Chin Trad Herbal Drugs 199829 Supp1–4.Chinese [Google Scholar]
- Chen J, Hu L, Jiang H, Gu J, Zhu W, Chen Z, et al. A 3D-QSAR study on ginkgolides and their analogues with comparative molecular field analysis. Bioorganic & Medicinal Chemistry Lett. 1998;8:1291–6. doi: 10.1016/s0960-894x(98)00205-4. [DOI] [PubMed] [Google Scholar]
- Hu L, Chen Z, Cheng X, Xie Y. Chemistry of ginkgolides: structure-activity relationship as PAF antagonists. Pure Appl Chem. 1999;71:1153–6. [Google Scholar]
- Zheng SX, Zhou LJ, Chen ZL, Yin ML, Zhu XZ. Bilobalide promotes expression of glial cell line-derived neurotrophic factor and vascular endothelial growth factor in rat astrocytes. Acta Pharmacol Sin. 2000;21:151–5. [PubMed] [Google Scholar]
- Zhang Y, Gu D, Mao S, Chen W.Protective effects of Ginkgo biloba extract on focal cerebral ischemia and thrombogenesis of carotid artery in rats Acta Pharm Sin 199833901–5.Chinese [PubMed] [Google Scholar]
- Yang YW.Ginkgo biloba extract: its effects on cognitive function in model rats with cerebral ischemia reperfusion Mod Trad Chin Med 20082864–5. 70Chinese [Google Scholar]
- Li FM, Duan XM, Wang JJ, Fang HL.Effects of Ginkgo biloba extract on MitoKATP in focal cerebral ischemia-reperfusion rats Chin Arch Tradit Chin Med 2008261462–3.Chinese [Google Scholar]
- Li XH, Zhang F, Zhao MX, Zhang QL, Zhao ZX. Effect of Ginkgo biloba on expression of N-methy-D-aspartate (NMDA) receptor 1 mRNA on post-ischemia reperfusion injury in the rat brain. Neurosci Bull. 2005;21:210–4. [Google Scholar]
- Szasz BK, Lenkey N, Barth AM, Mike A, Somogyvari Z, Farkas O, et al. Converging effects of Ginkgo biloba extract at the level of transmitter release, NMDA and sodium currents and dendritic spikes. Planta Med. 2008;74:1235–9. doi: 10.1055/s-2008-1081292. [DOI] [PubMed] [Google Scholar]
- Wang SY, Liu XM, Wang JX, Liu YX, Yuang DX.Effect of EGb on GFAP in focal cerebral ischemia/reperfusion injury rats Anatomy Res 200830115–7.Chinese [Google Scholar]
- Chan PC, Xia Q, Fu PP. Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25:211–44. doi: 10.1080/10590500701569414. [DOI] [PubMed] [Google Scholar]
- Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. Jama. 1997;278:1327–32. doi: 10.1001/jama.278.16.1327. [DOI] [PubMed] [Google Scholar]
- Kurz A, Van Baelen B. Ginkgo biloba compared with cholinesterase inhibitors in the treatment of dementia: a review based on meta-analyses by the cochrane collaboration. Dement Geriatr Cogn Disord. 2004;18:217–26. doi: 10.1159/000079388. [DOI] [PubMed] [Google Scholar]
- Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer's dementia in a randomized placebo-controlled double-blind study. Eur J Neurol. 2006;13:981–5. doi: 10.1111/j.1468-1331.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Yin M.Ginkgo leaves — active substances for dementia prevention and cure Intern Pharm Info 200139Chinese
- Schwarz K, Ternes W. Antioxidative constituents of Rosmarinus officinalis and Salvia officinalis. II. Isolation of carnosic acid and formation of other phenolic diterpenes. Z Lebensm Unters Forsch. 1992;195:99–103. doi: 10.1007/BF01201766. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomized and placebo-controlled trial. J Clin Pharm Ther. 2003;28:53–9. doi: 10.1046/j.1365-2710.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Biochem Pharmacol. 2007;74:1078–90. doi: 10.1016/j.bcp.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baricevic D, Sosa S, Della Loggia R, Tubaro A, Simonovska B, Krasna A, et al. Topical anti-inflammatory activity of Salvia officinalis L. leaves: the relevance of ursolic acid. J Ethnopharmacol. 2001;75:125–32. doi: 10.1016/s0378-8741(00)00396-2. [DOI] [PubMed] [Google Scholar]
