Abstract
Aim:
To investigate whether mitochondria permeability transition pore (mPTP) opening was involved in ginsenoside Rb1 (Gs-Rb1) induced anti-hypoxia effects in neonatal rat cardiomyocytes ex vivo.
Methods:
Cardiomyocytes were randomly divided into 7 groups: control group, hypoxia group (500 μmol/L CoCl2), Gs-Rb1 200 μmol/L group (CoCl2 intervention+Gs-Rb1), wortmannin (PI3K inhibitor) 0.5 μmol/L group, wortmannin+Gs-Rb1 group, adenine 9-β-D-arabinofuranoside (Ara A, AMPK inhibitor) 500 μmol/L group, and Ara A and Gs-Rb1 group. Apoptosis rate was determined by using flow cytometry. The opening of the transient mPTP was assessed by using co-loading with calcein AM and CoCl2 in high conductance mode. Expression of GSK-3β, cytochrome c, caspase-3 and poly (ADP-ribose) polymerase (PARP) was measured by using Western blotting. ΔGSK-3β was defined as the ratio of p-Ser9-GSK-3β to total GSK-3β.
Results:
CoCl2 significantly stimulated mPTP opening and up-regulated the level of ΔGSK-3β. There was a statistically significant positive correlation between apoptosis rate and mPTP opening, between apoptosis rate and ΔGSK-3β, and between mPTP opening and ΔGSK-3β. Gs-Rb1 significantly inhibited mPTP opening induced by hypoxia (41.3%±2.0%, P<0.001) . Gs-Rb1 caused a 77.3%±3.2% reduction in the expression of GSK-3β protein (P<0.001) and a significant increase of 1.182±0.007–fold (P=0.0001) in p-Ser9-GSK-3β compared with control group. Wortmannin and Ara A significantly inhibited the effect of Gs-Rb1 on mPTP opening and ΔGSK-3β. Gs-Rb1 significantly decreased expression of cytochrome c (66.1%±1.7%, P=0.001), caspase-3 (56.5%±2.7%, P=0.001) and cleaved poly ADP-ribose polymerase (PARP) (57.9%±1.4%, P=0.001).
Conclusion:
Gs-Rb1 exerted anti-hypoxia effect on neonatal rat cardiomyocytes by inhibiting GSK-3β-mediated mPTP opening.
Keywords: ginsenoside Rb1, cardiomyocytes, hypoxia, apoptosis, mitochondria permeability transition pore, glycogen synthase kinase-3β, cytochrome c, caspase 3, wortmannin
Introduction
By inducing cardiomyocyte apoptosis, hypoxia is an important factor involved in ischemic heart disease. Thus, improving the anti-hypoxia capability of cardiomyocytes has been an important issue within the cardiovascular field. Ginsenoside-Rbl (Gs-Rbl), a main component of Ginsenosides extracted from Ginseng (the root of Panax ginseng CA Meyer, family Araliaceae, a traditional medicine in Asian countries), has been reported to inhibit ischemic/reperfusion injury as a result of myocardial infarction in vivo 1, 2, 3, 4. Gs-Rbl may also protect neonatal rat cardiomyocytes from CoCl2-induced apoptosis depending on whether the PI3K or AMPK signaling cascade results in GLUT4 translocation ex vivo5.
The mitochondrial permeability transition pore (mPTP) is a mega-channel with permeability to all molecules of less than 1.5 kDa. The opening of the mPTP is often a common cause of cardiac cell apoptosis in numerous cardiac diseases. It may lead to cardiomyocyte apoptosis and/or necrosis6, 7, 8 by activating mitochondrial membrane depolarization, which has been indicated as a major contributor to ischemic/reperfusion injury9. Inhibition of mPTP opening by glycogen synthase kinase-3β (GSK-3β) or melatonin for example10, 11, may prevent cell apoptosis, which further implies that mPTP is a critical site for intervention6, 7, 8. To our knowledge, the anti-hypoxia capability of Gs-Rb1 through inhibiting mPTP opening has not been reported. It is well known that the mitochondria-mediated intrinsic pathways take part in the ischemic/reperfusion apoptosis of cardiomyocytes, during which cytochrome c release, caspase activation and poly ADP-ribose polymerase (PARP) cleavage play important roles in the apoptosis process. However, whether those proteins in hypoxic cardiomyocytes were influenced by Gs-Rb1 has not been reported.
GSK-3β, one of two GSK-3 isoforms, has a relatively higher activity than the other isoform in cardiac myocytes and is thought to be the crucial regulatory target of mPTP. In other words, the activity of GSK-3β is a threshold determinant for mPTP opening in cardiomyocytes apoptosis12, 13. However, whether the Gs-Rb1 effect in inhibiting mPTP opening, is mediated by GSK-3β remains unclear. Thus, we investigated whether Gs-Rb1 influenced the expression of GSK-3β and whether a correlation existed between GSK-3β and mPTP opening. In addition, considering that the anti-hypoxia ability of Gs-Rb1 is mediated by both phosphoinositide 3-kinase (PI3K) and AMP-activated protein kinase (AMPK) signaling5, we further evaluated whether the mechanisms contributing to adjust both mPTP opening and the activity of GSK-3β were mediated by both PI3K and AMPK signaling ex vivo.
Materials and methods
This study was performed in strict accordance with the institutional recommendations for animal care of China Medical University (CMU) and approved by the local animal research committee. All Wistar rats were purchased from the Laboratory Animal Center of CMU [SCXK (Liao) 2003-0009].
Cardiomyocytes culture
As in our previous study5, cardiomyocyte cultures were prepared from hearts of 1-3-d-old rats. In brief, ventricles had been separated and cut into 1 mm3 pieces and digested (37 °C, 10 min, two to three such cycles to achieve full digestion) with phosphate-buffered saline (PBS) containing 0.08% trypsin and 0.05% collagenase II (Gibco). Next, the cell suspension was collected, centrifuged (100×g, 10 min) and suspended in Dulbecco's modified Eagle's media (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and 0.1 mmol/L bromodeoxyuridine in 100 mL culture dishes (37 °C, 5% CO2, 95% O2; NuAire 8000 Autoflow CO2 Incubator; Thermo Electron Corporation, Franklin, MA) for 90 min to lyse fibroblasts. Finally, cardiomyocytes were seeded on culture flasks at a density of 1.0×105 cells per milliliter and incubated for 3 d.
