Abstract
Chronicity of hepatitis B (CHB) infection is characterized by a weak immune response to the virus. Entecavir (ETV) and adefovir dipivoxil (ADV) are effective in suppressing hepatitis B virus (HBV) replication. However, the underlying immune mechanism in the antiviral response of patients treated with nucleoside or nucleotide analogs is not clearly understood. In this study, regulatory T cells (Tregs) and intracellular cytokines, including IL-2, interferon (IFN)-γ, tumor-necrosis factor (TNF)-α and IL-4, were measured prior to and at 12, 24, 36 and 48 weeks after treatment with ETV or ADV. The cytokines were increased from 24 to 48 weeks after treatment. Higher levels of Th1 cytokines were observed with ETV (n=29) versus ADV (n=28) treatment. By contrast, the numbers of Tregs in both groups were decreased. The altered cytokine profile and cellular component was accompanied by a decrease in HBV DNA levels in both groups, which may contribute to their therapeutic effect in CHB infection. Our findings suggest that the antiviral effect of the drugs may be attributed not only to their direct effect on virus suppression but also to their immunoregulatory capabilities.
Keywords: ADV, cytokine, ETV, HBV, Treg
Introduction
Chronic hepatitis caused by hepatitis B virus (HBV), a hepadnavirus, is estimated to affect 400 million people globally.1 Chronic HBV infection can lead to the development of hepatitis, cirrhosis and hepatocellular carcinoma.2 Substantial evidence exists to indicate that host innate and adaptive immune responses play a crucial role in controlling HBV replication in vivo.1 Based on virological and biochemical parameters, chronic HBV infection can be divided into three natural stages: immune tolerance, immune clearance and inactive HBsAg carrier.1 Only patients in the immune clearance phase are candidates for antiviral therapy. Infection with HBV in adults usually results in self-limiting acute hepatitis, which confers protective immunity and causes no further disease.1 Patients with a chronic HBV infection lack a vigorous, polyclonal and multispecific T-cell response and instead exhibit a weak, ineffective or undetectable virus-specific T-cell response.1 The mechanisms responsible for T-cell tolerance in chronic HBV infection are not completely understood.
Previous studies suggest that the host protective immune response against HBV infection is mainly mediated by CD4+ and CD8+ T cells, which secrete IFN-γ and activated cytotoxic T lymphocytes, which directly eliminate infected cells.3, 4 In addition, type 2 cytokines, such as IL-4 and IL-5, may also be involved in the clearance of circulating virus by promoting the production of neutralizing antibodies against the HBV surface and core antigens.5 In chronic hepatitis B (CHB), the T-cell response and circulating cytokine profile are associated with viral replication and liver function.3 Low doses of virus may trigger Th1 and cytotoxic T lymphocyte responses, whereas high doses of virus induce a Th2-mediated, non-protective humoral response.6 The T-cell response is relatively mild and ineffective in chronically infected patients compared to acute incidence,7 suggesting the development of immune tolerance in these patients.
CD4+CD25+ regulatory T cells are immunosuppressive T cells that play an essential role in controlling immune responses and autoimmunity.4 Recent findings suggest that regulatory T cells (Tregs) may also play a role in regulating immune responses to HBV infection.8 High levels of Tregs have been detected in CHB and are thought to be responsible for the chronicity of hepatitis B infection, probably by inhibiting HBV-specific T-cell responses. However, Treg function also may be beneficial, limiting immune-mediated liver damage.2
The currently available therapeutic drugs for patients with CHB include an interferon-alpha (IFN-α)-based therapy and nucleoside or nucleotide analogs.2 IFN-α has marked immunomodulatory but less pronounced direct antiviral effects.2 Nucleoside and nucleotide analogs, such as lamivudine, adefovir dipivoxil (ADV) and entecavir (ETV), directly inhibit the viral reverse transcriptase and impair viral replication.2
Nucleoside and nucleotide analogs represent the following three structural classes with respect to the ribose isostere: l-ribose-configured nucleosides, such as lamivudine and telbivudine; acyclic or alkyl chain isosteres administered as prodrugs of phosphonates, such as ADV; and a novel cyclopentyl isostere, such as ETV. Only ETV has the hydrogen bond between F88 and the 3′-hydroxyl group. ADV and ETV are associated with a low frequency of resistance and strong antiviral capabilities. They inhibit both the priming and the elongation steps of viral DNA replication. In clinical studies, ETV displayed higher intrinsic potency than other nucleotide analogs.9, 10 In hepatitis e antigen (HBeAg)-positive patients, ADV has been reported to suppress HBV replication by stimulating virus-specific CD4+ T-cell reactivity and IFN-γ production.11 In a phase 3, double-blind clinical trial involving HBeAg-positive CHB patients, 52 weeks of ETV treatment led to a 21% seroconversion with no viral resistance.9 Continued clinical benefits, such as reduced HBV DNA levels and alanine aminotransferase (ALT) normalization, have been observed with 1 mg/day of ETV for up to 96 weeks in lamivudine refractory HBeAg-positive CHB patients.12
The relationship between the role of cytokines, Tregs, viral suppression and biochemical parameters in immune modulation after ETV or ADV therapy is not well established. The aim of this study was to evaluate the host immune response and compare the clinical effect of either ETV or ADV standard-of-care nucleoside/tide analog monotherapy in chronic HBV patients.
