Abstract
The hygiene hypothesis was proposed more than two decades ago, but its mechanism remains unclear. This review focuses on recent advances in the field, especially on the role played by dendritic cells (DCs) and their modulating effects on various infections and allergic diseases, including allergic asthma. DCs isolated from mice long after the resolution of an infection were reported to have a significant modulating effect on allergen-specific Th2 responses in both in vitro and in vivo systems. These DCs showed DC1-like and/or tolerogenic DC capacity, which allowed for the inhibition of allergic responses by immune deviation (enhancing Th1 response) and immune regulation (through regulatory T-cell and Th2 hyporesponsiveness) mechanisms. These findings represented a significant advance in the elucidation of the mechanisms underlying the hygiene hypothesis. Further investigation on the mechanisms by which DCs are ‘educated' by infectious agents and the influence of the type, time, and extent of infections on this ‘education' process will help us understand immune regulation in disease settings and in the rational design of preventive/therapeutic approaches to allergy/asthma and infections.
Keywords: allergy, asthma, DC, hygiene hypothesis, infection
Introduction
For decades, an inverse relationship between infections and allergic diseases has been observed in most developed countries. The ‘hygiene hypothesis' suggests that exposure to microbial infections or microbial products may inhibit the development or pathogenesis of allergic responses, including asthma.1, 2, 3, 4, 5 Many epidemiological observations related to various infections support this provocative hypothesis.6, 7, 8, 9, 10, 11, 12, 13, 14 In particular, a series of studies have described children who had close contact with farm animals and exhibited significantly lower levels of allergy and asthma later in life. In addition, previous research has found that some bacteria isolated from farm cowsheds have allergy-preventive properties.14, 15, 16, 17 These large scale, relatively well-controlled series of epidemiological studies have provided strong evidence for the imprinting effect of infection on the immune system and on the prevention of allergic diseases. Moreover, a recent, thoroughly performed systematic review and meta-analysis of 23 published papers (up to June 2008) examined the relationship between early life Mycobacterium bovis Bacillus Calmette-Guerin (BCG) exposure, as demonstrated by history of BCG vaccination, tuberculin response and scar diameter, and asthma; the review included 10 cohort, 5 case–control and 8 cross-sectional studies, and it found a clear inhibitory effect of BCG vaccination on the development of asthma.18
In addition to epidemiological observations, experimental studies have also provided support for the hygiene hypothesis.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 For example, BCG infection prevented the development of asthma-like reactions in a murine asthma model.19, 20 Some studies showed that systemic or mucosal administration of killed bacteria, introduction of bacterial CpG, and intramuscular infection with adenovirus also inhibit allergic reactions.21, 22, 23, 24, 25, 26 We have shown that the Chlamydia trachomatis mouse pneumonitis strain, more recently called C. muridarum, and BCG infections have a significant imprinting effect on newborn or young adult C57BL/6 and Balb/c mice; the imprinting inhibited allergy/asthma induced by natural or model allergens later in life.31, 32, 33, 34, 35, 36, 37, 38 In particular, C. muridarum infection had a strong imprinting effect on the inhibition of allergy, even after the infection had long been cleared.35, 36, 37, 38 Moreover, recent studies demonstrated an inhibitory effect of parasitic infections on allergy/asthma.27, 28, 29, 30
However, this hypothesis currently faces numerous challenges,39, 40, 41, 42, 43, 44, 45 because some epidemiological studies have been performed on populations with different genetic backgrounds, culture, economic development stage, health care system and geographical location, and they have shown either no relationship between infection and allergy, especially asthma, or an increased risk of allergic asthma in association with infection.40, 41, 42, 43, 44, 45, 46 In experimental models, some infections, such as acute infections, exacerbated already established allergy/asthma or enhanced allergic reactions when the allergen and infectious agents were co-administered.39 In general, the imprinting effect of previous bacterial infections on the inhibition of allergy and asthma development or pathogenesis is more commonly observed than the effect of recent or concurrent infections.
Immunological basis for infection-mediated inhibition of allergy
The underlying mechanisms for the inverse relationship between exposure to microbes or microbial substance and development of allergy are still ill defined. However, from an immunologic point of view, the involvement of innate and adaptive immune responses, especially the interaction between these two types of immune responses, is likely to occur. In particular, microbial infections may modulate Th2-like allergic responses by promoting immune deviation (toward a Th1 response) and/or enhancing immune regulation, depending on the nature of the infectious agents, the time and duration from exposure to infection, the extent and stages of the infection, and the genetic background of the host.
Immune deviation
Since allergy is a typical Th2-type disease, many bacterial infections induce a Th1 response, so earlier studies mainly focused on immune deviation.47 The shift from a Th1 to Th2 response was hypothesized to be due to a reduction in infection load and was discussed as the reason for the increased prevalence of allergic diseases. The groundbreaking study by Shirakwa et al. provided evidence to support that hypothesis by showing an inverse correlation between the intensity of tuberculin reaction and the likelihood of developing an atopic disease in later life.6 Many subsequent studies also showed supportive data for the importance of immune deviation in the prevention of allergy.19, 20, 24, 25, 26
Immune regulation
Although the relationship between the Th1-promoting effects of infections and the consequent inhibition of allergy provides an explanation for the hygiene hypothesis, the mechanism has been challenged because some Th1-type autoimmune diseases have also increased over the past decades. Moreover, some pro-Th2 parasitic infections were found to also inhibit allergic diseases. Therefore, a lack of, or diminished, Th1-type promoting effect induced by an infection is unlikely to be the predominant mechanism for the observed increase of allergic diseases in developed countries. In addition, although many epidemiological and experimental studies have shown that pro-Th1 infections/vaccinations, such as BCG, have an inhibitory effect on the development of allergic responses, not all studies showed the same effect. Rather, the lack of a regulatory mechanism for the development of allergic responses may be involved and may even be more relevant in certain circumstances.48, 49, 50, 51 The regulatory mechanism likely involves: (i) the induction of various types of regulatory T cells (Tregs), which produce/express IL-10 and/or transforming growth factor (TGF)-β and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4); (ii) the unresponsiveness of effector T cells; and (iii) the apoptosis of allergen-specific Th2 cells.
