Abstract
Aim:
To examine if steroid-like compounds found in many Chinese medicinal products conventionally used for the promotion of blood circulation may act as active components via the same molecular mechanism triggered by cardiac glycosides, such as ouabain.
Methods:
The inhibitory potency of ouabain and the identified steroid-like compounds on Na+/K+-ATPase activity was examined and compared. Molecular modeling was exhibited for the docking of these compounds to Na+/K+-ATPase.
Results:
All the examined steroid-like compounds displayed more or less inhibition on Na+/K+-ATPase, with bufalin (structurally almost equivalent to ouabain) exhibiting significantly higher inhibitory potency than the others. In the pentacyclic triterpenoids examined, ursolic acid and oleanolic acid were moderate inhibitors of Na+/K+-ATPase, and their inhibitory potency was comparable to that of ginsenoside Rh2. The relatively high inhibitory potency of ursolic acid or oleanolic acid was due to the formation of a hydrogen bond between its carboxyl group and the Ile322 residue in the deep cavity close to two K+ binding sites of Na+/K+-ATPase. Moreover, the drastic difference observed in the inhibitory potency of ouabain, bufalin, ginsenoside Rh2, and pentacyclic triterpenoids is ascribed mainly to the number of hydrogen bonds and partially to the strength of hydrophobic interaction between the compounds and residues around the deep cavity of Na+/K+-ATPase.
Conclusion:
Steroid-like compounds seem to contribute to therapeutic effects of many cardioactive Chinese medicinal products. Chinese herbs, such as Prunella vulgaris L, rich in ursolic acid, oleanolic acid and their glycoside derivatives may be adequate sources for cardiac therapy via effective inhibition on Na+/K+-ATPase.
Keywords: cardiac glycoside, molecular modeling, Na+/K+-ATPase, ouabain, steroid-like, Traditional Chinese Medicine
Introduction
Many Chinese medicinal products have been traditionally used for the treatment of cardiovascular diseases, and their curative effects have also been verified by a large number of clinical studies in the past few decades1, 2. A large number of these Chinese medicinal products seem to achieve their therapeutic effects via the promotion of blood circulation. Promoting blood circulation, including improvement of hemodynamic and hemorheology, and removing blood stasis, are already accepted concept in Traditional Chinese Medicine. However, little is known for the detailed molecular mechanisms how these Chinese medicines trigger the promotion of blood circulation.
Cardiac glycosides, such as ouabain and digoxin, are steroid-like compounds and have been used in the treatment of congestive heart failure and supraventricular arrhythmias. The therapeutic effect of cardiac glycosides lies in their reversible inhibition on the membrane-bound Na+/K+-ATPase located in human myocardium3. The inhibition on Na+/K+-ATPase leads to the elevation of intracellular Na+concentration, which in turn activates a Na+/Ca2+ exchanger resulting in an increase of intracellular Ca2+ concentration. The elevated intracellular Ca2+ concentration causes an increased inotropism, accentuating the force of myocardial contraction by increasing the velocity and extent of sarcomere shortening, thus translating into increased stroke work for a given filling volume of pressure.
Biosynthesis of steroid-like compounds proceeds from the cyclization of oxidosqualene in the isoprenoid pathway. After rearrangement and additional modifications of the carbon skeleton, three major end products, tetracyclic triterpenoids, pentacyclic triterpenoids and steroids are yielded. These structurally similar compounds are converted to saponins via sugar attachment at variable positions of their carbon skeleton, usually C-3 position. The core structure of cardiac glycosides consists of a tetracyclic steroidal framework, which is considered the pharmacophoric moiety responsible for their inhibition on Na+/K+-ATPase4. Amusingly, a number of steroid-like compounds, such as triterpenoids, steroids and saponins, are also found in many Chinese medicinal products used for promoting blood circulation, and regarded as the active ingredients responsible for their therapeutic effects5.
In a previous study, we demonstrated that ginsenosides with sugar moieties attached only to the C-3 position of their core steroid-like structure possessed inhibitory potency on Na+/K+-ATPase activity, and the inhibition was proposed to be partly, at least, responsible for the cardiac therapeutic effects of ginseng and sanqi via the promotion of blood circulation6. Therefore, we wonder if various steroid-like compounds found in other Chinese medicinal products may promote blood circulation via the same molecular mechanism. In this study, Na+/K+-ATPase inhibition by ouabain, ginsenoside Rh2 and several steroid-like compounds found in Chinese medicinal products used for promoting blood circulation was examined and compared. Molecular modeling and docking of these compounds to Na+/K+-ATPase were exhibited to reveal the observed difference in their inhibitory potency at molecular level.
Materials and methods
Chemicals and reagents
Ginsenoside Rh2, polygalacic acid and jujuboside B were purchased from Scientific Pharmaceutical Elite Company (Taiwan, China). Saikosaponin A and glycyrrhizin were purchased from Yoneyama Chemical Industry (Japan). Sarsasapogenin and astragaloside III were purchased from ChromaDex (USA). Bufalin was obtained from Wako Pure Chemical Industries (Japan). Ursolic acid, oleanolic acid, cholic acid, and ouabain were obtained from Sigma (USA). Phosphate assay kit was purchased from Amresco (USA).