- Chung YK, Heo HJ, Kim EK, Kim HK, Huh TL, Lim Y, et al. Inhibitory effect of ursolic acid purified from Origanum majorana L on the acetylcholinesterase. Mol Cells. 2001;11:137–43. [PubMed] [Google Scholar]
- Rutherford DM, Nielsen MP, Hansen SK, Witt MR, Bergendorff O, Sterner O. Isolation and identification from Salvia officinalis of two diterpenes which inhibit t-butylbicyclophosphoro[35S]thionate binding to chloride channel of rat cerebrocortical membranes in vitro. Neurosci Lett. 1992;135:224–6. doi: 10.1016/0304-3940(92)90441-9. [DOI] [PubMed] [Google Scholar]
- Eidi M, Eidi A, Bahar M. Effects of Salvia officinalis L (sage) leaves on memory retention and its interaction with the cholinergic system in rats. Nutrition. 2006;22:321–6. doi: 10.1016/j.nut.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Lo AH, Liang YC, Lin-Shiau SY, Ho CT, Lin JK. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis. 2002;23:983–91. doi: 10.1093/carcin/23.6.983. [DOI] [PubMed] [Google Scholar]
- Poeckel D, Greiner C, Verhoff M, Rau O, Tausch L, Hornig C, et al. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem Pharmacol. 2008;76:91–7. doi: 10.1016/j.bcp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Rau O, Wurglics M, Paulke A, Zitzkowski J, Meindl N, Bock A, et al. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med. 2006;72:881–7. doi: 10.1055/s-2006-946680. [DOI] [PubMed] [Google Scholar]
- Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–50. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, et al. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr. 2006;83:355–61. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- Perva-Uzunalic A, Skerget M, Knez Z, Weinreich B, Otto F, Gruner S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006;96:597–605. [Google Scholar]
- Bazinet L, Labbe D, Tremblay A. Production of green tea EGC- and EGCG-enriched fractions by a two-step extraction procedure. Sep Purif Technol. 2007;56:53–6. [Google Scholar]
- Hu B, Wang L, Zhou B, Zhang X, Sun Y, Ye H, et al. Efficient procedure for isolating methylated catechins from green tea and effective simultaneous analysis of ten catechins, three purine alkaloids, and gallic acid in tea by high-performance liquid chromatography with diode array detection. J Chromatogr A. 2009;1216:3223–31. doi: 10.1016/j.chroma.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Li Q, Li Y.Green tea polyphenols prevents the age-related decline of learning and memory ability of C57BL/6J mice Sci & Tech Review 20092726–31.Chinese [Google Scholar]
- Mandel SA, Amit T, Weinreb O, Reznichenko L, Youdim MB. Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci Ther. 2008;14:352–65. doi: 10.1111/j.1755-5949.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23:605–12. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- Hou RR, Chen JZ, Chen H, Kang XG, Li MG, Wang BR. Neuroprotective effects of (−)-epigallocatechin-3-gallate (EGCG) on paraquat-induced apoptosis in PC12 cells. Cell Biol Int. 2008;32:22–30. doi: 10.1016/j.cellbi.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–14. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello EJ, Savelev SU, Perry EK. In vitro anti-beta-secretase and dual anti-cholinesterase activities of Camellia sinensis L (tea) relevant to treatment of dementia. Phytother Res. 2004;18:624–7. doi: 10.1002/ptr.1519. [DOI] [PubMed] [Google Scholar]
- Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch Biochem Biophys. 1995;322:339–46. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- Li R, Huang YG, Fang D, Le WD. (−)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J Neurosci Res. 2004;78:723–31. doi: 10.1002/jnr.20315. [DOI] [PubMed] [Google Scholar]
- Li YK, Ouyang CH, Shu SJ.Protective effect of EGCG on focal cerebral ischemia-reperfusion injury in rats J Xianning College (Med Sci) 200620373–6.Chinese [Google Scholar]
- Yang S, Zhu GH, Xie DH.Epigallocatechin gallate ameliorates accumulated β-amyloid protein caused by oxidative free radicals in cortex neuron in vitro J Clin Res 2007241065–7.Chinese [Google Scholar]
- Mandel SA, Amit T, Kalfon L, Reznichenko L, Weinreb O, Youdim MB. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: special reference to epigallocatechin gallate (EGCG) J Alzheimers Dis. 2008;15:211–22. doi: 10.3233/jad-2008-15207. [DOI] [PubMed] [Google Scholar]
- Rietveld A, Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. J Nutr. 2003;133:3285S–92S. doi: 10.1093/jn/133.10.3285S. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, et al. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–87. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- Lee JW, Lee YK, Ban JO, Ha TY, Yun YP, Han SB, et al. Green tea (−)-epigallocatechin-3-gallate inhibits {beta}-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-{kappa}B pathways in mice. J Nutr. 2009;139:1987–93. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- He M, Zhao L, Wei MJ, Yao WF, Zhao HS, Chen FJ. Neuroprotective effects of (−)-epigallocatechin-3-gallate on aging mice induced by D-galactose. Biol Pharm Bull. 2009;32:55–60. doi: 10.1248/bpb.32.55. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kumar A. Effect of lycopene and epigallocatechin-3-gallate against 3-nitropropionic acid induced cognitive dysfunction and glutathione depletion in rat: A novel nitric oxide mechanism. Food Chem Toxicol. 2009;47:2522–30. doi: 10.1016/j.fct.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Liu H, Li B, Li YT.Comparative research on celery seeds essential oil prepared by different methods Food Sci Technol 2009182–5.Chinese
- Peng Y, Xing C, Lemere CA, Chen G, Wang L, Feng Y, et al. l-3-n-Butylphthalide ameliorates beta-amyloid-induced neuronal toxicity in cultured neuronal cells. Neurosci Lett. 2008;434:224–9. doi: 10.1016/j.neulet.2008.01.080. [DOI] [PubMed] [Google Scholar]
- Peng Y, Xu S, Chen G, Wang L, Feng Y, Wang X. l-3-n-Butylphthalide improves cognitive impairment induced by chronic cerebral hypoperfusion in rats. J Pharmacol Exp Ther. 2007;321:902–10. doi: 10.1124/jpet.106.118760. [DOI] [PubMed] [Google Scholar]
- Ma S, Xu S, Liu B, Li J, Feng N, Wang L, et al. Long-term treatment of l-3-n-butylphthalide attenuated neurodegenerative changes in aged rats. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:565–74. doi: 10.1007/s00210-009-0398-8. [DOI] [PubMed] [Google Scholar]
- Liu CL, Liao SJ, Zeng JS, Lin JW, Li CX, Xie LC, et al. dl-3-n-butylphthalide prevents stroke via improvement of cerebral microvessels in RHRSP. J Neurol Sci. 2007;260:106–13. doi: 10.1016/j.jns.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Feng YP. Effects of dl-3-n-butylphthalide on production of TXB2 and 6-keto-PGF1 alpha in rat brain during focal cerebral ischemia and reperfusion. Acta Pharmacol Sin. 1997;18:505–8. [PubMed] [Google Scholar]