Tregs play an important role in regulating allergic reactions.52 The two major groups of Tregs include naturally occurring CD4+CD25+ Tregs (nTregs), which mainly develop in the thymus,53, 54 and inducible Tregs, which develop in the periphery from conventional CD4+ T cells. Foxp3 is a typical marker initially identified in nTregs, but it is also expressed by some inducible Tregs.55, 56 The relationship between the Foxp3+ nTregs and Foxp3+ inducible Tregs remains unclear. The inducible Tregs may include IL-10-producing regulatory type 1 (Tr1) cells, TGF-β-producing Th3 cells and converted Foxp3+ Tregs. Th1-like Tregs have also been reported to produce both IL-10 and interferon (IFN)-γ.57 Different types of Tregs may inhibit allergic reactions through antigen-specific and/or antigen-non-specific mechanisms. The role of Tregs in infection-mediated inhibition of allergy has been documented in studies on parasitic infections, especially helminth infections.58, 59, 60, 61, 62, 63 The involvement of Tregs in bacterial infection-mediated modulation of allergy/asthma has been reported, but is much less studied.64, 65, 66 In particular, subcutaneous treatment with heat-killed Mycobacterium vaccae was reported to induce CD4+CD45RBlow T cells, which inhibited asthma through IL-10 and TGF-β production.65, 66
Therefore, microbial infections may modulate Th2-like allergic responses by promoting immune deviation (toward a Th1 response) and/or enhancing immune regulation depending on the nature of the infectious agents, the extent/stages of the infections and the genetic background of the hosts.
Contribution of dendritic cells (DCs) in infection-mediated inhibition of allergy/asthma
Although changes in immune deviation and immune regulation have been documented in numerous studies, until recently, the mechanisms for these changes remained largely unknown. In particular, how the immune deviation happened and how T-cell tolerance/Tregs were generated, as well as their roles in infection-mediated inhibition of allergy/asthma, were unknown. Recently, work from our group and others demonstrated that the modulating effect of infections on the function of DCs is likely the basis of infection-mediated inhibition of allergy and asthma. As a critical link in innate and adaptive immune responses, DCs provide a platform for microbial products to modulate Th2 cell responses.
T-cell responses and DC function
DCs are particularly important antigen-presenting cells because they induce primary T-cell responses and modulate T-cell polarization. DCs are heterogeneous in terms of cell lineage, stage of maturation and function. DCs isolated ex vivo can be grouped into lymphoid/plasmacytoid and myeloid lineages.67 In mice, the lymphoid DCs are CD8α+, while the myeloid lineages are CD8α−. In addition to these two conventional DCs, plasmacytoid DCs (pDCs) have also been identified in humans and mice. pDCs are characterized by their high expression of B220 and mouse pDC antigen, and mouse pDCs have a high level of IFN-α production.68 The relationship between DC phenotype/lineage and the role of DCs in immune regulation, in both humans and mice, is a controversial issue.69, 70, 71, 72, 73 In mice, splenic CD8α+ lymphoid DCs (DC1) were initially found to primarily promote a Th1 response, whereas the CD8α− myeloid DCs (DC2) were found to promote Th2 differentiation.74, 75 However, later studies challenged this one cell type-one type of response concept by showing that DCs with distinct functional properties may emerge from the same precursors,76, 77 and that CD8α+ DCs could also play a regulatory role. Therefore, DCs are believed to be largely plastic in function, and the expression of costimulatory markers and cytokines/molecules is likely to be more important for characterizing functional DC subsets.78, 79, 80, 81, 82, 83, 84, 85, 86
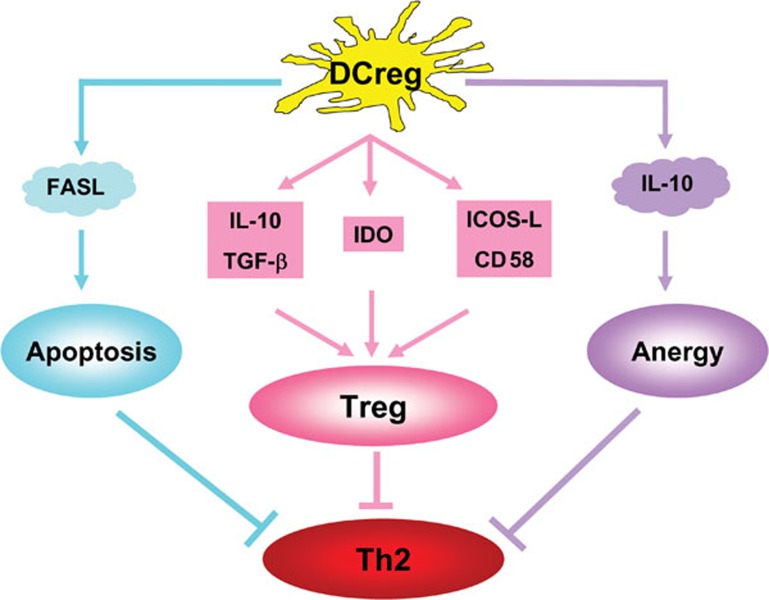
Tolerogenic/regulatory DCs (DCregs), which may cause T-cell tolerance by different mechanisms, including T-cell depletion (apoptosis), T-cell hyporesponsiveness (anergy) and Treg induction, may be involved in the inhibition of allergic Th2 responses (Figure 1). Some CD8α+ DCs express higher levels of Fas ligand (FasL) and are able to cause T-cell apoptosis.87, 88 The apoptosis is mediated by the interaction of FasL on CD8α+ DCs with Fas on activated T cells.89 DCs treated with IL-1090, 91, 92 may induce T-cell anergy. Immature DCs, or DCs with altered maturation status, may induce Treg development. Some specialized DCs can induce Tregs even in their fully mature state. Levings et al. proposed a model of two-step differentiation in Tregs:91 in the first step, naive T cells encounter antigen presented by DCs in the presence of IL-10 and possibly TGF-β, and they become hyporesponsive to the antigen through a cell–cell contact dependent process; in the second step, following repeated antigen exposure, these T cells gain their ability to produce IL-10 and TGF-β, and they mediate suppression through a cytokine-dependent, cell–cell contact-independent mechanism. The fully differentiated Tregs are more potent in their suppressive activity than anergic T cells.