CoCl2-induced chemical hypoxia and Gs-Rb1 intervention
According to our previous experimental results5, 500 μmol/L CoCl2, 200 μmol/L Gs-Rb1, 500 μmol/L adenine 9-β-D-arabinofuranoside (Ara A, AMPK inhibitor) and 0.5 μmol/L wortmannin (PI3K inhibitor) were freshly prepared. Cells were randomly divided into seven groups as follows: control group (normal oxygen group), hypoxia group (500 μmol/L CoCl2 intervention), Gs-Rb1 group (200 μmol/L Gs-Rb1 and 500 μmol/L CoCl2 intervention), W-1 group (0.5 μmol/L wortmannin and 500 μmol/L CoCl2 intervention), W-2 group (0.5 μmol/L wortmannin intervention basing on 200 μmol/L Gs-Rb1 and 500 μmol/L CoCl2 intervention), A-1 group (500 μmol/L Ara A and 500 μmol/L CoCl2 intervention) and A-2 group (500 μmol/L Ara A intervention basing on 200 μmol/L Gs-Rb1 and 500 μmol/L CoCl2 intervention). Before the above intervention, all cardiomyocytes were synchronized and then incubated, ex vivo, in L-DMEM supplemented with 10% fetal bovine serum and administrated with fresh CoCl2, Gs-Rb1, wortmannin or Ara A for 12 h.
Flow cytometry
The above cells were adjusted to 1.0×106/mL in each sample and then were analyzed by flow cytometry (FCM). Briefly, in a double variance scatterplot, single positive (FITC+/PI−) populations were referred to as apoptotic cells, and the quadrant percentage was regarded as the apoptosis ratio (AR). Annexin V FITC/PI kits were from Boehringer Mannheim biological technology Ltd.
Immunocytochemistry for GSK-3β and p-Ser9-GSK-3β (phosphorylation at Ser 9)
The immunofluorescence staining for GSK-3β and p-Ser9-GSK-3β, after being fixed in PBS (pH 7.2) with 4% paraformaldehyde (PFA) for 20 min at 4°C, was performed with primary antibodies against GSK-3β and p-Ser9-GSK-3β (Sigma) at a dilution of 1:200 (overnight at room temperature), and secondary antibodies FITC-conjugated goat anti-Rabbit lgG (Invitrogen) at a dilution of 1:500 for 45 min. Quality analysis was performed via SCION image software. Every cell at ×400 (five sections was chosen in each group) was calculated according to the formula [(mean gray value-Min/(Max-Min)×100].
Western blotting analysis of GSK-3β, caspase-3, PARP and cytochrome c
Western blotting was used to evaluate the expression of GSK-3β, caspase-3, PARP and cytochrome c in each group. Total protein was extracted with ice-cold lysis buffer and centrifugation (4 °C, 12 000×g, 10 min). A 20 μL protein solution for each sample was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto polyvinylidene difluoride membranes. Afterwards, the membrane was incubated with specific primary antibodies against GSK-3β (1:1000 dilution), p-Ser9-GSK-3β (1:1000 dilution), p-T216-GSK-3β (phosphorylation at Tyr 216; 1:1000 dilution), caspase-3 (1:1000 dilution), PARP (1:1000 dilution) and cytochrome c (1:1000 dilution) overnight at 4 °C and then with HRP-conjugated secondary antibody (1:2000 dilution) for 2 h. Integral optical density (IOD) was detected via SCION image software. Relevant band intensities were quantified after normalization to the amount of β-actin protein. To identify the exact effect of different interventions, ΔGSK-3β, expressed as the inactive form, was analyzed and defined as ΔGSK-3β= p-Ser9-GSK-3β/total GSK-3β14, 15.
Determination of mPTP opening
According to the previous report7, the opening of the transient mPTP was directly assessed by co-loading with calcein AM and CoCl2 in high conductance mode. The principle idea revolved around the fact that calcein AM is permeable to intact membranes but not to intact mitochondrial membranes and that mPTP opening leads to the exit of calcein in high conductance mode. Thus, the condition allows for monitoring of calcein fluorescence in mitochondria of intact cells. In view of hypoxia mode induced by CoCl2, the present study did not add CoCl2 intervention any longer than previous studies. In brief, cardiomyocytes were loaded for 15 min with 1 μmol/L calcein AM in working solution7 at room temperature and then washed free of calcein and CoCl2. The rate of calcein AM loading and exit was measured by recording the fluorescence signal every 5 min using Turner Quantech Digital Filter Fluorometer (excitation filter NB490 and emission filter SC515) and calculated as a percent change to maximal fluorescence signal. In addition, the present study recorded the fluorescence signal for 30 min, keeping in accordance with hypoxia intervention time according to our preliminary experiments.
Data analysis
All experiments were repeated at least three times, independently. All data in this study are presented as mean±SD. Statistical analysis was performed using analysis of variance with one-way ANOVA and linear regression analysis. P<0.05 were considered significant.
Results
Apoptosis rate
The apoptosis rate was 3.3%±0.1% in the control group; 39.2%±0.1% in hypoxia group; 14.9%±0.4% in Gs-Rb1 200 μmol/L treatment group; 40.7%±1.5% in 0.5 μmol/L wortmannin group; 36.7%±0.6% in wortmannin+Gs-Rb1 group; 41.3%±0.7% in Ara A 500 μmol/L group; and 29.6%±0.6% in Ara A+Gs-Rb1 group. Gs-Rb1 protected cardiomyocytes from hypoxia-induced apoptosis, which was in agreement with our previous study.