Measurement of Na+/K+-ATPase activity
The activity of Na+/K+-ATPase was determined by measuring the amount of inorganic phosphate (Pi) liberated from ATP. A commercial Na+/K+-ATPase from porcine cerebral cortex (Sigma, 0.3 units/mg) was incorporated into a reaction mixture of 500 μL containing 1 mmol/L ATP, 5 mmol/L MgCl2, 80 mmol/L NaCl, 20 mmol/L KCl, and 40 mmol/L Tris-HCl (pH 7.8); the enzymatic reaction was terminated by adding 250 μL of 30% (w/v) trichloroacetic acid after the incubation period. After centrifugation at 10000×g for 10 min at 4 °C, the supernatant was diluted 12.5-fold with deionized water and then added with 50 μL color development reagent provided by the phosphate assay kit. After 30 min of incubation at room temperature, the color intensity was measured at 620 nm on SpectraMax M2 reader (Molecular Devices, USA). Sodium pump activity was expressed as μmol Pi liberated from ATP by 1 mg of Na+/K+-ATPase in 1 h.
Statistical analysis
Data were expressed as mean±SEM of 5 replicates and the analysis of variance (One-way ANOVA) was performed on SPSS 12.0 for Windows. Differences were considered statistically significant at P<0.05.
Molecular modeling and docking
The crystal structures of pig renal Na+,K+-ATPase (PDB code 3B8E) and shark rectal gland Na+,K+-ATPase (PDB code 3A3Y) were downloaded from Protein Data Bank and found to posses equivalent structures7, 8. In order to facilitate docking process, the β and γ subunits of the Na+,K+-ATPase were removed, as well as the water molecules and counter-ions surrounding the remaining α subunit. The modified Na+,K+-ATPase after hydrogen saturation was minimized with CHARMm force field9 using the Discover Studio 2.0 package (http://accelrys.com/products/discovery-studio/). The 2D structures of ginsenosides used in this study were constructed by using the ChemDraw program, and their corresponding 3D structures were converted by the Chem3D program (http://www.cambridgesoft.com/). The pocket for binding cardiac glycosides in the Na+,K+-ATPase α subunit was defined among the extracellular loops linking transmembrane segments as reported by Qiu et al10 , and the subunit-binding domain was defined as the region of the sphere with 10 Å radius from the center of the binding pocket, which lies between Ile315 and Leu793 of the modified Na+,K+-ATPase. Docking of steroid-like compounds was performed in silico by employing the LibDock module11 in the Discover Studio 2.0 package. There are 100 hotspots identified in the binding pocket. The LibDock methodology effectively executed the docking of combinatorial libraries of compounds in a high throughput manner while keeping the protein structure fixed12. After LibDock, the protein-ligand complexes were further optimized by LigandFit and then smart minimizer algorithm to minimize with CHARMm forcefield. Among the candidate structures, reported by the docking simulation, the docking structure with highest Ligscore2 value, as computed by the score ligand pose module, was selected to represent each of those steroid-like compounds inside the binding pocket.
Results
Steroid-like compounds in Chinese medicinal products used for promoting blood circulation
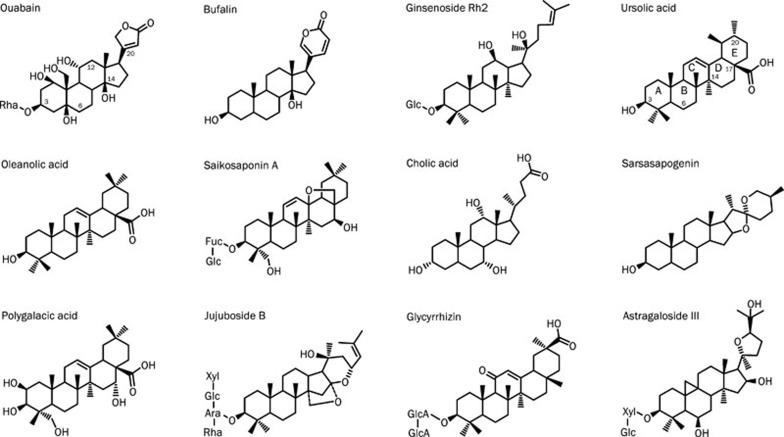
Eleven steroid-like compounds were selected for this study as they have been structurally determined and regarded as possible active ingredients in Chinese medicinal products used for the promotion of blood circulation and the treatment of cardiovascular diseases (Table 1)13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23. These 11 steroid-like compounds are structurally similar to ouabain, a cardiac glycoside, in the hydrophobic steroidal core regardless the different sugar units attached to the C-3 position and diverse oxidative groups modified at variable positions (Figure 1).
Table 1. Steroid-like compounds in Chinese medicines used for the promotion of blood circulation.
| Compound | Chinese medicinal sources | References |
|---|---|---|
| Bufalin | The dried venom of Bufo bufo gargarizans Cantoror B bufo melanostictus Schneider | 13 |
| Ginsenoside Rh2 | The dry root of Panax ginseng or P notoginseng FH Chen | 14 |
| Ursolic acid | Whole plant of Prunella vulgaris L with dry flowers | 15 |
| Oleanolic acid | Whole plant of Prunella vulgaris L with dry flowers | 15 |
| Saikosaponin A | The dry root of Bupleurum chinense DC or B scorzonerifolium Willd | 16, 17 |
| Cholic acid | The dried bile of Ursus arctos Linnaeus or Selenarctos thibetanus G Cuvier | 18 |
| Sarsasapogenin | The dry root of Anemarrhena asphodeloides Bge | 19 |
| Polygalacic acid | The dry root of Platycodon grandiflorum (Jacq) A DC | 20 |
| Jujuboside B | The mature seeds of Ziziphus jujube Mill var spinosa (Bunge) Hu ex HF Chou | 21 |
| Glycyrrhizin | The root of Glycyrrhiza uralensis Fisch G inflata Bat or G glabra L | 17, 22 |
| Astragaloside III | The dry root of Astragalus membranaceus (Fisch) Bge or var mongholicus (Bge) Hsiao | 23 |
Figure 1.