Figure 1.
The mechanisms by which tolerogenic DCs/DCregs inhibit allergic Th2 responses. DCregs induced by infection or exposure to bacterial components can inhibit allergic Th2 responses through three mechanisms: (i) DCregs can express FasL, which leads to Th2 cell apoptosis through FasL–Fas interaction (blue line); (ii) DCregs can induce allergen-specific and allergen-non-specific Tregs through the production of immunoregulatory cytokines such as IL-10 and TGF-β, enzymes such as IDO, and costimulatory surface markers (red line); and (iii) DCregs may induce Th2 cell anergy through the production of IL-10 and other cytokines. →: promoting effect; ⊣: inhibitory effect. DCreg, regulatory dendritic cell; FasL, Fas ligand; ICOS-L, inducible costimulatory ligand; IDO, indoleamine 2,3-dioxygenase; TGF, transforming growth factor; Treg, regulatory T cell.
The molecular basis for the capacity of tolerogenic DCs to induce Tregs may be related to their cytokine production and the expression of intracellular enzymes and surface molecules (Figure 1). The production of IL-10, TGF-β and IFN-α by DCs, either as individual cytokines or in combination, is particularly important for the induction of Tregs.93, 94, 95, 96, 97, 98, 99, 100 Interestingly, an intracellular enzyme indoleamine 2,3-dioxygenase (IDO), which catabolizes tryptophan and triggers cellular stress responses, also participates in the immune regulation of chronic infections.93 An IDO-expressing subpopulation of pDCs showed potent regulatory properties by inducing Tregs.94 In terms of surface markers, certain molecules such as inducible costimulatory ligand (ICOS-L), CD58, OX-2 and programmed death-1 ligands can promote the development of Tregs.93, 101, 102, 103 pDCs appear to be particularly relevant for tolerogenic DCs, which express high levels of ICOS-L, IDO, IFN-α and Toll-like receptor (TLR)-9.
Critical role of DCs in infection-mediated inhibition of allergy
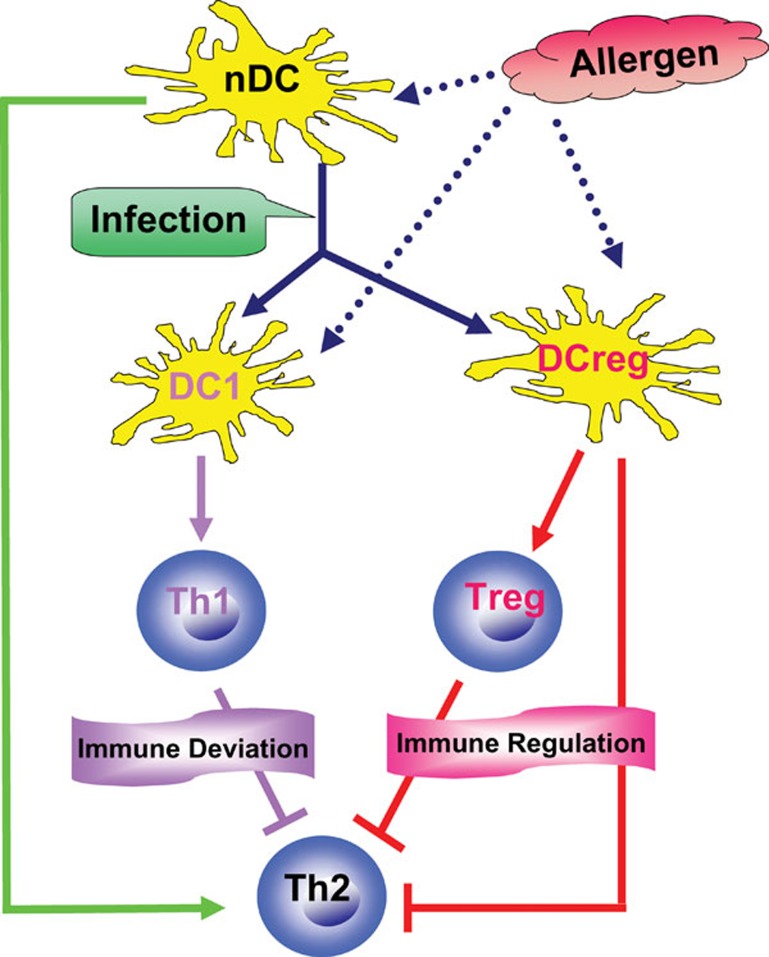
The critical importance of DCs in infection-mediated inhibition of allergy has been shown in recent reports.33, 34, 35, 36, 37, 38, 63 These studies suggested that different infections may ‘educate' DCs to become DC1-like cells, which promote a Th1 response and subsequently inhibit allergic Th2 responses (immune deviation), and/or these DC1-like cells become tolerogenic DCs/DCregs, which induce Tregs or have a direct inhibitory effect on allergic Th2 cells (immune regulation) (Figure 2). We first reported that DCs freshly isolated from C. muridarum-infected mice, but not DCs from naive mice, could inhibit the systemic and cutaneous eosinophilia inflammation normally induced by exposure to an allergen upon adoptive transfer.33 The reduction of eosinophilia inflammation was associated with a decrease in IL-5 receptor expression on bone marrow cells and a decrease in the production of IL-5 and IL-13 by T lymphocytes. Coculture of DCs with naive CD4+ cells from ovalbumin (OVA) peptide TCR-transgenic mice (DO11.10) showed that DCs from infected mice directed a Th1-dominant response, while DCs from naive mice directed a Th2-dominant, allergen-specific CD4+ T-cell response. Upon analysis, the DCs from infected mice were found to express significantly higher levels of TLR-9 and IL-12 compared with DCs from naive mice. More recently, we found that adoptively transferred DCs from BCG-infected mice, but not from naive mice, significantly inhibited established allergic airway eosinophilia and mucus overproduction.38 The inhibitory effect was correlated with a significant increase in Th1-related cytokines (IFN-γ and IL-12) and with a significant decrease in allergen-driven Th2 cytokines (IL-4, -5, -9 and -13). The DCs from BCG-infected mice produced significantly higher levels of IL-12 and expressed higher levels of TLRs than DCs from naive mice. The blockade of IL-12 dramatically reduced the Th1-enhancing effect of the adoptively transferred DCs, and this reduction was associated with a reversal of the inhibitory effect on allergic airway inflammation and Th2 cytokine responses. Therefore, DCs from mice infected with bacteria can modulate allergic responses through immune deviation.