mPTP opening
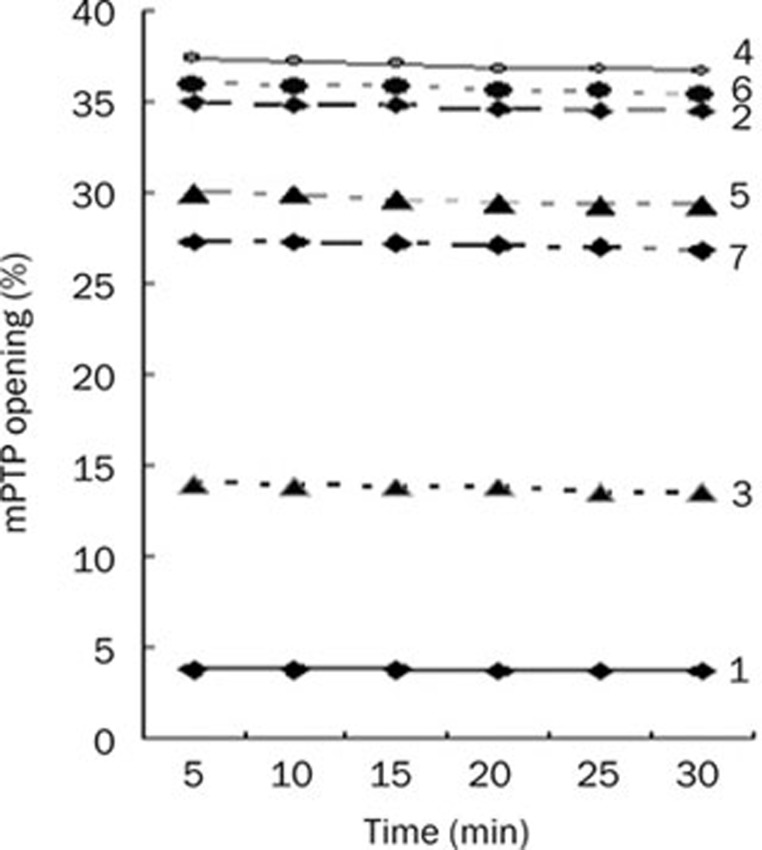
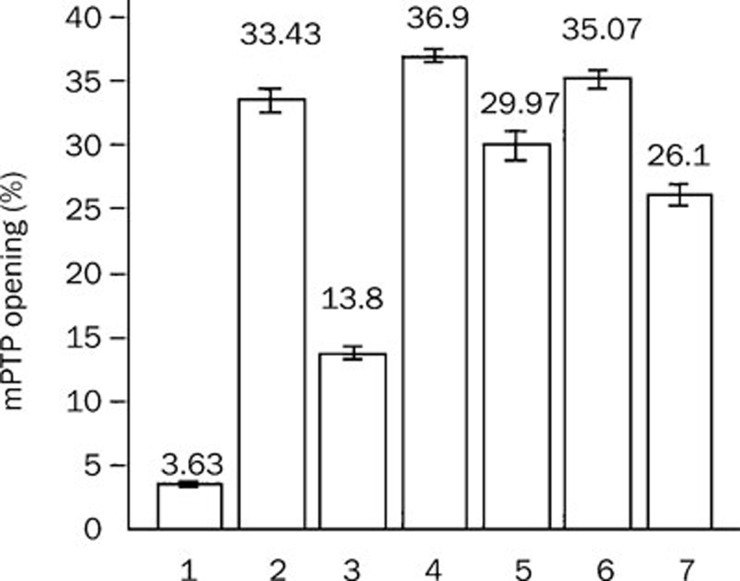
In each group, mPTP opening was not significantly changed at different time points during 30 min. Therefore, all data were presented as the mean in each group for comparison. mPTP opening was 3.6%±0.2%, 33.4%±0.9%, 13.8%±0.5%, 36.9%±0.6%, 30.0%±1.1%, 35.1%±0.8%, and 26.1%±0.9% in the control group, hypoxia group, Gs-Rb1 group, wortmannin group, wortmannin+Gs-Rb1 group, Ara A group, and Ara A+Gs-Rb1 group, respectively (Figure 1).
Figure 1.
Inhibitory effect of ginsenoside (Gs) Rb1 on mitochondria permeability transition pore (mPTP) opening from 5 to 30 min. (1) control group; (2) simple hypoxia group; (3) Gs-Rb1 200 mol/L treatment group; (4) 0.5 μmol/L wortmannin group; (5) wortmannin+Gs-Rb1 group; (6) Ara A 500 μmol/L treatment group; (7) Ara A+Gs-Rb1 group.
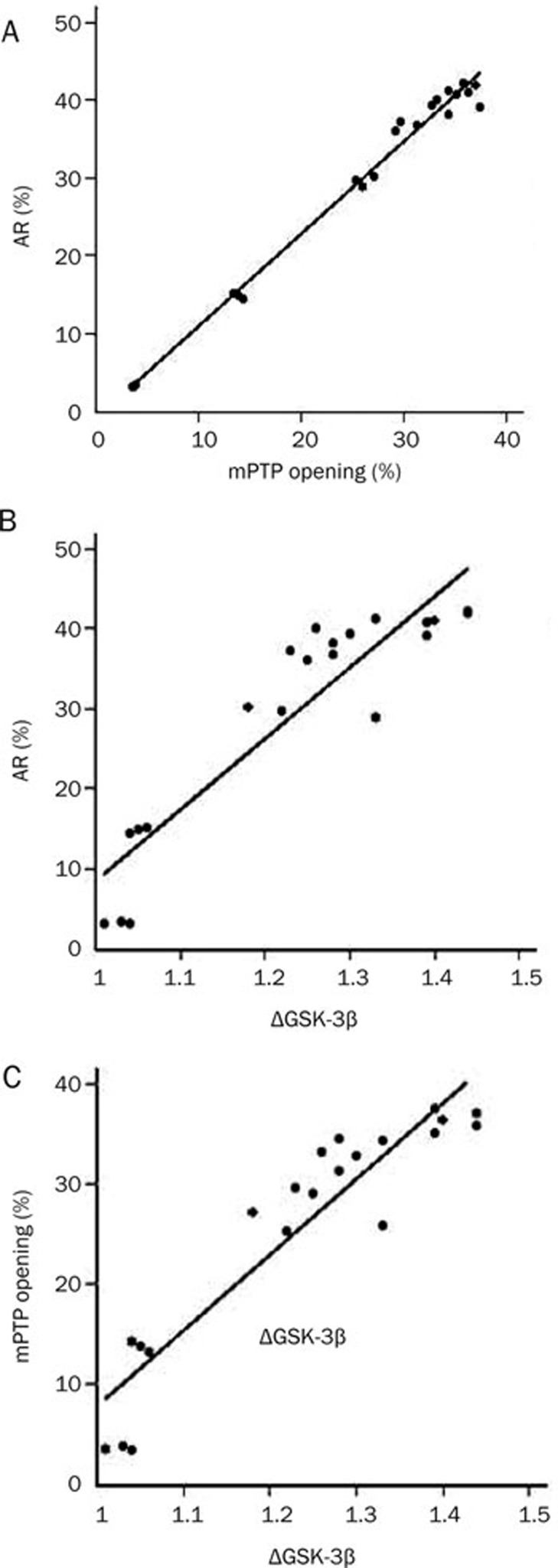
The correlation between apoptosis rate and ΔGSK-3β and/or mPTP opening
There were significant positive correlations between apoptosis rate and mPTP opening (Figure 2A), between AR and ΔGSK-3β (Figure 2B), and between mPTP opening and ΔGSK-3β (Figure 2C).