Chemical structures of ouabain and 11 steroid-like compounds found in Chinese medicinal products used for the promotion of blood circulation.
Inhibition of Na+,K+-ATPase by the selected steroid-like compounds
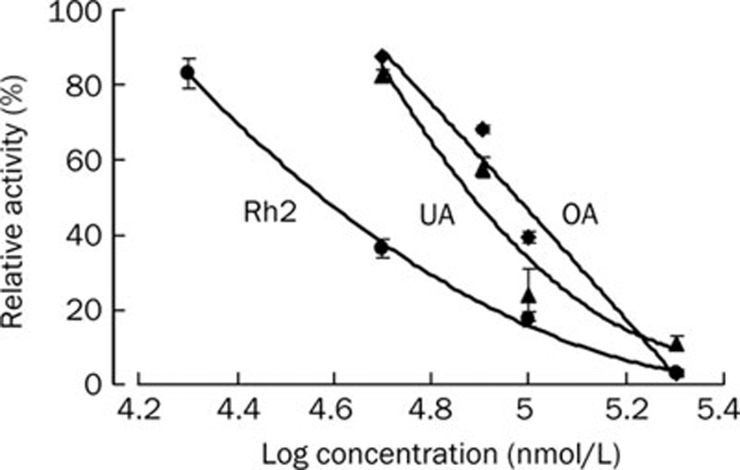
To examine whether the selected steroid-like compounds from Chinese medicinal herbs may be responsible for the effect of promoting blood circulation via the same mechanism triggered by ouabain, a commercial Na+,K+-ATPase from porcine cerebral cortex was used to evaluate the inhibitory potency of these compounds. All the examined steroid-like compounds displayed more or less inhibition on Na+/K+-ATPase in a dose-dependent manner (Figure 2). Among these steroid-like compounds, bufalin (structurally almost equivalent to ouabain with a unique lactone ring attached to the hydrophobic steroidal core) exhibited significantly higher inhibitory potency than the others. In the five examined pentacyclic triterpenoids, ursolic acid and oleanolic acid were detected as moderate inhibitors of Na+/K+-ATPase while saikosaponin A, polygalacic acid and glycyrrhizin only exhibited weak inhibition. The IC50 of ursolic acid (76.7 μmol/L) or oleanolic acid (94.3 μmol/L) was comparable to that of ginsenoside Rh2 (37.5 μmol/L) (Figure 3).
Figure 2.
(A) Inhibition of porcine Na+,K+-ATPase by 0.1 mmol/L of ouabain and the selected 11 steroid-like compounds. (B) For the 7 compounds showing low inhibitory potency, the concentration was increased to 0.2 mmol/L for the same assay. Inhibitory potency of these compounds, presented in the order as shown in Figure 1, was observed as the reduction of Pi liberation released from ATP by a constant amount of commercial porcine Na+,K+-ATPase. Data represent mean±SEM of 5 replicates. bP<0.05, cP<0.01 vs control group (CON; deionized water only).
Figure 3.
Inhibitory potency of ginsenoside Rh2, ursolic acid (UA) and oleanolic acid (OA) on porcine Na+,K+-ATPase. Inhibitory potency of various concentrations of ginsenoside Rh2, ursolic acid and oleanolic acid was observed as the reduction of Pi liberation released from ATP by a constant amount of commercial porcine Na+,K+-ATPase.
Molecular modeling and docking of steroid-like compounds to Na+,K+-ATPase
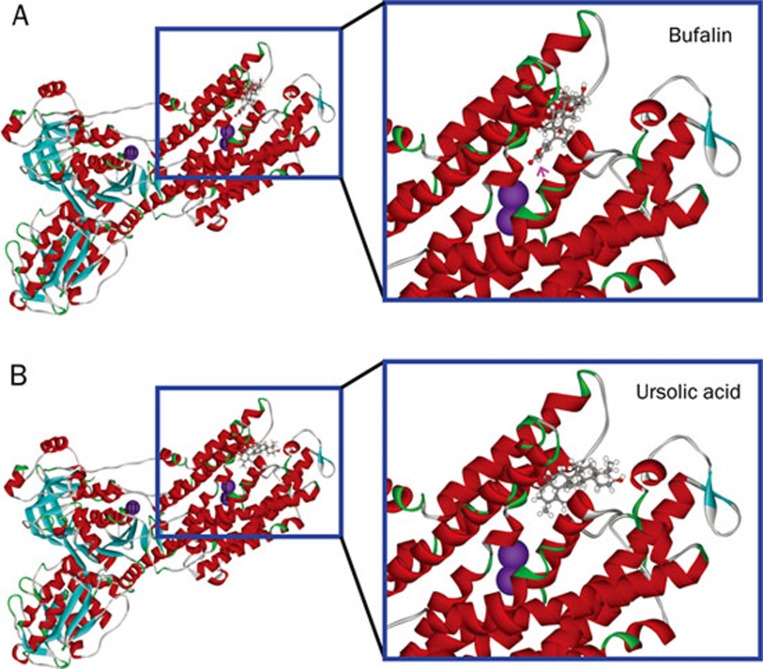
To reveal the observed difference in Na+,K+-ATPase inhibition at molecular level, the examined steroid-like compounds were subjected to molecular modeling and docking, as exemplified by bufalin and ursolic acid, to the extracellular domain of Na+,K+-ATPase α subunit. As expected from structural similarity, the interaction of bufalin with the binding pocket of Na+,K+-ATPase nearly matched to that of ouabain reported previously7 with the unique lactone ring (indicated by a pink arrow) penetrating deeply into the cavity close to two K+ binding sites (Figure 4A). Comparably, the hydrophobic steroidal core of ursolic acid was confined in a location similar to that of bufalin within the binding pocket of Na+,K+-ATPase (Figure 4B). However, no hydrophilic functional group attached to the hydrophobic steroidal core of ursolic acid was present in the location equivalently occupied by the lactone ring of bufalin in the deep cavity close to two K+ binding sites of Na+,K+-ATPase.