Figure 2.
The central role of DCs in infection-mediated inhibition of allergic responses. Allergens presented by nDCs induce allergic Th2 responses (green line). DCs educated by in vivo infection may become DC1-like cells (DC1) and/or tolerogenic DCs/DCregs. Allergens presented by DC1-like cells can activate Th1 cells, which inhibit allergic Th2 responses (immune deviation), whereas those presented by DCregs can induce Tregs or have a direct suppressing effect, thus inhibiting allergic Th2 responses (immune regulation). →: promoting effect; ⊣: inhibitory effect. DC, dendritic cell; DCreg, regulatory dendritic cell; nDC, normal dendritic cell; Treg, regulatory T cell.
In addition to the effect on immune deviation, DCs from infected individuals can play a role as tolerogenic DCs/DCregs that induce Tregs or directly inhibit allergic responses.34, 36, 38, 57, 63, 64, 66, 104 We recently showed that the adoptive transfer of DCs from Schistosoma japonicum-infected mice dramatically decreased airway allergic inflammation and was associated with a significant decrease in IL-4/IL-5 production and an increase in IL-10 production.63 The DCs' modulating effect on allergy was related to enhanced CD4+CD25+Foxp3+ Treg responses (Liu Z, Liu P, Yang X, unpubl. data). In addition, bacterial infections can also induce tolerogenic DCs, which inhibit allergic responses. Bacterial products, such as filamentous hemagglutinin expressed by Bordetella pertussis, have been found to enhance IL-10 production by DCs and promote Tr1 cells.64 Tr1 clones may express T1/ST2 and CC chemokine receptor 5 and secrete high levels of IL-10, but not IL-4 or IFN-γ. In addition, M. vaccae was reported to induce a population of IL-10- and TGF-β-producing pulmonary DCs, which may either directly inhibit asthma or indirectly inhibit asthma through the generation of IL-10-producing Tr1 cells.66 We found that DCs isolated from C. muridarum-infected mice during later stages of infection or after the resolution of the infection produced significantly higher levels of IL-10 and IL-12 than those from naive mice.34, 36, 38 Adoptive transfer of the DCs from the previously infected mice inhibited allergen-driven Th2 cytokine responses. Coculture of DCs from C. muridarum-infected mice with CD4+ T cells from OVA peptide TCR-transgenic (DO11.10) mice showed reduced Th2-like cytokine responses. Neutralization of either IL-10 or IL-12 in the coculture of DCs and T cells and in adoptive transfer experiments significantly reduced the inhibitory effect on allergen-driven Th2 responses. Moreover, we found that DCs from Chlamydia-infected mice expressed higher levels of ICOS-L,34 and we found that ICOS-L expression could potentiate the modulating effect by synergizing with the IL-10 produced by these DCs.34 In our study on the adoptive transfer of DCs from BCG-infected mice,38 we found that these DCs showed increased expression of CD8α, costimulatory markers and TLRs, and increased production of IL-10 and IL-12. In vivo neutralization of either IL-10 or IL-12 significantly reversed the inhibitory effect of these DCs on established allergic airway inflammation and Th2 cytokine responses.38 These results suggested that DCs from bacteria-infected mice might contain IL-10-producing tolerogenic DCs in addition to the IL-12-producing DC1-like cells mentioned above.
The imprinting effect of early life exposure to bacterial products on the inhibition of allergy and its relationship with DC function has also been explored.36 Neonatal exposure to killed bacteria has been reported to alter the surface marker, TLR expression and cytokine production by DCs in adulthood. More importantly, DCs from adult mice treated neonatally with killed bacteria induced significantly lower antigen-specific Th2 responses than DCs from sham-treated mice in experiments with the coculture of DCs and T cells and in adoptive transfer experiments. Interestingly, depletion of T cells in vivo largely abolished the phenotypic and functional alterations caused by bacterial exposure, suggesting that the T cells are involved in maintaining the tolerogenic function of DCs. These data demonstrated a central role for DCs in linking early-life exposure to microbial products and the balanced development of immune regulatory functions to allergic reactions and in the involvement of T cells in the imprinting of DCreg function.
An interesting study using Listeria monocytogenes investigated the relationship between DC subsets and the induction of Tregs.57 Specifically, co-injection of heat-killed L. monocytogenes, OVA-pulsed CD8α+ DCs and OVA-specific naive CD4+ T cells from TCR-transgenic DO11.10 mice induced Th1-like Tregs. The CD8α+ DC subset produced both IL-10 and IL-12, and the induced Th1-like Tregs produced IFN-γ and IL-10 and expressed ICOS, Foxp3 and T-bet. These Th1-like Tregs significantly inhibited allergic reactions.57 Consistently, we found that both Chlamydia and BCG infections preferentially enhanced CD8α+ DC expansion.38, 105 More interestingly, we found that CD8α+ DCs isolated from Chlamydia-infected mice produced significantly higher levels of IL-12 and IL-10.105 When the inhibition potency on allergic reactions was compared, CD8α+ DCs were more effective in inhibiting de novo and established allergic reactions (Bilenki L, Yang X, unpubl. data). However, it remains unclear whether these DCs belong to one DC subset or several subsets. It would be interesting to know whether the CD8α+ DC subset from Chlamydia-infected mice can induce Th1-like Tregs and whether these potential Tregs play a role in the DC-mediated inhibition of allergen-specific Th2 responses.