- Dong GX, Feng YP.Effects of NBP on ATPase and anti-oxidant enzymes activities and lipid peroxidation in transient focal cerebral ischemic rats Acta Acad Med Sin 20022493–7.Chinese [PubMed] [Google Scholar]
- Peng Y, Zeng X, Feng Y, Wang X. Antiplatelet and antithrombotic activity of L-3-n-butylphthalide in rats. J Cardiovasc Pharmacol. 2004;43:876–81. doi: 10.1097/00005344-200406000-00018. [DOI] [PubMed] [Google Scholar]
- Yan CH, Feng YP, Zhang JT. Effects of dl-3-n-butylphthalide on regional cerebral blood flow in right middle cerebral artery occlusion rats. Acta Pharmacol Sin. 1998;19:117–20. [PubMed] [Google Scholar]
- Dong GX, Feng YP.Effects of 3-N-butylphthalide on cortical calcineurin and calpain activities in focal cerebral ischemia rats Acta Pharm Sin 200035790–2.Chinese [PubMed] [Google Scholar]
- Liu XG, Feng YP.Protective effect of dl-3-n-butylphthalide on ischemic neurological damage and abnormal behavior in rats subjected to focal ischemia Acta Pharm Sin 199530896–903.Chinese [PubMed] [Google Scholar]
- Pan XD, Chen XC.Advances in the study of immunopharmacological effects and mechanisms of extracts of Tripterygium wilfordii Hook F in neuroimmunologic disorders Acta Pharm Sin 20084311796–85.Chinese [PubMed] [Google Scholar]
- Gao JP, Sun S, Li WW, Chen YP, Cai DF. Triptolide protects against 1-methyl-4-phenyl pyridinium-induced dopaminergic neurotoxicity in rats: implication for immunosuppressive therapy in Parkinson's disease. Neurosci Bull. 2008;24:133–42. doi: 10.1007/s12264-008-1225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HF, Liu XY, Niu DB, Li FQ, He QH, Wang XM. Triptolide protects dopaminergic neurons from inflammation-mediated damage induced by lipopolysaccharide intranigral injection. Neurobiol Dis. 2005;18:441–9. doi: 10.1016/j.nbd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Li FQ, Cheng XX, Liang XB, Wang XH, Xue B, He QH, et al. Neurotrophic and neuroprotective effects of tripchlorolide, an extract of Chinese herb Tripterygium wilfordii Hook F, on dopaminergic neurons. Exp Neurol. 2003;179:28–37. doi: 10.1006/exnr.2002.8049. [DOI] [PubMed] [Google Scholar]
- Hong Z, Wang G, Gu J, Pan J, Bai L, Zhang S, et al. Tripchlorolide protects against MPTP-induced neurotoxicity in C57BL/6 mice. Eur J Neurosci. 2007;26:1500–8. doi: 10.1111/j.1460-9568.2007.05766.x. [DOI] [PubMed] [Google Scholar]
- Yan H, Bian XY.Study on extraction of total flavonoids from safflower by ultrasonic-assisted method J Anhui Agri Sci 20083613675–6.Chinese [Google Scholar]
- Li R, Guo M, Zhang G, Xu X, Li Q. Neuroprotection of nicotiflorin in permanent focal cerebral ischemia and in neuronal cultures. Biol Pharm Bull. 2006;29:1868–72. doi: 10.1248/bpb.29.1868. [DOI] [PubMed] [Google Scholar]
- Huang JL, Fu ST, Jiang YY, Cao YB, Guo ML, Wang Y, et al. Protective effects of Nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats. Pharmacol Biochem Behav. 2007;86:741–8. doi: 10.1016/j.pbb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Hotta Y, Nagatsu A, Liu W, Muto T, Narumiya C, Lu X, et al. Protective effects of antioxidative serotonin derivatives isolated from safflower against postischemic myocardial dysfunction. Mol Cell Biochem. 2002;238:151–62. doi: 10.1023/a:1019992124986. [DOI] [PubMed] [Google Scholar]
- Cai J.Functional components and health function of Polygonum multiflorum Thunb Acad Periodical Farm Prod Proc 200941–3.Chinese [Google Scholar]