Figure 4.
Modeling of bufalin (A) and ursolic acid (B) binding to the extracellular pocket of Na+,K+-ATPase α subunit. The amino acid residues around the binding pocket of Na+,K+-ATPase are shown in ribbon structure, and bufalin and ursolic acid in scaled ball and stick. Three K+ binding sites are shown in purple balls. The position of the unique lactone ring of bufalin is indicated by a pink arrow.
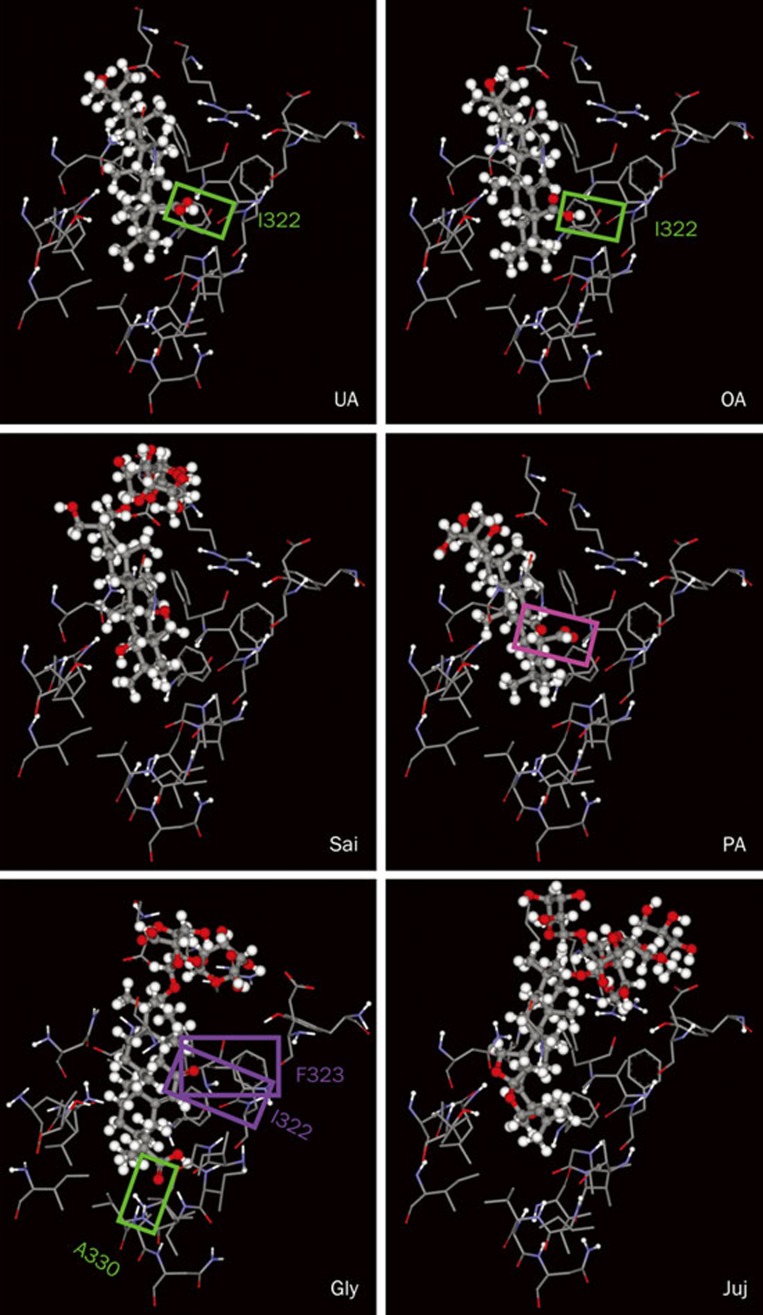
In accord with the results of Na+,K+-ATPase inhibition assay for the examined pentacyclic triterpenoids, the relatively high inhibitory potency of ursolic acid or oleanolic acid is due to the formation of a hydrogen bond between its carboxyl group at C-17 and the Ile322 residue in the deep cavity close to two K+ binding sites of Na+/K+-ATPase (Figure 5). This hydrogen bond is not found in the other three pentacyclic triterpenoids, saikosaponin A, polygalacic acid and glycyrrhizin, neither in another pentacyclic steroid-like compound, jujuboside B. The carboxyl group of ursolic acid or oleanolic acid forming a hydrogen bond with Ile322 of Na+/K+-ATPase is also correspondingly present in polygalacic acid; however, an intramolecular hydrogen bond between the carboxyl group and an adjacent hydroxyl group at C-16 is formed in polygalacic acid (indicated by a pink box) and impedes its formation of the intermolecular hydrogen bond with Na+/K+-ATPase. In contrast, a different hydrogen bond is formed between the carboxyl group of glycyrrhizin and Ala330 of Na+/K+-ATPase (indicated by a green box). Whereas, the repulsion between the hydrophilic carbonyl group at C-11 of glycyrrhizin and the hydrophobic Ile322 and Phe323 of Na+/K+-ATPase (indicated by two purple boxes) greatly reduces the stability of glycyrrhizin-Na+/K+-ATPase complex. As expected from the inhibition assay and chemical structures, no hydrogen bond is formed between Na+/K+-ATPase and cholic acid, sarsaspogenin or astragaloside III (data not shown).