Not all studies of DCs modified by bacterial antigens exhibited an inhibition of allergy. Schröder et al. reported that the adoptive transfer of DCs isolated from the lungs of C. pneumoniae-infected mice, but not those from phosphate-buffered saline-treated mice, induced allergic sensitization.106 Notably, the DCs were isolated during early stage infection (day 5), and the DCs were incubated with allergen overnight before adoptive transfer. More recently, a study on neonatal infection of mice with C. muridarum found that DCs isolated from infected mice induced higher IL-13 production by OVA-specific TCR-transgenic CD4+ T cells in a DC and T-cell coculture system, suggesting that IL-13 had a promoting effect on the DCs from infected mice.107 No adoptive transfer experiments were performed to test the in vivo function of the DCs in that study. We also found that, although DCs from adult mice with neonatal exposure to killed bacteria inhibited the development of allergic reactions in recipient mice after adoptive transfer, DCs from young mice with short exposure to the same bacteria failed to inhibit the development of allergic reactions; this failure was associated with the lack of IL-10 production by the latter DCs (Jiao L, Yang X, unpubl. data). In addition, in adult mice we found that the capacity of the DCs from infected mice to produce IL-10 was influenced by the stage of infection; an increase in IL-10 production by DCs was observed only in later stages of infection.33, 34 Therefore, the conclusion can vary depending on the model system and methodology used. Careful control of the experimental system and objective analysis of the advantages and disadvantages of different experimental systems, is critical for generating meaningful data to explain the mechanisms of the hygiene hypothesis.
Concluding remarks
The relationship between infection and allergy is likely influenced by multiple factors, including the type of infections/microbial substances, time and duration of exposure, and genetic factors, among other potential factors. In particular, different infectious organisms, and even various components of the same infectious agent, may have varying influences on allergy. Moreover, the modulating effect of infections on allergy and asthma may be potentiated or diminished by other factors. For example, although the inhibitory effect of BCG vaccination on asthma has been well documented,18 the prevalence of asthma has still increased significantly during the past two decades in developing countries, in which most people are vaccinated with BCG after birth.10 Compared to the relationship of infection with allergy, the relationship of infection with asthma is even more complicated because not all asthma cases are related to allergy and, even for those that are related, numerous processes may contribute to the development and exacerbation of the disease. Therefore, although the hygiene hypothesis is a credible explanation, it is unlikely to be the sole explanation for the observed increase in allergic diseases during the past decades. Therefore, it is not surprising that inconsistent findings are often reported on this topic. This inconsistency emphasizes the critical importance of mechanistic studies on the relationship between infection and allergy/asthma using well-established experimental models, especially those that are well controlled for the type of infection, condition of infection and host genetic background. At the same time, the difference between humans and animals should also be kept in mind when interpreting research data. The recent finding on the critical role of DCs in the infection-mediated inhibition of allergy and asthma represents a significant advance in elucidating the mechanism for the hygiene hypothesis. Moreover, although allergic asthma is mainly a Th2-related disease, recent studies have shown that the Th17 cell, a newly identified effector T cell, may also contribute to allergic inflammation.108, 109 DCs are important for Th17 cell development, but the role of post-infection DCs in modulating allergic Th17 responses has not been addressed. Further investigation into the mechanisms by which DCs are ‘educated' by infection at different stages of host development will enhance the understanding of immune regulation in disease settings and will be useful in the rational design of preventive/therapeutic approaches to allergy/asthma and infections. In particular, the following questions should be addressed:
What determines the heterogeneity of DCs (tolerogenic or DC1-like) in different types/conditions of infections, especially parasitic and bacterial infections?
How do different types of DCs that are ‘educated' by single or combined infections modulate allergic/asthmatic reactions?
How do the infection-imprinted DCs maintain their tolerogenic/regulatory functions in vivo?
On the other hand, although the hygiene hypothesis is unlikely the sole explanation for the observed increase in allergic diseases, the information generated from the epidemiological, experimental and clinical studies in this field is still very useful for the design of prevention and treatment approaches for allergic diseases. In particular, it is worthwhile to carefully test the protective capacity, safety and mechanisms of the infectious/microbial products that have been found in experimental studies to be protective for allergic diseases. Learning and mimicking the mechanisms by which these protective microbes modulate allergy, including the involvement of DCs in the process, is important. A good example is the testing of probiotics for the prevention of allergy in clinical trials.110, 111, 112 A double-blind, randomized, placebo-controlled trial in Finland has shown encouraging results that the ingestion of probiotics, in the form of a non-pathogenic microorganism such as Lactobacillus GG, by expectant mothers can decrease the incidence of atopic dermatitis.110, 111 This perinatal protection can last up to 7 years.111 Another trial showed improvement of atopic dermatitis in response to treatment with other strains of probiotics.112 The ultimate goal of this field of research is to develop new methods, or improve upon old methods, for the prevention and treatment of allergic diseases. If this goal can be met, then the validity of the hygiene hypothesis becomes less important.
Acknowledgments
This work was supported by operating grants from the Canadian Institutes for Health Research (CIHR) and the Manitoba Health Research Council (MHRC) to XY. XY is the Canada Research Chair in Infection and Immunity. XG is a recipient of the MHRC studentship.