Figure 2.
The correlation among apoptosis rate (AR), ΔGSK-3β and/or mPTP opening. (A) The correlation between AR and mPTP opening (r=0.993, P<0.001); (B) The correlation between AR and ΔGSK-3β (r=0.917, P<0.001); (C) The correlation between mPTP opening and ΔGSK-3β (r=0.931, P<0.001).
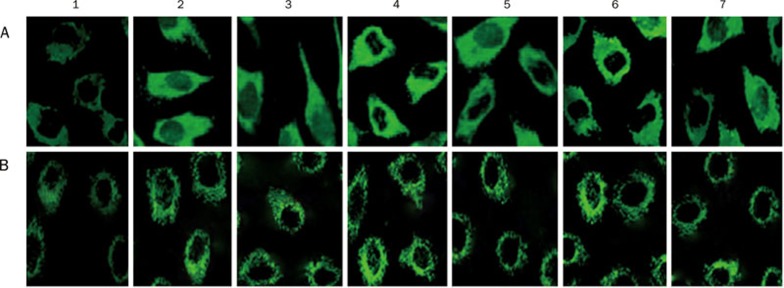
Immunofluorescence staining for GSK-3β protein and p-Ser9-GSK-3β protein
A positive expression of GSK-3β protein (Figure 3A) and p-Ser9-GSK-3β protein (Figure 3B) was observed in the cytoplasm of cardiomyocytes in each group. Gs-Rb1 significantly decreased the expression of GSK-3β induced by hypoxia. But the inhibitory effect of Gs-Rb1 was reversed by wortmannin and Ara A.
Figure 3.
Fluorescent immunocytochemical staining for GSK-3β protein in cardiomyocytes. Green fluorescent grains appeared in the cell plasma of cardiomyocytes (400×). (A) GSK-3β protein; (B) p-Ser9-GSK-3β (1) control group; (2) simple hypoxia group; (3) Gs-Rb1 200 μmol/L treatment group; (4) 0.5 μmol/L wortmannin group; (5) wortmannin+Gs-Rb1 group; (6) Ara A 500 μmol/L treatment group; (7) Ara A+Gs-Rb1 group.
Influence of hypoxia on mPTP opening and GSK-3β protein
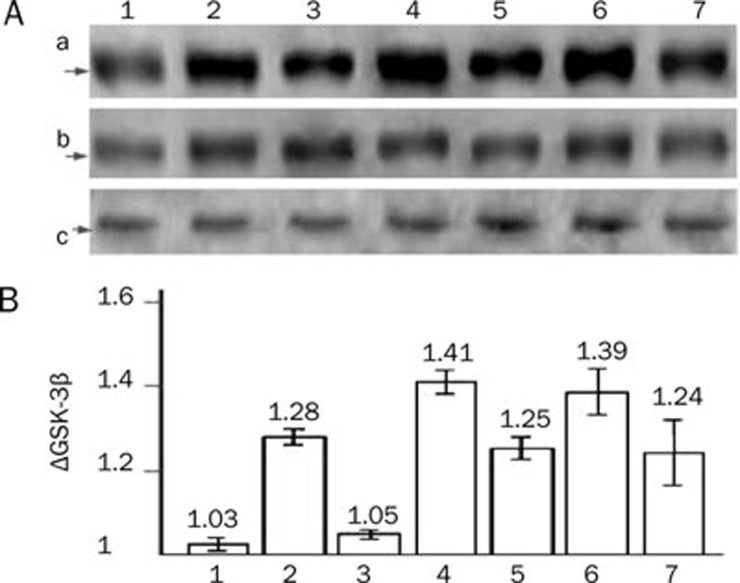
mPTP was not opened in normoxia cardiomyocytes and was significantly opened after CoCl2–induced hypoxia (9.25±0.95-fold, P<0.001; Figure 1, 4). Compared with the control group, hypoxia caused significant increase in GSK-3β protein (2.326±0.245–fold, P<0.001; Figure 5Aa) and its phosphorylation of Ser9 (ie, p-Ser9-GSK-3β, 1.602±0.115–fold, P<0.001; Figure 5Ab) but not T216 (ie p-T216-GSK-3β, P>0.05; Figure 5Ac), and significantly up-regulated ΔGSK-3β up to 1.25±0.01–fold (P<0.001; Figure 5B).
Figure 4.
Inhibitory effect of Gs-Rb1 on mPTP opening and GSK-3 activity in each group. (1) control group; (2) simple hypoxia group; (3) Gs-Rb1 200 μmol/L treatment group; (4) 0.5 μmol/L wortmannin group; (5) wortmannin+Gs-Rb1 group; (6) Ara A 500 μmol/L treatment group; (7) Ara A+Gs-Rb1 group.
Figure 5.
Western blotting results of GSK-3β, p-Ser9-GSK-3β and p-T216-GSK-3β in different groups. (A) Representatives of Western blotting. (a) GSK-3β protein; (b) p-Ser9-GSK-3β (c) p-T216-GSK-3β. (B) ΔGSK-3β values. (1) control group; (2) simple hypoxia group; (3) Gs-Rb1 200 μmol/L treatment group; (4) 0.5 mol/L wortmannin group; (5) wortmannin+Gs-Rb1 group; (6) Ara A 500 μmol/L treatment group; (7) Ara A+Gs-Rb1 group.