Figure 5.
Detailed molecular interactions between the extracellular pocket of Na+,K+-ATPase and ursolic acid (UA), oleanolic acid (OA), saikosaponin A (Sai), polygalacic acid (PA), glycyrrhizin (Gly), and jujuboside B (Juj). The amino acid residues of Na+,K+-ATPase close to the binding compounds are shown in wireframe, and the structures of the compounds in scaled ball and stick. A hydrogen bond formed between Na+,K+-ATPase and ursolic acid, oleanolic acid or glycyrrhizin is indicated by a green box. An intramolecular hydrogen bond in polygalacic acid is indicated by a pink box. The repulsion between the hydrophilic carbonyl group of glycyrrhizin and the hydrophobic Ile322 and Phe323 of Na+/K+-ATPase is indicated by two purple boxes.
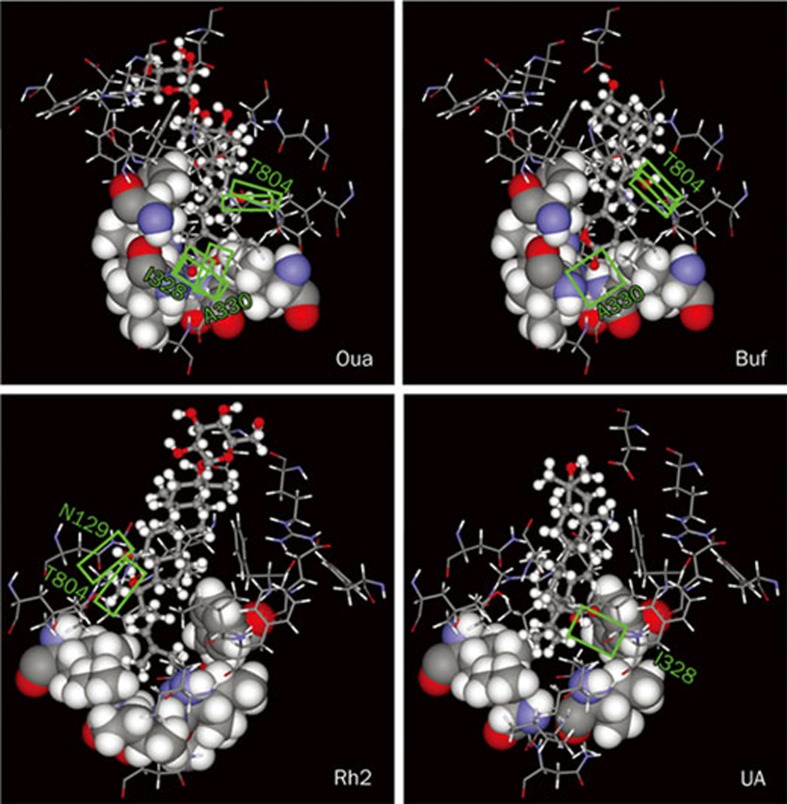
Regardless of their structural similarity and functional capability of inhibiting Na+/K+-ATPase activity, drastic difference was observed in the inhibitory potency of ouabain, bufalin, ginsenoside Rh2, and ursolic acid (Figure 2). The difference is ascribed mainly to the number of hydrogen bonds (H-bonds) and partially to the strength of hydrophobic interaction between the compounds and residues around the deep cavity close to the two K+ binding sites of Na+/K+- ATPase (Figure 6). Three H-bonds are formed between the lactone of ouabain and Ile328 (forming one H-bond) and Ala330 (forming two H-bonds) of Na+/K+-ATPase, and two H-bonds between the hydroxyl group at C-14 of ouabain and Thr804 of Na+/K+-ATPase. In contrast, one H-bond is formed between the lactone of bufalin and Ala330 of Na+/K+-ATPase, and two H-bonds between the hydroxyl group at C-14 of bufalin and Thr804 of Na+/K+-ATPase. Strong hydrophobic interaction is found between the lactone of ouabain or bufalin and six hydrophobic residues (Ile327, Ile328, Val329, Ile787, Phe790, and Ile807 shown in spacefill) around the deep cavity of Na+/K+-ATPase. Two H-bonds are formed between the hydroxyl groups at C-12 and C-20 of ginsenoside Rh2 and Asn129 and Thr804 of Na+/K+-ATPase, respectively; and strong hydrophobic interaction is found between the alkyl group of ginsenoside Rh2 and the same six hydrophobic residues around the deep cavity of Na+/K+-ATPase. One H-bond is formed between the carboxyl group of ursolic acid and Ile322 of Na+/K+-ATPase, and moderate hydrophobic interaction is found between the ring E of ursolic acid and four hydrophobic residues (Ile327, Val329, Phe790, and Ile807 shown in spacefill) of Na+/K+-ATPase. In addition, similar hydrophobic interaction is observed between the hydrophobic steroidal core of each examined compound and 8 other hydrophobic residues (Leu132, Tyr315, Ile322, Phe323, Ile325, Phe793, Ile794, and Leu802 located in the upper portions of the figures shown in wireframe) around the binding pocket of Na+/K+-ATPase.