- Qi AD.Optimum for extract processing of stilbene glucoside from Polygonum multiflorum Chin Trad Herbal Drugs 200233609–11.Chinese [Google Scholar]
- Zhang L, Xing Y, Ye CF, Ai HX, Wei HF, Li L. Learning-memory deficit with aging in APP transgenic mice of Alzheimer's disease and intervention by using tetrahydroxystilbene glucoside. Behav Brain Res. 2006;173:246–54. doi: 10.1016/j.bbr.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Liu LL, Li L, Zhao L, Zhang L, Li YL, Ye CF.Effects of 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside on learning and memory abilities of rats with chronic cerebral ischemia Chin J Pharmacol Toxicol 200822108–15.Chinese [Google Scholar]
- Zhang L, Ye CF, Zhu YQ, Li B, Li L.Effects of tetrahydroxystilbene glucoside on cholinergic system in dementia rats model induced by ibotenic acid Chin Pharm J 200540749–53.Chinese [Google Scholar]
- Chu J, Ye CF, Li L, Zhang L.Effects of Stilbene-glycoside on behavior and cholinergic function of Dementia Model Mice Induced by β-Amyloid Basic & Clin Med 200626197–8.Chinese [Google Scholar]
- Tan P, Yang Q, Tan X.Effects of Polygonum multiflorum Thumb on patients with vascular dementia J Hubei Inst Nationalities (Medical Edition) 20021015–7.Chinese [Google Scholar]
- Gao XQ, Liu MY, Dong C, Wang YF, Li XJ.Study on extraction process of curcumin in Rhizoma Curcumae Longae China Pharmaceu 20081756–7.Chinese [Google Scholar]
- Niu SY, He FG, Xu QY.The extracting and application of the curcumin J Henan Inst Sci Technol 20083658–61.Chinese [Google Scholar]
- Butterfield D, Castegna A, Pocernich C, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer's disease. J Nutr Biochem. 2002;13:444. doi: 10.1016/s0955-2863(02)00205-x. [DOI] [PubMed] [Google Scholar]
- Singhal SS, Awasthi S, Pandya U, Piper JT, Saini MK, Cheng JZ, et al. The effect of curcumin on glutathione-linked enzymes in K562 human leukemia cells. Toxicol Lett. 1999;109:87–95. doi: 10.1016/s0378-4274(99)00124-1. [DOI] [PubMed] [Google Scholar]
- Kuner P, Schubenel R, Hertel C. Beta-amyloid binds to p57NTR and activates NFkappaB in human neuroblastoma cells. J Neurosci Res. 1998;54:798–804. doi: 10.1002/(SICI)1097-4547(19981215)54:6<798::AID-JNR7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–7. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002;61:554–61. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–12. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- Hafner-Bratkovic I, Gaspersic J, Smid LM, Bresjanac M, Jerala R. Curcumin binds to the alpha-helical intermediate and to the amyloid form of prion protein — a new mechanism for the inhibition of PrP(Sc) accumulation. J Neurochem. 2008;104:1553–64. doi: 10.1111/j.1471-4159.2007.05105.x. [DOI] [PubMed] [Google Scholar]
- Pan R, Qiu S, Lu DX, Dong J. Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chin Med J (Engl) 2008;121:832–9. [PubMed] [Google Scholar]
- Isik AT, Celik T, Ulusoy G, Ongoru O, Elibol B, Doruk H, et al. Curcumin ameliorates impaired insulin/IGF signalling and memory deficit in a streptozotocin-treated rat model. Age (Dordr) 2009;31:39–49. doi: 10.1007/s11357-008-9078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res. 2005;2:131–6. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin RC, Li GW, Ma SL, Zhou ST, Zhao GL.Ferulic acid in Angelica sinensis extracting technology Chin Trad Pat Med 200830516–9.Chinese [Google Scholar]