Patients and methods
Study patients
A total of 57 CHB patients at Jilin University First Hospital and 20 healthy controls were included in the study. CHB patients were randomly assigned to an initial treatment regimen of either ETV (0.5 mg per os, once a day) or ADV (10.0 mg per os, once a day) for 48 weeks. Twenty-nine patients were treated with ETV and 28 patients were treated with ADV. Th1 and Th2 cytokines, including IL-2, IFN-γ, tumor-necrosis factor (TNF)-α and IL-4, and Tregs were measured before treatment and at 24 and 48 weeks after treatment. Viral suppression was evaluated by measurement of HBV DNA along with the biochemical markers AST and ALT (see supplementary materials).
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review committee. Informed consent was obtained from all study subjects.
Flow cytometric analysis and intracellular cytokine staining
Blood cells were stimulated and cytokine-secreting cells were analyzed using flow cytometry according to a previously reported protocol.13 For the analysis of intracellular cytokines, 1000 µl of blood was diluted with Iscove's modified Dulbecco's medium (1∶1 volume). The diluted whole blood was stimulated with 50 ng/ml of phorbol 12-myristate 13-acetate (Sigma Chemical Co., St Louis, MO, USA) and 2 µg/ml of ionomycin for 6 h. Two hours before the cells were collected, 10 µg/ml of brefeldin A was added. The cells were then stained with antibodies to surface markers (anti-CD3-PerCP, anti-CD8-FITC or anti-CD8-PE). After cells were fixed and permeabilized with the fixation reagent (Caltag Laboratories, Burlingame, CA, USA) and permeabilized reagent (Caltag Laboratories) anti-IL-2-FITC, anti-TNF-α-FITC, anti-IL-4-PE, anti-IFN-γ-PE or isotype-matched control antibodies were added for 30 min. Data were acquired on a flow cytometer (FACSCalibur; Beckton Dickinson, San Jose, CA, USA), and analysis was conducted using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Estimation of T-cell subsets and Tregs
The CD4+ and CD8+ T lymphocyte subsets were determined in uncoagulated peripheral blood of CHB patients and healthy controls. Fluorescence-conjugated antibodies against CD4, CD8 and CD3 (BD Biosciences or eBioscience, San Diego, CA, USA) were added to tubes containing 100 µl of whole blood. The samples were gently vortexed in an upright position for 5 s and incubated at room temperature for 1 h. The absolute number of CD3+CD4+ and CD3+CD8+ T lymphocytes was estimated on a FACSCalibur using FlowJo software.
Treg numbers were estimated based on the previously published staining method of Liu et al.14 Each sample of isolated lymphocytes was washed and resuspended in a staining buffer containing phosphate-buffered saline and 1% bovine serum albumin, and stained with PerCP-conjugated anti-CD4 and FITC-conjugated anti-CD25 (Becton Dickinson). The cells were fixed and permeabilized with Fix/Perm buffer (eBioscience), washed with permeabilization buffer (eBioscience), blocked with normal rat serum, stained with PE-conjugated anti-Foxp3 (Foxp3 Staining Set, clone PCH101; eBioscience) and analyzed on a fluorescence-activated cell sorter (FACSCalibur) using FlowJo software.
Virological assessments and biochemical measurements
HBV DNA levels were measured using a luciferase quantitation kit (Roche, Mannheim, Germany) at 24 and 48 weeks after treatment. HBsAg, anti-HBs, HBeAg and anti-HBe were determined by commercial MUREX enzyme immunoassay kits (Abbott Laboratories, North Chicago, IL, USA). ALT and aspartate aminotransferase were also measured at baseline and at 24 and 48 weeks after treatment.
Statistical analyses
All data were analyzed using SAS 8.0 software. Student's t-test and chi-square test were used for comparison of demographical data, and the signed-rank test, Kruskal–Wallis test and Spearman correlation were used for analysis of immune and antiviral parameters from ADV or ETV treated groups. Cytokines and Treg values in conjunction with HBeAg status (+ve or −ve) were analyzed in patients and healthy control groups with Kruskal–Wallis, chi-square and Fisher's exact tests. Values of P<0.05 were considered statistically significant.