Figure 6.
Detailed molecular interactions between the extracellular binding pocket of Na+,K+-ATPase and ouabain (Oua), bufalin (Buf), ginsenoside Rh2, and ursolic acid (UA). The hydrophobic amino acid residues around the deep cavity of Na+,K+-ATPase interacting with the binding compounds are shown in spacefill, the rest of amino acid residues of Na+,K+-ATPase close to the binding compounds in wireframe, and the compounds in scaled ball and stick. The hydrogen bonds formed between Na+,K+-ATPase and the compounds are indicated by green boxes.
Discussion
On the basis of this study, the therapeutic effects of many cardiac Chinese medicinal products may be partly, at least, attributed to various steroid-like compounds that promote blood circulation via the same molecular mechanism triggered by ouabain, that is, accentuating the force of myocardial contraction by elevating calcium concentration via the inhibition of Na+,K+-ATPase. Bufalin, the active but toxic component in Chansu (a traditional Chinese medicine used to rescue patients with heart failure) has a chemical structure highly similar to ouabain, particularly with a unique lactone ring attached to the hydrophobic steroidal core, and thus possesses strong inhibitory potency on Na+,K+-ATPase. Comparable to ginsenosides as reported previously6, ursolic acid and oleanolic acid are moderate inhibitors of Na+,K+-ATPase among the examined pentacyclic triterpenoids, possessing IC50 approximately 100 times higher than that of ouabain. It seems that detection of inhibitory potency on Na+,K+-ATPase together with the theoretical modeling of docking may be a suitable platform for the first screening of potential drug compounds from the abundant sources of Chinese medicinal products traditionally used for the treatment of cardiovascular diseases.
Ursolic acid and oleanolic acid are widely present in food, medicinal herbs and other plants24. So far, most pharmacological investigation of these two pentacyclic triterpenoids focused on their anti-inflammatory, anti-hyperlipidemic and hepatoprotective properties25, 26. The early understanding of their anti-inflammatory and hepatoprotective properties facilitated their use as remedies for the treatment of liver-related pathologies. In light of the results shown in this study, we propose that ursolic acid and oleanolic acid may also possess cardiac therapeutic effects as a consequence of their inhibitory potency on Na+,K+-ATPase.
Prunella vulgaris L, a commonly used Chinese medicinal herb, also known as self-heal, has a wide range of reported medicinal activities27, 28. This herb with antioxidant, antimicrobial and anti-inflammatory properties has a long history of use as a remedy for cardiovascular diseases29, 30. Phytochemical analyses showed that ursolic acid, oleanolic acid and their glycoside derivatives were the major components of P vulgaris L31. Commonly, the glycoside derivatives tend to be metabolized to their aglycone by intestinal bacterial deglycosylation after oral administration, and the metabolites are easily absorbed by the intestines due to the increase of hydrophobicity32, 33. Therefore, it is likely that the cardiac therapeutic effects of P vulgaris L are partly, at least, attributed to the effective inhibition of Na+,K+-ATPase by their major triterpenoidal ingredients, ursolic acid and oleanolic acid as well as their glycoside derivatives. Similarly, ginsenoside Ro, an oleanolic acid glycoside may partly contribute to the cardiac therapeutic effect of ginseng after deglycosylation by intestinal bacteria34.
Experimental observation and theoretical modeling revealed that an oxygen-containing function group attached to ring C or D of steroid-like compounds is crucial for their inhibitory potency on Na+,K+-ATPase, ie, the oxidized functional group may form hydrogen bonds with Na+,K+-ATPase within the binding pocket very close to the K+ binding sites. As shown in this study, the carboxyl group of ursolic acid or oleanolic acid, the hydroxyl group at C-14 of ouabain or bufalin, and the hydroxyl groups at C-12 of ginsenoside Rh2 formed hydrogen bonds with Na+,K+-ATPase. On the other hand, lactone ring of ouabain and bufalin is definitely the key moiety to their potent inhibition on Na+,K+-ATPase, hence the steroid-like compounds attached with flexible functional group structurally similar to lactone at ring D could be selected as potent inhibitors of Na+,K+-ATPase. The moiety connected to ring D of steroid-like compound should be the target of chemical modification for increasing its inhibitory potency on Na+,K+-ATPase. Although our experimental observation and theoretical modeling were executed using porcine and shark Na+,K+-ATPase, the observed inhibitory potency of steroid-like compounds was presumably applicable to human Na+,K+-ATPase since isoforms of this enzyme in diverse species were highly conserved throughout evolution35. Of course, the precise interaction of the steroid-like compounds in the binding pocket of Na+,K+-ATPase should be further elucidated by their co-crystal 3D structure.