- Sudheer AR, Muthukumaran S, Devipriya N, Devaraj H, Menon VP. Influence of ferulic acid on nicotine-induced lipid peroxidation, DNA damage and inflammation in experimental rats as compared to N-acetylcysteine. Toxicology. 2008;243:317–29. doi: 10.1016/j.tox.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–50. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Choi SJ, Lim ST, Kim HK, Heo HJ, Kim EK, et al. Ferulic acid supplementation prevents trimethyltin-induced cognitive deficits in mice. Biosci Biotechnol Biochem. 2007;71:1063–8. doi: 10.1271/bbb.60564. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Kise M, Morikawa K. Ferulic acid attenuated cognitive deficits and increase in carbonyl proteins induced by buthionine-sulfoximine in mice. Neurosci Lett. 2008;430:115–8. doi: 10.1016/j.neulet.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Gao DY, Huang YS, He HW.A clinical study on 36 cases of Alzheimer disease treated with Danggui Shaoyao San Chin Gen Pract 20047782–3.Chinese [Google Scholar]
- Boyd B. Ongoing progress in the Alzheimer's disease arena. Drug News Perspect. 2000;13:425–38. [PubMed] [Google Scholar]
- Liu Y, Shi CZ, Zhang JT.Anti-lipidperoxidation and cerebral protective effects of clausenamide Acta Pharm Sin 199126166–70.Chinese [PubMed] [Google Scholar]
- Jiang ZC, Bi GN, Shi SL.Effect of clausenamide on the expression of Bcl-2 protein and apoptosis after focal cerebral ischemia/reperfusion in renovascular hypertensive rats Chin Crit Care Med 200517289–92.Chinese [PubMed] [Google Scholar]
- Xu L, Liu SL, Zhang JT. (–)-Clausenamide potentiates synaptic transmission in the dentate gyrus of rats. Chirality. 2005;17:239–44. doi: 10.1002/chir.20150. [DOI] [PubMed] [Google Scholar]
- Wu L, Liu KX, Feng JL, Zeng KY, Li H.Effects of sinomenine on expressions of cyclooxygenase-2 and prostaglandin E2 content following cerebral ischemic reperfusion injury in rats China J Rehab Med 200924293–6.Chinese [Google Scholar]
- Zhou SX, Xia N, Liang YZ.Effect of sinomenine on expression of Bcl-2 and Bax following cerebral ischemic reperfusion injury in diabetic rats J Emerg Tradit Chin Med 2009763–4.Chinese [Google Scholar]
- Lin L, Zhang J, Fu Q, He LC, Li Y. Concentration and extraction of sinomenine from herb and plasma using a molecularly imprinted polymer as the stationary phase. Anal Chim Acta. 2006;561:178–82. [Google Scholar]
- Yan FL, Wang YQ, Lu G, Hong Z. Effects of Puerarin on protein expression of hyperphosphorylated tau and ChAT in hippocampus of Alzheimer's disease rats. J Clin Neurol. 2006;19:191–3. [Google Scholar]
- Yang DX, Tang Y, Hu XM, Liu JX, Chen Y, Jin YY.Effects of puerarin on learning and memory of model mouse with beta amyloid peptide-induced dementia Chin J Clin Rehabil 20059169–71.Chinese [Google Scholar]
- Zhang BQ, Wang YL.Effects of puerarin on hippocampal pyramidal cell and the expression of BDNF in vascular dementia rats Chin J Neuroanat 200723615–20.Chinese [Google Scholar]
- Dong WY, Sun J, Ding T, Zhao JR, Xia SJ, Lin SR, et al. Effect of puerarin injected into superior cervical sympathetic ganglia on learning and memory function of mice with dementia J Chin Med Univ 200635235–6.Chinese [Google Scholar]
- Qiu F, Liu Y, Zhang P, Tian Y, Zhao J, Kang Q.The effect of ligustrazine on cells proliferation in cortex and striatum after focal cerebral ischemia in adult rats J Chin Med Mater 20061196–200.Chinese [PubMed] [Google Scholar]
- Zhang T.Protective effect of ligustrazine on brain J Chin Med Res 20066993–4.Chinese [Google Scholar]