Results
Demographic background
All of the 57 CHB patients completed the 48 weeks of treatment (29 on ETV and 28 on ADV). The mean ages of the study participants were 40.64±9.26 years in the ADV-treated group and 43.10±8.10 years in the ETV-treated group. There was no statistically significant difference in mean age between the two groups. Male patients predominated, with 82.1% in the ADV group and 82.8% in the ETV group, and 50.0% of the ADV- and 58.6% of the ETV-treated patients were nucleoside-naive. Sixty-nine percent of ETV and 82.1% of ADV-treated patients were HBeAg-positive (Table 1).
Table 1. Demographic profile of study participantsa.
| Parameter | Adefovir | Entecavir |
|---|---|---|
| No. of patients | 28 | 29 |
| Age (years) | ||
| Mean | 40.64 | 43.10 |
| SD | 9.26 | 8.10 |
| Range | 22–57 | 30–61 |
| Sex, N (%) | ||
| Male | 23 (82.1) | 24 (82.8) |
| Female | 5 (17.9) | 5 (17.2) |
| HBV DNA (copies/ml) | ||
| Mean | 190 941 617 | 1 171 036 392 |
| SD | 407 881 925 | 5 296 696 665 |
| Prior nucleoside exposure | ||
| Naive | 14 (50.0) | 17 (58.6) |
| Experienced | 14 (50.0) | 12 (41.4) |
| HBeAg | ||
| Positive | 23 (82.1) | 20 (69.0) |
| Negative | 5 (17.9) | 9 (31.0) |
Demographic variables between treatment groups were not statistically significant.
Abbreviations: HBeAg, hepatitis e antigen; HBV, hepatitis B virus.
Th1 and Th2 cytokine-secreting T cells and Tregs in chronic HBV patients
The cytokine profile in CHB patients and healthy controls was measured. At baseline, the levels of Th1 and Th2 cytokines, IL-2, IFN-γ, TNF-α and IL-4 were markedly lower in CHB patients compared to healthy controls. This study also confirmed that both Th1 and Th2 immune responses were functionally impaired in chronic HBV patients. Before treatment, Tregs and total CD3+CD4+ T cells were higher in both treatment groups than in healthy controls. However, the CD3+CD8+ T-cell subset was lower in both treatment groups compared to controls, suggesting a dominant immune tolerance in the CHB patients (Table 2).
Table 2. Cytokine-producing T cells and T-cell subsets in patients and healthy controls.
| Parameter | Patient (no. 57) | Control (no. 20) |
|---|---|---|
| IL-4 (% CD3+CD4+) | 1.97 (0.54–5.2) | 5.69 (1.99–14.22) |
| IFN-γ (% CD3+CD4+) | 2.18 (0.53–8.02) | 5.73 (3.78–12.09) |
| IL-2 (% CD3+CD4+) | 2.01 (0.17–6.2) | 12.16 (4.44–17.82) |
| TNF-α (% CD3+CD4+) | 2.48 (0.92–13.21) | 15.75 (7.25–27.41) |
| CD3+CD4+ (% lymphocytes) | 55.57 (21.89–77.78) | 38.6 (18.9–45.6) |
| CD3+CD8+ (% lymphocytes) | 16.61 (7.95–51.3) | 29.95 (11.4–35.2) |
| CD4+CD25+Foxp3+ (% lymphocytes) | 9.52 (4.13–17.02) | 3.65 (1.52–7.14) |
Data show the median (range); P values for all patients were statistically different from controls (P<0.0001).
Abbreviations: IFN, interferon; TNF, tumor-necrosis factor.
Th1 and Th2 cytokine production in patients treated with ADV and ETV
The cytokine profile associated with the nucleoside analog treatment was investigated. Treatment with ADV or ETV for up to 48 weeks continuously increased both Th1 and Th2 cytokine production (Table 3). After 24 and 48 weeks of therapy, the levels of IFN-γand IL-2 were significantly increased in the ETV treatment group compared to those in the ADV group (P<0.001, P<0.001, P=0.0006 and P<0.001). Among the Th1 cytokines, the increase in IFN-γ production was more pronounced in the ETV group compared to that in the ADV group. After 24 and 48 weeks of treatment, the mean change in IFN-γ was twofold higher in the ETV group compared to that in the healthy controls. TNF-α levels were also increased in both treatment groups, and ETV treatment induced higher TNF-α secretion than ADV treatment at 24 weeks (P=0.0034). However, the difference was less significant at 48 weeks.