More and more evidence supports that Na+,K+-ATPase can be a drug target for the treatment of several diseases, including congestive heart failure, ischemic stroke, neurodegenerative diseases and even cancer36. Recently several compounds showing inhibitory potency on Na+,K+-ATPase, such as cardiac glycosides, ginsenosides, and magnesium lithospermate B (the major water soluble ingredient in the dried roots of medicinal plant Salvia miltiorrhiza), were demonstrated to provide neuroprotection against ischemic stroke in a cortical brain slice-based compound screening platform6, 37, 38. Similarly, oleanolic acid, showing inhibitory potency on Na+,K+-ATPase in this study, has also been demonstrated to display neuroprotective effect against focal cerebral ischemic injury39. The cumulated evidence suggests that inhibiting Na+,K+-ATPase may provide neuroprotection in the context of ischemia as well as other neurodegenerative conditions though it cannot be ignored that the neuroprotective effects of those compounds against cerebral ischemic injury may be partly attributed to their anti-oxidative properties. Moreover, it has been hypothesized that blockade of Na+,K+-ATPase may provide neuroprotection in ischemia through ATP conservation and modulating intracellular calcium levels just as the cardiac glycosides do in the heart contraction cycle40.
Author contribution
Jason TC TZEN designed the research; Ronald JY CHEN and Tse-yu CHUNG performed the research; Feng-yin LI, Wei-hung YANG, and Tzyy-rong JINN contributed new analytical tools and reagents; Jason TC TZEN wrote the paper.
Acknowledgments
Project supported by a grant to Jason TC TZEN from the National Science Council, Taiwan, China (No 96-2752-B-005-008-PAE).
We thank Prof Chih-ning SUN (Department of Entomology, National Chuang Hsing University) for critical reading of the manuscript.
References
- Gong X, Sucher NJ. Stroke therapy in traditional Chinese medicine (TCM): prospects for drug discovery and development. Trends Pharmacol Sci. 1999;20:191–6. doi: 10.1016/s0165-6147(98)01276-0. [DOI] [PubMed] [Google Scholar]
- Kim H. Neuroprotective herbs for stroke therapy in traditional eastern medicine. Neurol Res. 2005;27:287–301. doi: 10.1179/016164105X25234. [DOI] [PubMed] [Google Scholar]
- Li-Saw-Hee FL, Lip GY. Digoxin revisted. QJM. 1998;91:259–64. doi: 10.1093/qjmed/91.4.259. [DOI] [PubMed] [Google Scholar]
- Schönfeld W, Menke KH, Schönfeld R, Repke KR. 5 Beta, 14 beta-androstane-3 beta, 14-diol binds to the digitalis receptor site on Na/K-ATPase. J Enzyme Inhib. 1987;2:37–45. doi: 10.3109/14756368709030355. [DOI] [PubMed] [Google Scholar]
- Zhou J. Bioactive glycosides from Chinese medicines. Mem Inst Oswaldo Cruz. 1991;86:231–4. doi: 10.1590/s0074-02761991000600051. [DOI] [PubMed] [Google Scholar]
- Chen RJ, Chung TY, Li FY, Lin NH, Tzen JT. Effect of sugar positions in ginsenosides and their inhibitory potency on Na+/K+-ATPase activity. Acta Pharmacol Sin. 2009;30:61–9. doi: 10.1038/aps.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morth JP, Pedersen BP, Toustrup-Jensen MS, Sørensen TL, Petersen J, Andersen JP, et al. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–9. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Shinoda T, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc Natl Acad Sci USA. 2009;106:13742–7. doi: 10.1073/pnas.0907054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. CHARMM: A program for macromolecular energy minimization and dynamics calculations. J Comp Chem. 1983;4:187–217. [Google Scholar]
- Qiu LY, Krieger E, Schaftenaar G, Swarts HG, Willems PH, De Pont JJ, et al. Reconstruction of the complete ouabain-binding pocket of Na,K-ATPase in gastric H,K-ATPase by substitution of only seven amino acids. J Biol Chem. 2005;280:32349–55. doi: 10.1074/jbc.M505168200. [DOI] [PubMed] [Google Scholar]
- Dixon SL, Merz KM., Jr One-dimensional molecular representations and similarity calculations: methodology and validation. J Med Chem. 2001;44:3795–809. doi: 10.1021/jm010137f. [DOI] [PubMed] [Google Scholar]
- Rao SN, Head MS, Kulkarni A, LaLonde JM. Validation studies of the site-directed docking program LibDock. J Chem Inf Model. 2007;47:2159–71. doi: 10.1021/ci6004299. [DOI] [PubMed] [Google Scholar]
- Yang Z, Luo H, Wang H, Hou H. Preparative isolation of bufalin and cinobufagin from Chinese traditional medicine ChanSu. J Chromatogr Sci. 2008;46:81–5. doi: 10.1093/chromsci/46.1.81. [DOI] [PubMed] [Google Scholar]
- Jia WW, Bu X, Philips D, Yan H, Liu G, Chen X, et al. Rh2, a compound extracted from ginseng, hypersensitizes multidrug-resistant tumor cells to chemotherapy. Can J Physiol Pharmacol. 2004;82:431–7. doi: 10.1139/y04-049. [DOI] [PubMed] [Google Scholar]
- Lee IK, Kim do H, Lee SY, Kim KR, Choi SU, Hong JK, et al. Triterpenoic acids of Prunella vulgaris var lilacina and their cytotoxic activities in vitro. Arch Pharm Res. 2008;31:1578–83. doi: 10.1007/s12272-001-2154-6. [DOI] [PubMed] [Google Scholar]