Effect of Gs-Rb1 on mPTP opening
Gs-Rb1 significantly inhibited mPTP opening (41.3%±2.0%, P<0.001) induced by hypoxia; but the difference between Gs-Rb1 group and the control group was significant (P<0.001, Figure 4).
Effect of Gs-Rb1 on the expression of GSK-3β protein
Gs-Rb1 intervention caused a 77.3%±3.2% reduction in the expression of GSK-3β protein (P<0.001) and a significant increase of 1.182±0.007–fold (P<0.0001) in p-Ser9-GSK-3β, except for p-T216-GSK-3β (P>0.05) in hypoxic cardiomyocytes. Gs-Rb1 significantly decreased ΔGSK-3β (82.0%±1.7%, P<0.001) induced by hypoxia, but the level of ΔGSK-3β in Gs-Rb1 group was still higher than that in the control group (P<0.001, Figure 5).
Effect of wortmannin on mPTP opening and GSK-3β protein
Wortmannin 0.5 μmol/L significantly increased the mPTP opening and the level of ΔGSK-3β compared with control group (P<0.001). Gs-Rb1 inhibited the effects of wortmannin (Figure 1, 4, 5).
Effect of Ara A on GSK-3β protein and mPTP opening
Ara A significantly increased the opening of mPTP and the level of ΔGSK-3β (P<0.001) compared with control group. Gs-Rb1 inhibited the effects of Ara A (Figure 1, 4, 5).
Expression of cytochrome c, caspase-3 and PARP
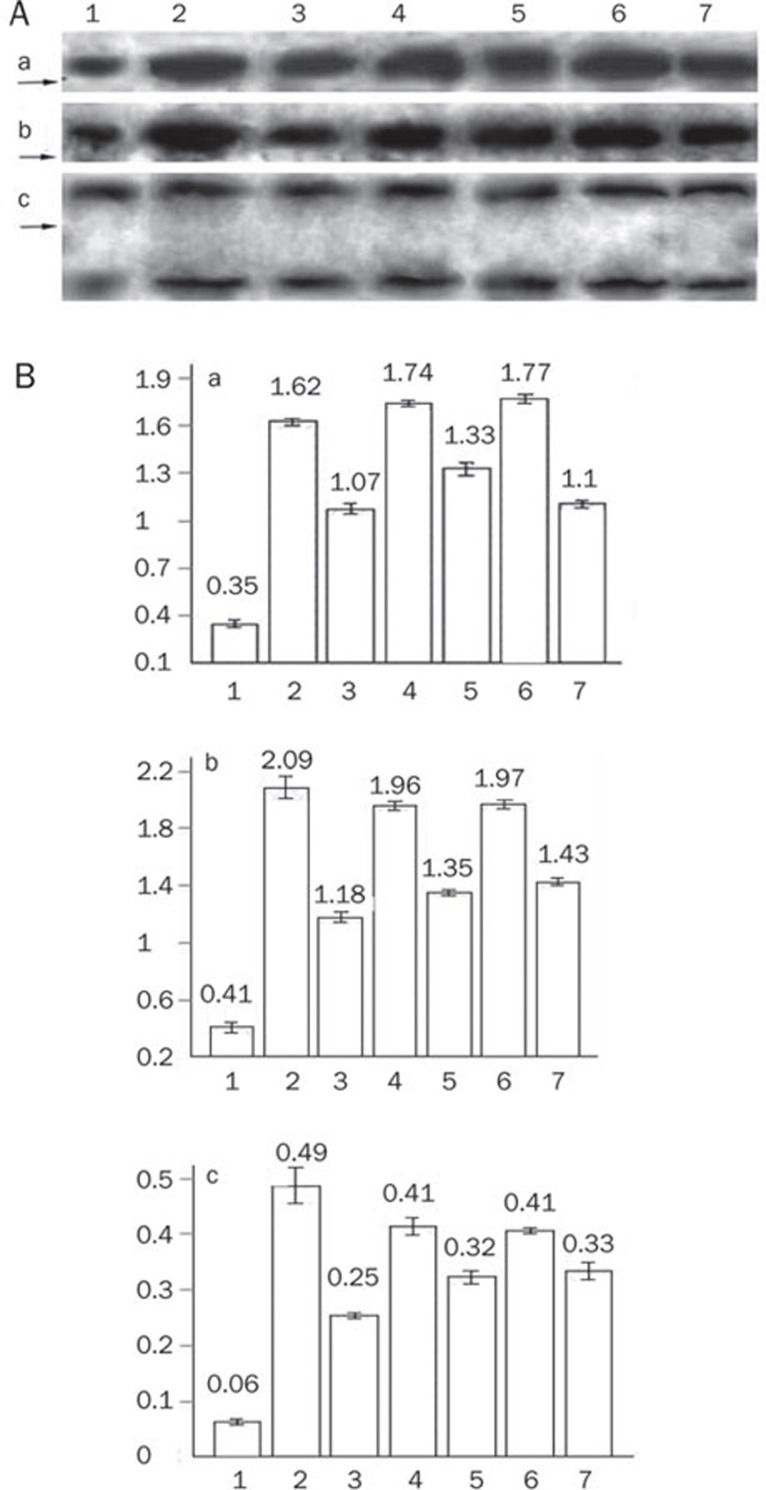
Compared to the hypoxia group, Gs-Rb1 significantly decreased expression of cytochrome c (66.1%±1.7%, P=0.001), caspase-3 (56.5%±2.7%, P=0.001) and cleaved PARP (57.9%±1.4%, P=0.001), all of which were significantly diminished by wortmannin and Ara A. However, there was no difference in total PARP content between the three groups (P>0.05, Figure 6).
Figure 6.
Western blotting results of cytochrome c, caspase-3, and PARP. (A) Representatives for Western blotting. (a) Cytochrome c protein; (b) caspase-3; (c) PARP (upper for total PARP 116 kDa, lower for cleaved PARP 85 kDa). (1) control group; (2) simple hypoxia group; (3) Gs-Rb1 200 mol/L treatment group; (4) 0.5 μmol/L wortmannin group; (5) wortmannin+Gs-Rb1 group; (6) Ara A 500 μmol/L treatment group; (7) Ara A+Gs-Rb1 group.