References
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299 6710:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:969–977. doi: 10.1016/j.jaci.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Erb KJ. Atopic disorders: a default pathway in the absence of infection. Immunol Today. 1999;20:317–320. doi: 10.1016/s0167-5699(99)01475-9. [DOI] [PubMed] [Google Scholar]
- Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402 6760 Suppl:B2–B4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- Sweiss ST. Eat dirt—the hygiene hypothesis and allergic diseases. N Engl J Med. 2002;347:930–931. doi: 10.1056/NEJMe020092. [DOI] [PubMed] [Google Scholar]
- Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275 5296:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- Aaby P, Shaheen SO, Heyes CB, Goudiaby A, Hall AJ, Shiell AW, et al. Early BCG vaccination and reduction in atopy in Guinea-Bissau. Clin Exp Allergy. 2000;30:644–650. doi: 10.1046/j.1365-2222.2000.00803.x. [DOI] [PubMed] [Google Scholar]
- Shaheen SO, Aaby P, Hall AJ, Barker DJ, Heyes CB, Shiell AW, et al. Measles and atopy in Guinea-Bissau. Lancet. 1996;347 9018:1792–1796. doi: 10.1016/s0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- Bodner C, Anderson WJ, Reid TS, Godden DJ. Childhood exposure to infection and risk of adult onset wheeze and atopy. Thorax. 2000;55:383–387. doi: 10.1136/thorax.55.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AH, Murphy JR. Hygiene hypothesis: fact or fiction. J Allergy Clin Immunol. 2003;11:471–478. doi: 10.1067/mai.2003.172. [DOI] [PubMed] [Google Scholar]
- Matricardi PM, Rosmini F, Ferrigno L, Nisini R, Rapicetta M, Chionne P, et al. Cross sectional retrospective study of prevalence of atopy among Italian military students with antibodies against hepatitis A virus. BMJ. 1997;314 7086:999–1003. doi: 10.1136/bmj.314.7086.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296 5567:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- Araujo MI, Hoppe B, Medeiros M, Alcantara L, Almeida MC, Schriefer A, et al. Impaired T helper 2 response to aeroallergen in helminth-infected patients with asthma. J Infect Dis. 2004;190:1797–1803. doi: 10.1086/425017. [DOI] [PubMed] [Google Scholar]
- von Mutius E, Braun-Fahrlander C, Schierl R, Riedler J, Ehlermann S, Maisch S, et al. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy. 2000;30:1230–1234. doi: 10.1046/j.1365-2222.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blumer N, von Mutius E, et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol. 2007;119:1514–1521. doi: 10.1016/j.jaci.2007.03.023. [DOI] [PubMed] [Google Scholar]
- El-Zein M, Parent ME, Benedetti A, Rousseau MC. Does BCG vaccination protect against the development of childhood asthma? A systematic review and meta-analysis of epidemiological studies. Int J Epidemiol. 2010;39:469–486. doi: 10.1093/ije/dyp307. [DOI] [PubMed] [Google Scholar]
- Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187:561–569. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz U, Gerhold K, Gruber C, Braun A, Wahn U, Renz H, et al. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998;102:867–874. doi: 10.1016/s0091-6749(98)70030-2. [DOI] [PubMed] [Google Scholar]
- Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, et al. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998;161:7054–7062. [PubMed] [Google Scholar]
- Kline JN, Waldschmidt TJ, Businga TR, Lemish JE, Weinstock JV, Thorne PS, et al. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J Immunol. 1998;160:2555–2559. [PubMed] [Google Scholar]
- Stampfli MR, Ritz SA, Neigh GS, Sime PJ, Lei XF, Xing Z, et al. Adenoviral infection inhibits allergic airways inflammation in mice. Clin Exp Allergy. 1998;28:1581–1590. doi: 10.1046/j.1365-2222.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- Wang CC, Rook GA. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. . Immunology. 1998;93:307–313. doi: 10.1046/j.1365-2567.1998.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung VP, Gieni RS, Umetsu DT, DeKruyff RH. Heat-killed Listeria monocytogenes as an adjuvant converts established murine Th2-dominated immune responses into Th1-dominated responses. J Immunol. 1998;161:4146–4152. [PubMed] [Google Scholar]
- Hansen G, Yeung VP, Berry G, Umetsu DT, DeKruyff RH. Vaccination with heat-killed Listeria as adjuvant reverses established allergen-induced airway hyperreactivity and inflammation: role of CD8+ T cells and IL-18. J Immunol. 2000;164:223–230. doi: 10.4049/jimmunol.164.1.223. [DOI] [PubMed] [Google Scholar]
- Smits HH, Hammad H, van Nimwegen M, Soullie T, Willart MA, Lievers E, et al. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J Allergy Clin Immunol. 2007;120:932–940. doi: 10.1016/j.jaci.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Dittrich AM, Erbacher A, Specht S, Diesner F, Krokowski M, Avagyan A, et al. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J Immunol. 2008;180:1792–1799. doi: 10.4049/jimmunol.180.3.1792. [DOI] [PubMed] [Google Scholar]
- Mangan NE, Rooijen NV, McKenzie AN, Fallon PG. Helminth-modified pulmonary immune response protects mice from allergen-induced airway hyperresponsiveness. J Immunol. 2006;176:138–147. doi: 10.4049/jimmunol.176.1.138. [DOI] [PubMed] [Google Scholar]
- Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminthes protect in a murine model of asthma. J Immunol. 2006;177:1628–1635. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang S, Fan Y, Zhu L. Systemic mycobacterial infection inhibits antigen-specific immunoglobulin E production, bronchial mucus production and eosinophilic inflammation induced by allergen. Immunology. 1999;98:329–337. doi: 10.1046/j.1365-2567.1999.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilenki L, Fan Y, Wang S, Yang J, Han X, Yang X. Chlamydia trachomatis infection inhibit asthma-like reaction induced by ragweed. Clin Immunol. 2002;102:28–36. doi: 10.1006/clim.2001.5144. [DOI] [PubMed] [Google Scholar]
- Han X, Fan Y, Wang S, Yang J, Bilenki L, Qiu H, et al. Dendritic cells from Chlamydia-infected mice show altered Toll-like receptor expression and play a crucial role in inhibition of allergic responses to ovalbumin. Eur J Immunol. 2004;34:981–989. doi: 10.1002/eji.200324387. [DOI] [PubMed] [Google Scholar]