Table 3. Effect of ADV and ETV treatments on cytokine production.
| Parameter (% CD3+CD4+) | Adefovir (no. of patient 28) | Entecavir (no. of patient 29) | Difference between treatments (P) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 24 weeks | 48 weeks | Baseline | 24 weeks | 48 weeks | ||
| IL-2 | 2.22 (0.53–6.2) | 6.01 (2.11–11.34) | 10.77 (4.53–42.26) | 1.84 (0.17–6.12) | 10.04 (2.3–27.27) | 14.83 (8.15–55.34) | <0.001* 0.0006† |
| IFN-γ | 2.18 (0.65–5.57) | 6.64 (3.49–13.19) | 12.0 (7.61–18.6) | 2.18 (0.53–8.02) | 15.16 (9.13–34.02) | 22.23 (9.92–38.56) | <0.001* <0.001** |
| TNF-α | 2.18 (1.05, 5.11) | 9.35 (3.96–25.33) | 14.41 (5.27–31.45) | 2.67 (0.92–13.21) | 14.06 (3.95–60.83) | 18.69 (8.02–40.39) | 0.0034* 0.1649** |
| IL-4 | 2.11 (0.54–3.77) | 9.16 (4.56–20.42) | 13.35 (5.42–26.59) | 1.90 (0.68–5.2) | 10.74 (4.93–44.54) | 13.07 (8.93–23.48) | 0.1254* 0.8606** |
Data show the median (range); P values for all treatment groups were statistically different from baseline (P<0.0001).
*P values for baseline and 24 weeks.
**P values for baseline and 48 weeks.
Changes were calculated as the week 24 or 48 value minus the baseline value.
Abbreviations: ADV, adefovir dipivoxil; ETV, entecavir; IFN, interferon; TNF, tumor-necrosis factor.
Analysis of Th2 cytokines showed higher IL-4 production in both treatment groups compared to that in the healthy controls at both 24 and 48 weeks but no significant difference between the treatment groups.
Effect of ETV and ADV treatments on Tregs and T-cell subsets
The effect of ADV and ETV treatments on Tregs and T-cell subsets in peripheral blood after 24 and 48 weeks of treatment is shown in Table 4. The CD3+CD8+ T-cell numbers were higher in the ETV group than in the ADV group after 48 weeks of treatment (P=0.0071). The CD3+CD4+ and CD4+CD25+ Foxp3+ T-cell subsets showed no significant changes between the ETV and the ADV treatment groups.
Table 4. Effect of ADV and ETV treatments on Tregs and T-cell subsets.
| Parameter (% lymphocytes) | Adefovir (no. of patient 16) | Entecavir (no. of patient 22) | Difference between treatments (P) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 24 weeks | 48 weeks | Baseline | 24 weeks | 48 weeks | ||
| CD3+CD4+ | 58.20 (35.44–76.20) | 56.68 (32.35–74.34) | 62.46 (44.65–84.97) | 54.72 (21.89–77.72) | 52.79 (32.75–72.65) | 60.66 (48.06–70.83) | 0.4965*0.4965** |
| P | 0.782a | 0.029b | 0.782a | 0.1838b | |||
| CD3+CD8+ | 18.76 (10.98–37.09) | 19.75 (7.79–32.17) | 15.78 (5.51–39.97) | 14.27 (7.95–51.30) | 19.67 (11.94–29.66) | 22.98 (7.44–40.45) | 0.1393*0.0071** |
| P | 0.9399a | 0.3028b | 0.0074a | 0.001b | |||
| CD4+CD25+Foxp3+ | 8.72 (5.58–17.02) | 6.72 (2.79–11.30) | 6.47 (2.78–9.89) | 10.10 (4.13–14.72) | 6.55 (3.97–8.09) | 4.83 (1.88–9.78) | 0.2089*0.1157** |
| P | 0.011a | 0.0162b | <0.001a | <0.001b | |||
| Ratio (Tregs/CD3+CD4+) | 0.15 (0.097–0.168) | 0.12 (0.09–0.15) | 0.10 (0.06–0.11) | 0.18 (0.14–0.21) | 0.12 (0.1–0.15) | 0.08 (0.04–0.11) | 0.4247*0.1738** |
| P | 0.021a | 0.0122b | <0.001a | <0.001b | |||
Data show medians (range).
Statistical changes at 0–24 weeks.
Statistical changes at 0–48 weeks.
*P values for baseline and 24 weeks.
**P values for baseline and 48 weeks.
Abbreviations: ADV, adefovir dipivoxil; ETV, entecavir; IFN, interferon; TNF, tumor-necrosis factor; Tregs, regulatory T cells.
In addition, the ratio of Tregs within the CD3+CD4+ T-cell subset decreased after treatment with ADV or ETV for 48 weeks, but the ratio showed no statistically significant differences between the two treatment groups at 24 or 48 weeks (P=0.4247 and P=0.1738, respectively) (Figure 1).
Figure 1.