Discussion
Our previous study demonstrated that Gs-Rb1, by improving glucose uptake, plays an important role in protecting neonatal rat cardiomyocytes from CoCl2-induced apoptosis ex vivo5. This role may be controlled by the translocation of GLUT-4 mediated by both AMPK and/or PI3K signaling cascade. However, the full set of anti-apoptotic mechanisms of Gs-Rb1 remains unclear. Therefore, the questions of whether the effect of Gs-Rb1 was mediated by inhibiting mitochondrial permeability transition pore (mPTP), what role glycogen synthase kinase-3β (GSK-3β) plays in the mPTP opening, and whether the AMPK and PI3K signaling cascade mediated those roles were investigated in the present study.
It is well known that mitochondria play a key role in determining cell fate by controlling the balance between the survival signal and death signal6, 7, 8. The mPTP, being composed of the voltage dependent anion channel (VDAC, in the outer mitochondrial membrane), the adenine nucleotide translocase (ANT, in the inner mitochondrial membrane) and the phosphate carrier (PiC), has been an important mediator and end effector in mitochondria-mediated death pathways in cardiomyocytes as a result of ischemic/reperfusion injury6, 7, 8, 9, 16, 17, 18, 19, 20, 21. Some studies have shown that there is a strong negative correlation between cardiomyocyte survival and the fraction of depolarized mitochondria (ie mPTP opening)22 and have suggested that the mPTP, closed under physiological conditions, is opened due to cellular stress. A previous study showed that the mPTP remains closed throughout ischemia23; however, we found that CoCl2 may result in the opening of mPTP, which plays a key role in hypoxia-induced apoptosis of cardiomyocytes ex vivo. The reason for these differences could be due to experimental methods, particularly the hypoxia model. The mechanisms of mPTP opening mediating cell apoptosis may involve the collapse of the membrane potential, uncoupling of the respiratory chain, efflux of cytochrome c, Ca2+ overload, or other pro-apoptotic factors6, 7, 8, 9, 10, 11, 17, 18, 19, 20, 21, 22, 24. The fact that Gs-Rb1 inhibited the opening of mPTP suggests that the effect of Gs-Rb1 protecting neonatal rat cardiomyocytes from hypoxia-induced apoptosis was performed by virtue of inhibiting mPTP opening. However, it is unknown how Gs-Rb1 modulates mPTP opening.
GSK-3β, as the exact regulatory target of mPTP opening, was viewed as a possible therapeutic target for cardiomyocyte protection12, 13. The mechanisms that cause GSK-3β to provoke mPTP opening is not fully understood; some evidence to date, however, suggests that preservation of hexokinase-II in the mPTP complex, inhibition of cyclophilin-D-ANT binding, inhibition of p53 and inhibition of ANT in the mitochondria contribute to these mechanism10, 25. The positive correlation between hypoxia apoptosis and GSK-3β in our study supported previous conclusions12. It is noteworthy that the expression of GSK-3β, being significantly increased by hypoxia, may be significantly inhibited by Gs-Rb1 to the extent shown in the present results. The results suggested that the high activity of GSK-3β mediates hypoxia cardiomyocyte injury, in agreement with previous studies25. Therefore, we suggested that the effect of Gs-Rb1, inhibiting expression of GSK-3β, may be an important mechanism in improving the survival of hypoxia cells. GSK-3β phosphorylation is a crucial step in suppressing mPTP opening for protecting cardiomyocytes from apoptosis10. In the present study, we found both hypoxia and Gs-Rb1 significantly modulated the phosphorylation of GSK-3β at serine9 but not on T216 site; in addition, the expression level of p-Ser9-GSK-3β was significantly increased in the Gs-Rb1 group than in the hypoxia group. All of the above showed that Gs-Rb1 may down-regulate the activation of GSK-3β via phosphorylation at its serine9 site and that the phosphorylation of serine216 seems to play an insignificant role. Thus, we concluded that the phosphorylation at Ser9 may be a key step in preventing the activity of GSK-3β. In a word, the effect of Gs-Rb1 works in association with GSK-3β inactivation to inhibit cardiomyocyte apoptosis. However, further investigations of how Gs-Rb1 inhibits mPTP opening via GSK-3β are required.
Akt is one of the best described survival kinases. It is activated by receptor ligands, and its activation preserves mitochondrial integrity and protects cardiomyocytes against necrosis and apoptosis12, 26, 27. The role of PI3K-PKB/Akt via its downstream target, GSK-3β, mediates the convergence of myocyte protection signaling12, 26, 27. Many studies have suggested that PI3K/Akt may inhibit mPTP opening through inhibiting GSK-3β activity26, 27, 28, 29, 30, 31. In the present study, we found that the effect of Gs-Rb1, which inhibits the activity of GSK-3β and mPTP opening, was at least partially mediated by PI3K-PKB/Akt. According to the results, the influence of Gs-Rb1 on mPTP opening or GSK-3β activity is partially abrogated by PI3K activity inhibitors, which suggests that the PI3K-PKB/Akt-GSK-3β pathway is an important one in anti-hypoxia apoptosis of Gs-Rb1 ex vivo. However, it is just partly responsible for the efficacy of Gs-Rb1, in accordance with our previous study5. The mechanisms responsible for Gs-Rb1 controlling GSK-3β activity and mPTP opening via the PI3K/Akt pathway have not been fully elucidated in the present study. There exists, however, accumulating evidences that multiple Akt target molecules are recruited through both transcriptional32, 33, 34 and post-transcriptional35, 36 mechanisms to directly impinge upon and protect mitochondria27. Akt, once activated at the plasma membrane, may translocate to sub-cellular compartments, such as the nucleus and the mitochondria, and further preserve mitochondrial integrity in cardiomyocytes37, regulate the expression level of Bcl-2 family proteins in cardiomyocytes38, phosphorylate directly GSK-3β at Ser9, increase cellular hexokinase37, 39, 40, inhibit cytosolic Ca2+ overload and so on. Therefore, we suggested that the effect mediated by Gs-Rb1, inhibiting GSK-3β activity and mPTP opening, may be performed via these pathways too, which is adjusted by PI3K/Akt. The exact mechanisms, however, remain to be determined.