- Han X, Wang S, Fan Y, Yang J, Jiao L, Qiu H, et al. Chlamydia infection induces ICOS ligand-expressing and IL-10-producing dendritic cells that can inhibit airway inflammation and mucus overproduction elicited by allergen challenge in BALB/c mice. J Immunol. 2006;176:5232–5239. doi: 10.4049/jimmunol.176.9.5232. [DOI] [PubMed] [Google Scholar]
- Han X, Fan Y, Wang S, Jiao L, Qiu H, Yang X. NK cells contribute to intracellular bacterial infection-mediated inhibition of allergic responses. J Immunol. 2008;180:4621–4628. doi: 10.4049/jimmunol.180.7.4621. [DOI] [PubMed] [Google Scholar]
- Jiao L, Han X, Wang S, Fan Y, Yang M, Qiu H, et al. Imprinted DC mediate the immune-educating effect of early-life microbial exposure. Eur J Immunol. 2009;39:469–480. doi: 10.1002/eji.200838367. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang S, Fan Y, Han X, Yang J, Chen L. Mycobacterial infection inhibits established allergic responses by modifying cytokine production and adhesion molecule expression. Immunology. 2002;105:336–343. doi: 10.1046/j.0019-2805.2002.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilenki L, Gao X, Wang S, Yang J, Fan Y, Han X, et al. Dendritic cells from Mycobacteria-infected mice inhibits established allergic airway inflammatory responses to ragweed via IL-10- and IL-12-secreting mechanisms. J Immunol. 2010;184:7288–7296. doi: 10.4049/jimmunol.0902829. [DOI] [PubMed] [Google Scholar]
- Ramsey CD, Celedon JC. The hygiene hypothesis and asthma. Curr Opin Pulm Med. 2005;11:14–20. doi: 10.1097/01.mcp.0000145791.13714.ae. [DOI] [PubMed] [Google Scholar]
- Linneberg A, Ostergaard C, Tvede M, Andersen LP, Nielsen NH, Madsen F, et al. IgG antibodies against microorganisms and atopic disease in Danish adults: the Copenhagen Allergy Study. J Allergy Clin Immunol. 2003;111:847–853. doi: 10.1067/mai.2003.1335. [DOI] [PubMed] [Google Scholar]
- Rennie DC, Lawson JA, Kirychuk SP, Paterson C, Willson PJ, Senthilselvan A, et al. Assessment of endotoxin levels in the home and current asthma and wheeze in school-age children. Indoor Air. 2008;18:447–453. doi: 10.1111/j.1600-0668.2008.00543.x. [DOI] [PubMed] [Google Scholar]
- Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol. 2004;5:337–343. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med. 2001;163:322–328. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- Hogg JC. Childhood viral infection and the pathogenesis of asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 1999;160:S26–S28. doi: 10.1164/ajrccm.160.5.8. [DOI] [PubMed] [Google Scholar]
- Cramer C, Link E, Horster M, Koletzko S, Bauer CP, Berdel D, et al. Elder siblings enhance the effect of filaggrin mutations on childhood eczema: results from the 2 birth cohort studies LISAplus and GINIplus. J Allergy Clin Immunol. 2010;125:1254–1260. doi: 10.1016/j.jaci.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Oh SY, Zheng T, Kim YK. Immunomodulating effects of endotoxin in mouse models of allergic asthma. Clin Exp Allergy. 2010;40:536–546. doi: 10.1111/j.1365-2222.2010.03477.x. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Immunologic influences on allergy and the Th1/Th2 balance. J Allergy Clin Immunol. 2004;113:395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Garn H, Renz H. Epidemiological evidence for the hygiene hypothesis. Immunobiology. 2007;212:441–452. doi: 10.1016/j.imbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- Wohlleben G, Trujillo C, Muller J, Ritze Y, Grunewald S, Tatsch U, et al. Helminth infection modulates the development of allergen-induced airway inflammation. Int Immunol. 2004;16:585–596. doi: 10.1093/intimm/dxh062. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhao J, Yang Y, Zhang L, Yang X, Zhu X, et al. Schistosoma japonicum egg antigens stimulate CD4 CD25 T cells and modulate airway inflammation in a murine model of asthma. Immunology. 2006;120:8–18. doi: 10.1111/j.1365-2567.2006.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, et al. Antigen-specific regulatory T cells develop via the ICOS–ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Bandeira A. On the ontogeny and physiology of regulatory T cells. Immunol Rev. 2001;182:5–17. doi: 10.1034/j.1600-065x.2001.1820101.x. [DOI] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Indentifying Foxp3-epxressing suppressor T cells with bicistronic reporter. PNAS. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naïve T cells. J Ex Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat Immunol. 2004;5:1149–1156. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006;177:1628–1635. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169:3284–3292. doi: 10.4049/jimmunol.169.6.3284. [DOI] [PubMed] [Google Scholar]
- Wohlleben G, Trujillo C, Muller J, Ritze Y, Grunewald S, Tatsch U, Erb KJ. Helminth infection modulates the development of allergen-induced airway inflammation. Int Immunol. 2004;16:585–596. doi: 10.1093/intimm/dxh062. [DOI] [PubMed] [Google Scholar]
- Montagnoli C, Fallarino F, Gaziano R, Bozza S, Bellocchio S, Zelante T, et al. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J Immunol. 2006;176:1712–1723. doi: 10.4049/jimmunol.176.3.1712. [DOI] [PubMed] [Google Scholar]
- Liu P, Li J, Yang X, Shen Y, Zhu Y, Wang S, et al. Helminth infection inhibits airway allergic reaction and dendritic cells are involved in the modulation process. Parasite Immunol. 2010;32:57–66. doi: 10.1111/j.1365-3024.2009.01161.x. [DOI] [PubMed] [Google Scholar]
- McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. . J Exp Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuany-Amorim C, Manlius C, Trifilieff A, Brunet LR, Rook G, Bowen G, et al. Long-term protective and antigen-specific effect of heat-killed Mycobacterium vaccae in a murine model of allergic pulmonary inflammation. J Immunol. 2002;169:1492–1499. doi: 10.4049/jimmunol.169.3.1492. [DOI] [PubMed] [Google Scholar]
- Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8:625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Tang H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr Opin Immunol. 2008;20:61–67. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- Wu L, Liu YJ. Development of dendritic cell lineages. Immunity. 2007;26:741–750. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39:26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587–4595. [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R, de Smedt T, Pajak B, Heirman C, Thielemans K, Leo O, et al. Role of CD8alpha+ and CD8alpha− dendritic cells in the induction of primary immune responses in vivo. . J Leukoc Biol. 1999;66:242–246. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. . Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AD, Manickasingham SP, Sporri R, Diebold SS, Schulz O, Sher A, et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–3660. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- Manickasingham SP, Edwards AD, Schulz O, Reis e Sousa C. The ability of murine dendritic cell subsets to direct T helper cell differentiation is dependent on microbial signals. Eur J Immunol. 2003;33:101–107. doi: 10.1002/immu.200390001. [DOI] [PubMed] [Google Scholar]
- Hilkens C, Snijders A, Vermeulen H, van der Meide P, Wierenga E, Kapsenberg M. Accessory cell-derived interleukin-12 and prostaglandin E2 determine the level of interferon-gamma produced by activated human CD4+ T cells. Ann NY Acad Sci. 1996;795:349–350. doi: 10.1111/j.1749-6632.1996.tb52689.x. [DOI] [PubMed] [Google Scholar]
- Hilkens CM, Snijders A, Vermeulen H, van der Meide PH, Wierenga EA, Kapsenberg ML. Accessory cell-derived IL-12 and prostaglandin E2 determine the IFN-gamma level of activated human CD4+ T cells. J Immunol. 1996;156:1722–1727. [PubMed] [Google Scholar]
- Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–2849. [PubMed] [Google Scholar]
- Kalinski P, Hilkens CM, Schuitemaker JH, Kapsenberg ML.Prostaglandin E2 promotes the generation of dendritic antigen-presenting cells that induce type 2 cytokines in Th cells. J Immunol 199715928–35.9200435 [Google Scholar]
- Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162:6473–6481. [PubMed] [Google Scholar]
- Buelens C, Willems F, Delvaux A, Pierard G, Delville JP, Velu T, et al. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25:2668–2672. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Kosiewicz MM, Alard P, Streilein JW. On the mechanisms by which transforming growth factor-beta 2 alters antigen-presenting abilities of macrophages on T cell activation. Eur J Immunol. 1997;27:1648–1656. doi: 10.1002/eji.1830270709. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Granucci F, Citterio S, Foti M, Ricciardi-Castagnoli P. Coordinated events during bacteria-induced DC maturation. Immunol Today. 1999;20:200–203. doi: 10.1016/s0167-5699(98)01427-3. [DOI] [PubMed] [Google Scholar]
- Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4+ and CD8+ anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–2476. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263–276. doi: 10.1159/000067596. [DOI] [PubMed] [Google Scholar]
- Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. . J Exp Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath VL, Murphy EE, Crain C, Tomlinson MG, O'Garra A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol. 2000;30:2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gorczynski R, Bransom J, Cattral M, Huang X, Lei J, Min W, et al. Dendritic cells expressing TGFbeta/IL-10, and CHO cells with OX-2, increase graft survival. Transplant Proc. 2001;33:1565–1566. doi: 10.1016/s0041-1345(00)02595-1. [DOI] [PubMed] [Google Scholar]
- Wakkach A, Cottrez F, Groux H. Differentiation of regulatory T cells 1 is induced by CD2 costimulation. J Immunol. 2001;167:3107–3113. doi: 10.4049/jimmunol.167.6.3107. [DOI] [PubMed] [Google Scholar]
- Lu L, Bonham CA, Liang X, Chen Z, Li W, Wang L, et al. Liver-derived DEC205+B220+CD19− dendritic cells regulate T cell responses. J Immunol. 2001;166:7042–7052. doi: 10.4049/jimmunol.166.12.7042. [DOI] [PubMed] [Google Scholar]
- Njau F, Geffers R, Thalmann J, Haller H, Wagner AD. Restriction of Chlamydia pneumoniae replication in human dendritic cell by activation of indoleamine 2,3-dioxygenase. Microbes Infect. 2009;11:1002–1010. doi: 10.1016/j.micinf.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Adams VC, Hunt JR, Martinelli R, Palmer R, Rook GA, Brunet LR. Mycobacterium vaccae induces a population of pulmonary CD11c+ cells with regulatory potential in allergic mice. Eur J Immunol. 2004;34:631–638. doi: 10.1002/eji.200324659. [DOI] [PubMed] [Google Scholar]
- Bilenki L, Wang SH, Yang J, Fan YJ, Jiao L, Joyee AG, et al. Adoptive transfer of CD8alpha+ dendritic cells (DC) isolated from mice with Chlamydia trachomatis are more potent in inducing protective immunity than CD8alpha− DC. J Immunol. 2006;177:7067–7075. doi: 10.4049/jimmunol.177.10.7067. [DOI] [PubMed] [Google Scholar]
- Schröder NW, Crother TR, Naiki Y, Chen S, Wong MH, Yilmaz A, et al. Innate immune responses during respiratory tract infection with a bacterial pathogen induce allergic airway sensitization. J Allergy Clin Immunol. 2008;122:595–602. doi: 10.1016/j.jaci.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat JC, Starkey MR, Kim RY, Phipps S, Gibson PG, Beagley KW, et al. Early-life chlamydial lung infection enhances allergic airways disease through age-dependent differences in immunopathology. J Allergy Clin Immunol. 2010;125:617–625. doi: 10.1016/j.jaci.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Vazquez-Tello A, Semlali A, Chakir J, Martin JG, Leung DY, Eidelman DH, et al. Induction of glucocorticoid receptor-beta expression in epithelial cells of asthmatic airways by T-helper type 17 cytokines. Clin Exp Allergy. 2010;40:1312–1322. doi: 10.1111/j.1365-2222.2010.03544.x. [DOI] [PubMed] [Google Scholar]
- Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104:1131–1137. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1019–1021. doi: 10.1016/j.jaci.2006.12.608. [DOI] [PubMed] [Google Scholar]
- Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–198. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]