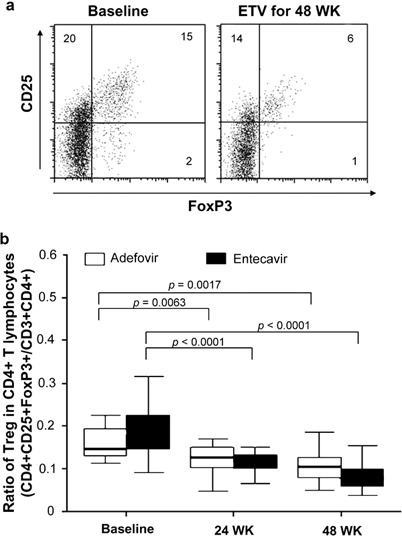
Analysis of Tregs and T-cell subsets during antiviral therapy. (a) FACS was performed to assess the populations of Tregs and T-cell subsets in PBL. Data shown are representative of baseline and post-treatment. (b) The effect of ADV and ETV treatments on the ratio of CD4+CD25+ Foxp3+ and CD3+CD4+ T cells was analyzed. ADV, adefovir dipivoxil; ETV, entecavir; FACS, fluorescence-activated cell sorting; PBMC, peripheral blood lymphocytes; Tregs, regulatory T cells.
Correlation of cytokines and Tregs with HBeAg status
We further determined the correlation between cytokine levels and Treg numbers with HBeAg status. Most of the cytokines at baseline and at 24 and 48 weeks of treatment were largely comparable between the two treatment groups in conjunction with their HBeAg status. However, at 48 weeks, a significantly higher level of IFN-γ was observed in the HBeAg-negative patients than in the HBeAg-positive patients in the ETV-treated group (P=0.0495) (Figure 2). Furthermore, IL-2 and TNF-α levels were significantly higher in HBeAg-negative patients than in HBeAg-positive patients prior to ETV treatment (P=0.0095 and P=0.0142, respectively). In the ADV group at week 48, the level of IL-4 was significantly higher in HBeAg-negative patients than in HBeAg-positive patients (P=0.0363). There was no statistically significant correlation between cellular component and HBeAg status at baseline or after 24 or 48 weeks of treatment.
Figure 2.
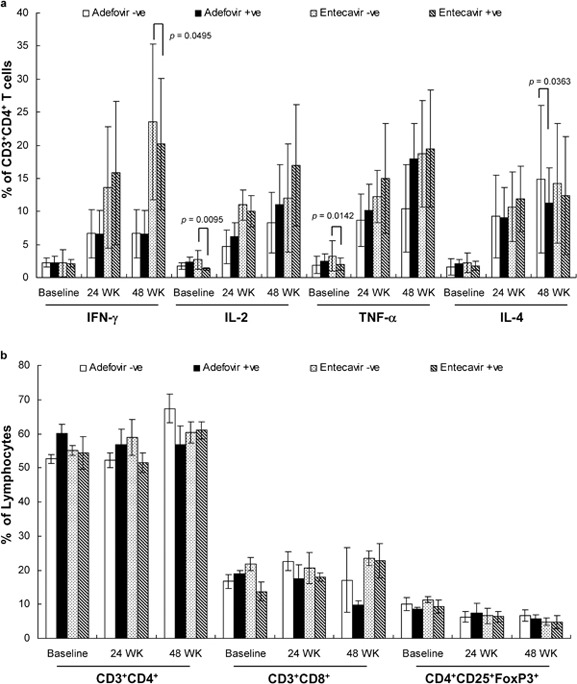
Effect of ADV and ETV treatments on cytokine production in patients with positive and negative HBeAg. Cytokine production (a) and T-cell subsets from total lymphocytes (b) were assessed at the time points of treatment as indicated and analyzed according to the initial measure of HBeAg in each patient's serum. HBeAg-positive and -negative are indicated as +ve and −ve, respectively. ADV, adefovir dipivoxil; ETV, entecavir; HBeAg, hepatitis e antigen; IFN, interferon; TNF, tumor-necrosis factor.
The effect of ADV or ETV treatment on Th1 and Th2 cytokines in patients with undetectable or detectable loads of HBV DNA
Both ETV and ADV treatments were found to suppress viral DNA at the end of 24 and 48 weeks of therapy. There was no statistically significant difference in mean HBV DNA values between the two treatment groups at baseline or after treatment. More patients in the ETV group showed undetectable viral loads (i.e., HBV DNA was at 0 or <300 copies/ml) compared to those in the ADV group at both 24 weeks (45% in the ETV group versus 32% in the ADV group) and 48 weeks (59% in the ETV group versus 43% in the ADV group). The percentage of HBeAg-positive patients was comparable in ADV (61%) and ETV (48%) groups at 48 weeks of treatment (Table 5).
Table 5. Effect of ADV and ETV on HBV DNA load and HBeAg status.
| Adevofir (no. of patients 28) | Entecavir (no. of patients 29) | Difference between treatments (P) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 24 weeks | 48 weeks | Baseline | 24 weeks | 48 weeks | |||
| HBV DNA (log10 copies/ml) | 50% median | 7.46 | 4.01 | 4.38 | 7.33 | 4.05 | 3.26 | 0.345* |
| 5% quantiles | 3.87 | 2.46 | 2.46 | 4.13 | 2.46 | 2.46 | 0.258** | |
| P | 0.0001a | 0.0001b | 0.0001a | 0.0012b | ||||
| HBV DNA | ||||||||
| <300 copies/ml | N | 0 | 9 | 12 | 0 | 13 | 17 | 0.325* |
| >300 copies/ml | N | 28 | 19 | 16 | 29 | 16 | 12 | 0.234** |
| P | 0.001a | 0.0001b | 0.0001a | 0.0001b | ||||
| HBeAg status | ||||||||
| +ve | N | 23 | 17 | 17 | 20 | 16 | 14 | 0.6718* |
| −ve | N | 5 | 11 | 11 | 9 | 13 | 15 | 0.3459** |
| P | 0.076a | 0.076b | 0.279a | 0.110b | ||||
Data show median (range).