A growing body of literature indicates that AMP-activated protein kinase (AMPK) may protect cardiomyocytes from ischemic/reperfusion by modulating energy generating metabolic pathways41, 42, enhancing glucose uptake and glycolysis43, 44, stimulating the oxidation of FFAs (free fatty acid)45 and so on. Our previous result suggested that AMPK activity is an important regulator in Gs-Rb1 anti-hypoxia effect, which may be performed via the translocation and the expression of GLUT-45. Some previous studies showed that the AMPK pathway associates with inactivation of GSK-3β by Ser9 phosphorylation, which improves mitochondrial dysfunction46, 47, 48. Our present study supports the conclusion that Gs-Rb1 may phosphorylate the Ser9 site (non Ser216 site) of GSK-3β through the AMPK pathway and then further inhibit the opening of mPTP. In summary, the effect of Gs-Rb1 on hypoxia cardiomyocytes can be partly mediated by the AMPK-GSK-3β-mPTP system, in which Ser9 phosphorylation of GSK-3β may play a key role. However, whether the AMPK effect of Gs-Rb1 mediating GSK-3β was performed by other signaling pathways needs further investigation.
Previous studies demonstrated that ischemia may lead to the release of cytochrome c from mitochondria into cytosol9, 49, 50, 51, 52, 53, which was shown in the present study. Here we showed that Gs-Rb1 can significantly inhibit cytochrome c release to the cytoplasm, PARP cleavage and caspase-3 activation in hypoxia cardiomyocytes, which may be partly inhibited by wortmannin or Ara A. It appears that mPTP opening is a main mechanism mediating cytochrome c release17, 20, 54; then cytochrome c release and PARP cleavage activate the caspase cascade, setting apoptosis in motion54; thus, the effects of Gs-Rb1 were evident in those pathways at least. However, whether other pathways take part in mediating the Gs-Rb1 effect has been unknown. Overall, the mitochondrial pathway is an important one in Gs-Rb1 helping cardiomyocytes resist hypoxia apoptosis55, 56.
In the present study, we found that neither Ara A nor wortmannin completely inhibited the activation of GSK-3β and the opening of mPTP mediated by Gs-Rb1, which suggested that other pathways exist that help to control the effect of Gs-Rb1 on GSK-3β and mPTP. Some studies have shown that the activation of intracellular kinase cascades, especially PKC57, PKG58 and mTOR/p70s6K59, 60, 61, plays a key role in preventing cardiomyocytes from ischemic/reperfusion injury. However, whether these signal transduction networks, together with AMPK and PI3K pathways, modulate the Gs-Rb1 inhibition of mPTP opening requires further study.
The present data strongly suggest that inhibiting mPTP opening is an important mechanism by which Gs-Rb1 protects cardiomyocytes from hypoxia-induced apoptosis; this is partially controlled by the downregulation of GSK-3β. In addition, the GSK-3β and/or the mPTP effects of Gs-Rb1 may be partly regulated by both the AMPK and PI3K signaling pathways; however, it is unclear whether there are other pathways, particularly death receptor pathways, taking part in the effects of Gs-Rb1 anti-apoptosis. In conclusion, our findings highlight a novel anti-apoptosis mechanism of Gs-Rb1 and suggest the importance to further ascertain the exact mechanism of how Gs-Rb1 improves hypoxic cardiomyocytes in resisting apoptosis in vivo.
Author contribution
Hong-liang KONG and Zhan-quan LI designed the experiments; Hong-liang KONG, Yao FU, Tong LI and Hui-jun LI performed the experimental protocols; Zhan-quan LI, Hong-liang KONG, Ying-jun ZHAO and Shu-mei ZHAO contributed new analytical tools and reagents; Hong-liang KONG and Yao FU analyzed data; Hong-liang KONG, Shu-mei ZHAO and Li ZHU wrote the paper.
Acknowledgments
We thank Prof Guo-xian QI (Department of Cardiology, the First Affiliated Hospital of China Medical University) for providing technological support.
References
- Liu YF, Liu SW, Liu ZX. Effects of ginsenoside Rb1 on blood vessel regeneration after ischemia and reperfusion in rats. Chin J Histochem Cytochem. 2008;17:39–44. [Google Scholar]
- Zeng HS, Liu ZX. Influence of ginsenoside Re on cardiomyocyte apoptosis and Fas expression after acute ischemia-reperfusion in rats. J Huazhong Univ Sci Tech [Health Sci] 2004;33:286–8. [Google Scholar]
- Guan L, Li WZ, Liu ZX. Effect of Ginsenoside-Rb1 on cardiomyocyte apoptosis after ischemia and reperfusion in rats. J Huazhong Univ Sci Technol [Med Sci] 2002;22:212–5. doi: 10.1007/BF02828182. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Liu XC. Effect of Ginsenoside Rb1 and Re on cardiomyocyte apoptosis after ischemia and reperfusion in rats. Chin J Histochem Cytochem. 2002;11:374–506. doi: 10.1007/BF02828182. [DOI] [PubMed] [Google Scholar]
- Kong HL, Wang JP, Li ZQ, Zhao SM, Dong J, Zhang WW. Anti-hypoxic effect of ginsenoside Rb1 on neonatal rat cardiomyocytes is mediated through the specific activation of glucose transporter-4 ex vivo. Acta Pharmacol Sin. 2009;30:396–403. doi: 10.1038/aps.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JW, Pagel AL, Means CK, Oksenberg D, Armstrong RC, Brown JH. Cardiomyocyte apoptosis induced by Galphaq signaling is mediated by permeability transition pore formation and activation of the mitochondrial death pathway. Circ Res. 2000;87:1180–7. doi: 10.1161/01.res.87.12.1180. [DOI] [PubMed] [Google Scholar]
- Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol. 2007;42:150–8. doi: 10.1016/j.yjmcc.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104:1240–52. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borutaite V, Brown GC. Mitochondria in apoptosis of ischemic heart. FEBS Lett. 2003;541:1–5. doi: 10.1016/s0014-5793(03)00278-3. [DOI] [PubMed] [Google Scholar]
- Miura T, Miki T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ J. 2009;73:1184–92. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Colantuono G, Moro N, Ruggiero FM, Tiravanti E, Di Venosa N, et al. Melatonin protects against heart ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am J Physiol Heart Circ Physiol. 2009;297:H1487–1493. doi: 10.1152/ajpheart.00163.2009. [DOI] [PubMed] [Google Scholar]
- Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, et al. Glycogen synthase kinase-3 mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–8. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in the aged rat heart: Roles of PKCdelta, PKCepsilon, Akt, and GSK3beta. Am J Physiol Regul Integr Comp Physiol. 2007;292:R800–9. doi: 10.1152/ajpregu.00374.2006. [DOI] [PubMed] [Google Scholar]
- Javadov S, Rajapurohitam V, Kilić A, Zeidan A, Choi A, Karmazyn M. Anti-hypertrophic effect of NHE-1 inhibition involves GSK-3beta-dependent attenuation of mitochondrial dysfunction. J Mol Cell Cardiol. 2009;46:998–1007. doi: 10.1016/j.yjmcc.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem. 2008;283:26312–23. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 2009;1787:1402–15. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Duchen MR, McGuinness O, Brown LA, Crompton M. On the involvement of a cyclosporin A sensitive mitochondrial pore in myocardial reperfusion injury. Cardiovasc Res. 1993;27:1790–4. doi: 10.1093/cvr/27.10.1790. [DOI] [PubMed] [Google Scholar]