- Tan LL, Cai X, Hu ZH, Ni XL. Localization and dynamic change of saikosaponin in root of Bupleurum chinense. J Integr Plant Biol. 2008;50:951–7. doi: 10.1111/j.1744-7909.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Zhou QL, Zhang ZQ, Nagasawa T, Hiai S.The structure activity relationship of saikosaponins and glycyrrhizin derivatives for Na+,K+-ATPase inhibiting action Yao Xue Xue Bao 199631496–501.Chinese. [PubMed] [Google Scholar]
- Wang FS, Xu LX, Zhao YJ, Liu AR, Jin LZ, Zhang XQ.Determination of bile acids in bear gall drainage by thin layer chromatographic scanning Yao Xue Xue Bao 198924397–400.Chinese. [PubMed] [Google Scholar]
- Liu Y, Chen W, Qiao C, Zhao N.Determination of sarsasapogenin in Anemarrhena asphodeloides Bunge by GC Zhongguo Zhong Yao Za Zhi 199924554–5.Chinese. [PubMed] [Google Scholar]
- Fu WW, Hou WB, Dou DQ, Hua HM, Gui MH, Fu R, et al. Saponins of polygalacic acid type from Platycodon grandiflorum Yao Xue Xue Bao 200641358–60.Chinese. [PubMed] [Google Scholar]
- Zhang M, Zhang Y, Xie J. Simultaneous determination of jujuboside A, B and betulinic acid in semen Ziziphi spinosae by high performance liquid chromatography-evaporative light scattering detection. J Pharm Biomed Anal. 2008;48:1467–70. doi: 10.1016/j.jpba.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Sun ZR, Zhai MP, Wang WQ, Li YR.Effects of density on seedling growth and glycyrrhizinic acid content in Glycyrrhiza uralensis Zhongguo Zhong Yao Za Zhi 2007322222–6.Chinese. [PubMed] [Google Scholar]
- Xu Q, Ma X, Liang X. Determination of astragalosides in the roots of Astragalus spp using liquid chromatography tandem atmospheric pressure chemical ionization mass spectrometry. Phytochem Anal. 2007;18:419–27. doi: 10.1002/pca.997. [DOI] [PubMed] [Google Scholar]
- Fai YM, Tao CC. A review of presence of oleanolic acid in natural products. Natura Proda Medica. 2009;2:77–290. [Google Scholar]
- Liu J. Oleanolic acid and ursolic acid: research perspectives. J Ethnopharmacol. 2005;100:92–4. doi: 10.1016/j.jep.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Zheng J, He J, Ji B, Li Y, Zhang X. Antihyperglycemic activity of Prunella vulgaris L in streptozotocin-induced diabetic mice. Asia Pac J Clin Nutr. 2007;16:427–31. [PubMed] [Google Scholar]
- Kageyama S, Kurokawa M, Shiraki K. Extract of Prunella vulgaris spikes inhibits HIV replication at reverse transcription in vitro and can be absorbed from intestine in vivo. Antivir Chem Chemother. 2000;11:157–64. doi: 10.1177/095632020001100207. [DOI] [PubMed] [Google Scholar]
- Psotov J, Kolár M, Sousek J, Svagera Z, Vicar J, Ulrichová J. Biological activities of Prunella vulgaris extract. Phytother Res. 2003;17:1082–7. doi: 10.1002/ptr.1324. [DOI] [PubMed] [Google Scholar]
- Psotov J, Chlopcíková S, Miketová P, Simánek V. Cytoprotectivity of Prunella vulgaris on doxorubicin-treated rat cardiomyocytes. Fitoterapia. 2005;76:556–61. doi: 10.1016/j.fitote.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Kajima H, Ogura H. Triterpenoids from Prunella vulgaris. Phytochemistry. 1986;25:729–33. [Google Scholar]
- Kobashi K, Akao T. Relation of intestinal bacteria to pharmacological effects of glycosides. Biosci Microflora. 1997;16:1–7. [Google Scholar]
- Akao T, Hayashi T, Kobashi K, Kanaoka M, Kato H, Kobayashi M, et al. Intestinal bacterial hydrolysis is indispensable to absorption of 18 beta-glycyrrhetic acid after oral administration of glycyrrhizin in rats. J Pharm Pharmacol. 1994;46:135–7. doi: 10.1111/j.2042-7158.1994.tb03756.x. [DOI] [PubMed] [Google Scholar]
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–93. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Rose AM, Qazzaz HM, Zolotarjova N, Mellett BJ, Martin AW, Valdes R., Jr Sodium pump isoforms in xenotransplantation: importance of biochemical compatibility. Clin Chem. 2000;46:234–41. [PubMed] [Google Scholar]
- Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7:926–35. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- Wang JK, Portbury S, Thomas MB, Barney S, Ricca DJ, Morris DL, et al. Cardiac glycosides provide neuroprotection against ischemic stroke: discovery by a brain slice-based compound screening platform. Proc Natl Acad Sci USA. 2006;103:10461–6. doi: 10.1073/pnas.0600930103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzen JT, Jinn TR, Chen YC, Li FY, Cheng FC, Shi LS, et al. Magnesium lithospermate B possesses inhibitory activity on Na+,K+-ATPase and neuroprotective effects against ischemic stroke. Acta Pharmacol Sin. 2007;28:609–15. doi: 10.1111/j.1745-7254.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Cho SO, Ban JY, Kim JY, Ju HS, Lee IS, Song KS, et al. Anti-ischemic activities of Aralia cordata and its active component, oleanolic acid. Arch Pharm Res. 2009;32:923–32. doi: 10.1007/s12272-009-1615-1. [DOI] [PubMed] [Google Scholar]
- Annunziato L.editor. New strategies in stroke interventionNew York: Springer; 2009