Statistical changes at 0–24 weeks.
Statistical changes at 0–48 weeks.
*P values for baseline and 24 weeks.
**P values for baseline and 48 weeks.
Abbreviations: ADV, adefovir dipivoxil; ETV, entecavir; HBeAg, hepatitis e antigen; HBV, hepatitis B virus.
In both the ADV and ETV groups separated based on viral load at 48 weeks, IFN-γ levels were higher in patients exhibiting HBV DNA at 0 or <300 copies/ml compared to those in patients with HBV DNA at >300 copies/ml (P=0.019 and P=0.0335, respectively).
The correlation between cytokines and HBV DNA
The correlation between cytokines and HBV DNA in both treatment groups was further analyzed (Figure 3). In the ADV group, HBV DNA decreased from 7.46log10 copies (baseline) to 4.38log10 copies (48 weeks), and the mean level of cytokines (IL-4, IFN-γ, TNF-α and IL-2) was increased in the given order. Similarly, in the ETV group, HBV DNA decreased from 7.33log10 copies (baseline) to 3.26log10 copies (48 weeks), and cytokines (IFN-γ, TNF-α and IL-2 in decreasing order) increased from baseline to 24 weeks and began to stabilize at 48 weeks.
Figure 3.
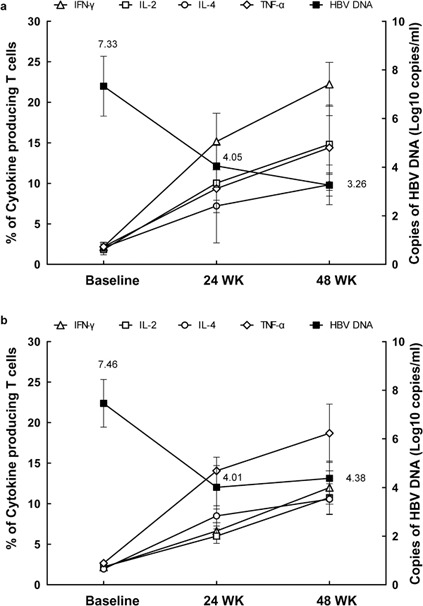
Correlation between cytokine production and HBV DNA load in patients treated with ADV and ETV. The mean values of HBV DNA and indicated cytokines in patients treated with ADV (a) and ETV (b) were shown. ADV, adefovir dipivoxil; ETV, entecavir; HBV, hepatitis B virus; IFN, interferon; TNF, tumor-necrosis factor.
Discussion
The success of CHB therapy is characterized by the suppression of HBV DNA replication and normalization of hepatic transaminases, with a treatment regimen targeting the biochemical and virological responses to reduce disease progress.15 Both ETV and ADV are new generation antiviral drugs.16 However, the therapeutic effect and immunomodulatory function of the drugs have not been systemically studied and compared in CHB patients. In a double-blind phase 3 trial in HBeAg-positive CHB patients, secondary efficacy end points of ETV treatment (0.5 mg, once daily) at 48 weeks revealed that 67% of patients had undetectable HBV DNA.9 In a comparative study of ETV and lamivudine in 709 HBeAg-positive CHB patients, none of the ETV-treated patients had HBV DNA levels at ≥105 copies/ml at the end of the first year, whereas 10% of lamivudine-treated patients had HBV DNA levels at ≥105 copies/ml at the end of the second year.17 Lee et al. have reported that viral suppression was higher after 48 weeks of ADV therapy in treatment-naive patients (n=38) than in lamivudine-resistant patients (n=57).18 In our study, we systematically compared the effect of treatment with ETV and ADV on CHB infection and confirmed that both therapies effectively led to viral remission. However, the therapeutic effect was modestly better in the ETV group compared to that in the ADV group at 48 weeks. This is consistent with a previous finding (ETV-079, E.A.R.L.Y.), which showed the superiority of ETV over ADV treatment for reducing HBV DNA loads as early as day 10 after therapy.19 At baseline, the HBV DNA level was higher in the ETV group than in the ADV group, but the difference was not statistically significant (Table 1). However, at week 24, 45% of ETV patients had <300 copies/ml of HBV DNA compared to 32% in the ADV group at week 24. This shows a 1.4-fold difference between the ETV and ADV groups. At week 48, 59% of the ETV-treated patients had <300 copies/ml of HBV DNA compared to 43% in the ADV-treated group. These results demonstrate the superior antiviral capacity of ETV, which could be due to its more efficient drug activity compared to ADV (Table 5).