- Griffiths EJ, Halestrap AP. Protection by cyclosporin A of ischemia/reperfusion induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–9. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–25. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–24. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, et al. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia but open upon reperfusion. Biochem J. 1995;307:93–8. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol. 2009;104:181–8. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann MW, Rechner C, Freund C, Baurand A, El Jamali A, Dietz R. Statins inhibit reoxygenation-induced cardiomyocyte apoptosis: role for glycogen synthase kinase 3beta and transcription factor beta-catenin. J Mol Cell Cardiol. 2004;37:681–90. doi: 10.1016/j.yjmcc.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Lee S, Chanoit G, McIntosh R, Zvara DA, Xu Z. Molecular mechanism underlying Akt activation in zinc-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2009;297:H569–75. doi: 10.1152/ajpheart.00293.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Murphy AN, Brown JH. Akt mediated mitochondrial protection in the heart: metabolic and survival pathways to the rescue. J Bioenerg Biomembr. 2009;41:169–80. doi: 10.1007/s10863-009-9205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Li L, Li L, Gao C, Shi C. Sevoflurane postconditioning protects chronically-infarcted rat hearts against ischemia-reperfusion injury by activation of pro-survival kinases and inhibition of mitochondrial permeability transition pore opening upon reperfusion. Biol Pharm Bull. 2009;32:1854–61. doi: 10.1248/bpb.32.1854. [DOI] [PubMed] [Google Scholar]
- Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103:987–95. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, et al. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92:873–80. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem. 2002;277:22528–33. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- Craig R, Wagner M, McCardle T, Craig AG, Glembotski CC. The cytoprotective effects of the glycoprotein 130 receptor-coupled cytokine, cardiotrophin-1, require activation of NF-kappa B. J Biol Chem. 2001;276:37621–9. doi: 10.1074/jbc.M103276200. [DOI] [PubMed] [Google Scholar]
- Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–75. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–65. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–9. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- Uchiyama T, Engelman RM, Maulik N, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109:3042–9. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- Chiara F, Castellaro D, Marin O, Petronilli V, Brusilow WS, Juhaszova M, et al. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS One. 2008;3:e1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Shukair S, Naik TJ, Moazed F, Ardehali H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol Cell Biol. 2008;28:1007–17. doi: 10.1128/MCB.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, et al. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–8. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- Hardie D, Scott J, Pan D, Hudson E. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–20. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–55. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- Fryer LG, Foufelle F, Barnes K, Baldwin SA, Woods A, Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J. 2002;363:167–74. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder W, Hardie D. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly (ADP-ribose) polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–95. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- Horike N, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Kamata H, et al. AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem. 2008;283:33902–10. doi: 10.1074/jbc.M802537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Hueckstaedt LK, Ren J. The protease inhibitor UCF-101 ameliorates streptozotocin-induced mouse cardiomyocyte contractile dysfunction in vitro: role of AMP-activated protein kinase. Exp Physiol. 2009;94:984–94. doi: 10.1113/expphysiol.2009.049189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Borutaite V, Budriunaite A, Brown GC. Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim Biophys Acta. 2000;1459:405–12. doi: 10.1016/s0005-2728(00)00178-x. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Budriunaite A, Morkuniene R, Brown GC. Release of mitochondrial cytochrome c and activation of cytosolic caspases induced by myocardial ischaemia. Biochim Biophys Acta. 2001;1537:101–9. doi: 10.1016/s0925-4439(01)00062-x. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Jekabsone A, Morkuniene R, Brown GC. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. J Mol Cell Cardiol. 2003;35:357–66. doi: 10.1016/s0022-2828(03)00005-1. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H2770–8. doi: 10.1152/ajpheart.2001.280.6.H2770. [DOI] [PubMed] [Google Scholar]
- Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–47. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- Kawata H, Yoshida K, Kawamoto A, Kurioka H, Takase E, Sasaki Y, et al. Ischemic preconditioning upregulates vascular endothelial growth factor mRNA expression and neovascularization via nuclear translocation of protein kinase C epsilon in the rat ischemic myocardium. Circ Res. 2001;88:696–704. doi: 10.1161/hh0701.088842. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhao S, Song J. Caspase-dependent apoptosis and -independent poly (ADP-ribose) polymerase cleavage induced by transforming growth factor beta1. Int J Biochem Cell Biol. 2004;36:223–34. doi: 10.1016/s1357-2725(03)00215-2. [DOI] [PubMed] [Google Scholar]
- Javadov S, Choi A, Rajapurohitam V, Zeidan A, Basnakian AG, Karmazyn M. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc Res. 2008;77:416–24. doi: 10.1093/cvr/cvm039. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Amit T, Falach-Yogev M, Bar Am O, Maruyama W, Naoi M. The essentiality of Bcl-2, PKC and proteasome-ubiquitin complex activations in the neuroprotective-antiapoptotic action of the anti-Parkinson drug, rasagiline. Biochem Pharmacol. 2003;66:1635–41. doi: 10.1016/s0006-2952(03)00535-5. [DOI] [PubMed] [Google Scholar]
- Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–36. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–76. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, et al. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;380:795–804. doi: 10.1042/BJ20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–71. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]