- Suzuki K, Koike T. Resveratrol abolishes resistance to axonal degeneration in slow Wallerian degeneration (WldS) mice: activation of SIRT2, an NAD-dependent tubulin deacetylase. Biochem Biophys Res Commun. 2007;359:665–71. doi: 10.1016/j.bbrc.2007.05.164. [DOI] [PubMed] [Google Scholar]
- Sharma M, Gupta YK. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002;71:2489–98. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- Sonmez U, Sonmez A, Erbil G, Tekmen I, Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci Lett. 2007;420:133–7. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- Xie GQ, Sun XL, Tian SP, Chen QS.Studies on the preventive and therapeutic effects of rhodosin on rats with AIzheimer's disease Chin J Behav Med Sci 20031218–20.Chinese [Google Scholar]
- Jiang WH, Meng XR, Hao LM, Cui L, Dong ZY, Wang SL.Study of anti-aging and anti-dementia effects of rhodosin on aging rats and experimental dementia rats J N Bethune Univ Med Sci 200127127–9.Chinese [Google Scholar]
- Abdel-Hafez AA, Meselhy MR, Nakamura N, Hattori M, Watanabe H, Murakami Y, et al. Effects of paeoniflorin derivatives on scopolamine-induced amnesia using a passive avoidance task in mice; structure-activity relationship. Biol Pharm Bull. 1998;21:1174–9. doi: 10.1248/bpb.21.1174. [DOI] [PubMed] [Google Scholar]
- Xiao L, Wang YZ, Liu J, Luo XT, Ye Y, Zhu XZ. Effects of paeoniflorin on the cerebral infarction, behavioral and cognitive impairments at the chronic stage of transient middle cerebral artery occlusion in rats. Life Sci. 2005;78:413–20. doi: 10.1016/j.lfs.2005.04.069. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhong SZ, Ma SP.Paeoniflorin ameliorates cognitive impairment and neurodegeneration in senescent mice induced by D-galactose and sodium nitrite Pharm & Clin Res 200917172–6.Chinese [Google Scholar]
- Jiang Y, Fang YQ, Zou YY.Effects of the β-asarone on learning and memory ability and SOD, GSH-Px, and MDA levels in AD mice Chin J Geront 2007271126–7.Chinese [Google Scholar]
- Zhang LD, Gong XJ, Hu MM, Li YM, Liu GQ.Anti-vascular dementia effect of gastrodin and its mechanisms of action Chin J Nat Med 20086130–4.Chinese [Google Scholar]
- Wang ZL, Wu GW, Wan HJ, Liu J, Mao SW.Clinical observation on gastrodin in cognitive impairment due to stroke Zhejiang JITCWM 200515675–6.Chinese [Google Scholar]
- Li L, Zhou QX, Shi JS. Protective effects of icariin on neurons injured by cerebral ischemia/reperfusion. Chin Med J (Engl) 2005;118:1637–43. [PubMed] [Google Scholar]
- Luo Y, Nie J, Gong QH, Lu YF, Wu Q, Shi JS. Protective effects of icariin against learning and memory deficits induced by aluminium in rats. Clin Exp Pharmacol Physiol. 2007;34:792–5. doi: 10.1111/j.1440-1681.2007.04647.x. [DOI] [PubMed] [Google Scholar]
- Hsieh MT, Hsieh CL, Wang WH, Chen CS, Lin CJ, Wu CR. Osthole improves aspects of spatial performance in ovariectomized rats. Am J Chin Med. 2004;32:11–20. doi: 10.1142/S0192415X04001758. [DOI] [PubMed] [Google Scholar]
- Shen LX, Jin LQ, Zhang DS, Xue GP.Effect of Osthol on memory impairment of mice in AlCl3-induced acute senile model Acta Pharm Sin 200237178–80.Chinese [PubMed] [Google Scholar]
- Shen LX, Zhang DS, Zhang L, Ren LM, Chang JQ.Action of Osthol on learning and memory and its mechanism analysis Acta Pharm Sin 199934405–9.Chinese [Google Scholar]
- Zhu JQ, Tuo XP, Chen HS, Lv JY, Jia LY, Zhou J.Protective effect of Schisandrone on β-amyloid protein-induced stress injury of hippocampal neurons Acad J Sec Mi Med Univ 2007281015–6.Chinese [Google Scholar]