A weak T-cell response is a major feature of CHB and is thought to be responsible for the perpetuation of HBV replication.11 However, the causes of immune tolerance in CHB are still unclear. Our study demonstrated that the impaired immunity in CHB patients was accompanied by an augmented Treg population. It has been reported that elevated numbers of Tregs can inhibit HBV-specific immune responses in a dose-dependent manner.8 Depletion of Tregs has been shown to increase IFN-γ production by HBV Ag-stimulated peripheral blood mononuclear cells.20 Therefore, it is likely that the HBV-mediated enhancement in Tregs may, at least in part, contribute to immune tolerance and persistent infection in CHB patients.
Interestingly, we found that the decline in HBV DNA replication during ETV and ADV treatments was accompanied by increased immune activation (Figure 3), suggesting that both therapies are able to break virus-mediated immune tolerance. This was shown by the increased types I and II cytokine production and decreased Treg numbers. Enhanced production of Th1 cytokines (IFN-γ, TNF-α and IL-12) and reduced viremia have been reported in CHB patients treated with a combination therapy of ribavirin and IFN-γ.21 ETV therapy in particular led to a twofold increase in IFN-γ production compared to healthy controls at 24 and 48 weeks of treatment. In contrast, the proportion of Tregs dramatically declined to levels comparable to healthy controls after 24 weeks of treatment with ETV. IFN-γ is a major protective cytokine and has been shown to inhibit HBV DNA transcription and replication.11 Studies in HBV-transgenic mice crossed with mice genetically deficient for IFN-γ and TNF-α have shown that IFN-γ downregulates HBV replication by IFN-α/β-independent pathways.22 ADV treatment has been reported to increase IFN-γ production by CD4+ T cells.11 Our study showed that, at 48 weeks, IFN-γ was significantly higher in HBeAg-negative patients than in HBeAg-positive patients in the ETV treatment group, and ETV therapy reduced the number of HBeAg-positive patients from 20 to 14 (Table 5). These data suggest that ETV treatment promoted higher IFN-γ production, and this may be responsible for improved HBeAg clearance. Increased HBeAg and HBV DNA clearance and improved clinical outcome have been reported with IFN-γ therapy in HBeAg-positive CHB patients.15, 23 IFN-γ appears to have the ability to skew the HBeAg-specific Th1/Th2 cell balance toward the Th1 subset.5
TNF also decreases the secretion of viral particles, thus inhibiting hepatic disease progression by HBV infection.24 Moreover, HBV gene expression is inhibited by IL-2 administration through TNF-α induction.25 In the current study, we noted that treatment with both ETV and ADV increased IL-2 and TNF-α production, and the effect of ETV on IL-2 and TNF-α production at 24 and 48 weeks was more pronounced than that of ADV. This suggests an immunological mechanism for the greater antiviral effect of ETV treatment compared to ADV treatment in CHB patients.
HBeAg loss is another important feature of effective CHB therapy.15 ADV and ETV treatments effectively reduced the HBeAg-positive status of patients. ADV has been reported to induce a T-cell response and reduce HBeAg levels11 ADV treatment has also been shown to result in a loss of HBeAg in 24% of CHB patients with a seroconversion rate of 12%.26 Similarly, ETV has been shown to cause HBeAg seroconversion in 11% of the CHB patients in the second year of therapy.17 However, the loss of HBeAg status in combination with incomplete viral suppression may lead to the emergence of drug resistant mutants.27
Treatment with ADV and ETV decreased the number of patients with ALT >80 U/l. As elevated ALT levels in CHB indicate hepatocellular damage, lowering ALT levels by both ADV and ETV treatments suggests functional hepatic recovery.
The enhanced immune response, especially the type I T-cell response, associated with ETV and ADV treatments, suggests that the antiviral effect of the drugs may be attributed not only to their direct effect on virus suppression but also to their immunoregulatory capabilities. Although the underlying mechanism remains to be understood, reduced viral load and diminished HBV-related Treg numbers may favor the restoration of antiviral immunity in CHB. Furthermore, the altered cytokine levels and T-cell subsets in CHB patients, in particular the Tregs, may provide additional biomarkers for disease diagnosis and prediction. We also noted that ETV triggers a stronger type I immune response and antiviral effect than ADV. Further study is needed to understand the mechanism underlying the immunoregulatory function of these molecules.
Acknowledgments
We gratefully acknowledge Dr Voo (The University of Texas MD Anderson Cancer Center, USA) for technical assistance and help in developing the content of the manuscript. This work was supported by grants from the Eleventh Five-Year Plan for AIDS and viral hepatitis, prevention and treatment of infectious diseases and other major science and technology (No. 2008ZX10002-004), the Ministry of Health Clinical Disciplines (No. 20073531), the National Natural Science Foundation of China (No. 30771912, 30972610 and 30972611), Jilin Province Science and Technology Agency (No. 200705128) and a BMS grant for manuscript development.
Supplementary Information
References
- WHO . Hepatitis B. Geneva; WHO; 2008. [Google Scholar]
- Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439–1449. doi: 10.1099/vir.0.81920-0. [DOI] [PubMed] [Google Scholar]
- Huang CF, Lin SS, Ho YC, Chen FL, Yang CC. The immune response induced by hepatitis B virus principal antigens. Cell Mol Immunol. 2006;3:97–106. [PubMed] [Google Scholar]
- Billerbeck E, Bottler T, Thimme R. Regulatory T cells in viral hepatitis. World J Gastroenterol. 2007;13:4858–4864. doi: 10.3748/wjg.v13.i36.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich DR, Schodel F, Hughes JL, Jones JE, Peterson DL. The hepatitis B virus core and e antigens elicit different Th cell subsets: antigen structure can affect Th cell phenotype. J Virol. 1997;71:2192–2201. doi: 10.1128/jvi.71.3.2192-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- Chisari FV. Cytotoxic T cells and viral hepatitis. J Clin Invest. 1997;99:1472–1477. doi: 10.1172/JCI119308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop JN, van der Molen RG, Kuipers EJ, Kusters JG, Janssen HL. Inhibition of viral replication reduces regulatory T cells and enhances the antiviral immune response in chronic hepatitis B. Virology. 2007;361:141–148. doi: 10.1016/j.virol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Chang TT, Gish RG, de MR, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- Cooksley H, Chokshi S, Maayan Y, Wedemeyer H, Andreone P, Gilson R, et al. Hepatitis B virus e antigen loss during adefovir dipivoxil therapy is associated with enhanced virus-specific CD4+ T-cell reactivity. Antimicrob Agents Chemother. 2008;52:312–320. doi: 10.1128/AAC.00467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M, Yurdaydin C, Simsek H, Silva M, Liaw YF, Rustgi VK, et al. Entecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeks. Hepatology. 2008;48:99–108. doi: 10.1002/hep.22323. [DOI] [PubMed] [Google Scholar]
- Duramad P, McMahon CW, Hubbard A, Eskenazi B, Holland NT. Flow cytometric detection of intracellular TH1/TH2 cytokines using whole blood: validation of immunologic biomarker for use in epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2004;13:1452–8. [PubMed] [Google Scholar]
- Liu W, Putnam AL, Zhou XY, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34:1225–1241. doi: 10.1053/jhep.2001.29401. [DOI] [PubMed] [Google Scholar]
- Buster EH, Janssen HL. Antiviral treatment for chronic hepatitis B virus infection—immune modulation or viral suppression. Neth J Med. 2006;64:175–185. [PubMed] [Google Scholar]
- Gish RG, Lok AS, Chang TT, de Man RA, Gadano A, Sollano J, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133:1437–1444. doi: 10.1053/j.gastro.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385–1391. doi: 10.1002/hep.21189. [DOI] [PubMed] [Google Scholar]
- Leung N, Peng CY, Sollano J, Lesmana L, Yuen MF, Jeffers L, et al. Entecavir (ETV) results in higher HBV DNA reduction versus adefovir (ADV) in antiviral-naive HBeAg+ adults with high HBV DNA: week 96 results (E.A.R.L.Y. Study) [abstract] J Hepatol. 2008;48 (Suppl 2):S373–S374. [Google Scholar]
- Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, et al. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- Ren FY, Jin H, Piao XX, Piao FS. Ribavirin and IFN-alpha combination therapy induces CD4+ T-cell proliferation and Th1 cytokine secretion in patients with chronic hepatitis B. World J Gastroenterol. 2007;13:5440–5445. doi: 10.3748/wjg.v13.i41.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClary H, Koch R, Chisari FV, Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol. 2000;74:2255–2264. doi: 10.1128/jvi.74.5.2255-2264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, et al. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med. 1996;334:1422–1427. doi: 10.1056/NEJM199605303342202. [DOI] [PubMed] [Google Scholar]
- Puro R, Schneider RJ. Tumor necrosis factor activates a conserved innate antiviral response to hepatitis B virus that destabilizes nucleocapsids and reduces nuclear viral DNA. J Virol. 2007;81:7351–7362. doi: 10.1128/JVI.00554-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Guilhot S, Chisari FV. Interleukin-2 and alpha/beta interferon down-regulate hepatitis B virus gene expression in vivo by tumor necrosis factor-dependent and -independent pathways. J Virol. 1994;68:1265–1270. doi: 10.1128/jvi.68.3.1265-1270.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- Marcellin P, Chang TT, Lim SG, Sievert W, Tong M, Arterburn S, et al. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2008;48:750–758. doi: 10.1002/hep.22414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


