Abstract
Salmonella enterica serovar Typhi is a pathogen that only infects humans. Currently, there is no animal model for studying this pathogen. Recently, alymphoid RAG-2−/−/γc−/− mice engrafted with human leukocytes, known as humanized mice, have been successfully utilized to develop experimental models for several human-specific viral infections, including HIV, human-like dengue fever and hepatitis C virus. Little is known about the usefulness and feasibility of the humanized mouse model for the study of human-specific bacterial pathogens, such as S. typhi. The aim of this study was to determine if Salmonella enterica serovar Typhi could establish productive infection in humanized mice. Here we report that intravenous inoculation of S. typhi into humanized mice, but not controls, established S. typhi infections. High bacterial loads were found in the liver, spleen, blood and bone marrow of mice reconstituted with human leukocytes, but not in the unreconstituted control mice. Importantly, S. typhi-infected humanized mice lost significant body weight, and some of the infected mice displayed neurological symptoms. Our data suggest, for the first time, that humanized mice are susceptible to S. typhi challenge and that this model can be utilized to study the pathogenesis of S. typhi to develop novel therapeutic strategies.
Keywords: animal model, humanized mice, RAG2−/−γc−/−, Salmonella typhi
Introduction
Salmonella enterica serovar Typhi (S. typhi) is a human-restricted facultative intracellular bacterium that causes a life-threatening systemic infection called typhoid fever.1 S. typhi causes significant morbidity and mortality across the globe, resulting in an estimated 21.5 million cases2 and 200 000 deaths3 each year, the majority of which occur in developing countries.2 Although the incidence of typhoid fever has decreased in recent years through strict sanitary and hygienic measures, the treatment of infected patients has become complicated due to emergence of antimicrobial-resistant strains of S. typhi.3, 4 The major sources of S. typhi infections in developed countries, including the United States and the United Kingdom, are from travelers returning from the Indian subcontinent or other endemic regions.4, 5 With the increasing threat of antimicrobial resistance, the prevention and development of appropriate empirical treatments of typhoid fever remain major public health goals.
A major impediment to studying the pathogenesis of S. typhi has been the lack of an animal model. Humanized mice have recently emerged as an essential tool in the study of human-specific pathogens in vivo [reviewed in Ref. 6]. These mice are generated by engrafting human hematopoietic stem cells into highly immunocompromised mice, resulting in the development of human immune cell populations that can be either susceptible to and/or respond to human-specific infectious agents.7 Other animal models such as non-human primates are often unable to reproduce the exact pathophysiology of human disease patterns.8 Recently, the humanized mouse model has been successfully utilized to study several human-specific viral infections including HIV,9 human-like dengue fever,10, 11 hepatitis C virus12 and herpes simplex virus-2.13 Thus far, most humanized mouse model studies have been limited to human-specific viral agents. Little is known about the usefulness and feasibility of the humanized mouse model for the study of human-specific bacterial pathogens, such as S. typhi.
Currently, there are no animal models that support productive infection with S. typhi. A recent study that attempted to establish an in vivo S. typhi infection in Balb/c mice and an in vitro infection in murine macrophage cells demonstrated bacterial clearance in both systems within 4–24 h of infection.14 Our current investigation was aimed at examining whether human S. typhi bacteria can establish an infection in humanized mice. Here we report, for the first time, that S. typhi survives and replicates in multiple organs, including the liver and spleen, in humanized mice.
Materials and methods
Humanized and control mice
Balb/c mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA).Breeding pairs of Balb/c-RAG2−/−γc−/− mice were kindly provided by M. Ito (Central Institute of Experimental Animals, Kawasaki, Japan). All mice were maintained under specific pathogen-free conditions that received ethics board approval and that followed the recommendations of the Central Animal Care Facility of McMaster University. Umbilical cord blood was obtained with parental consent from patients at the Department of Labor and Delivery of the McMaster University Medical Centre. The samples were processed using the RosetteSep Human Cord Blood Progenitor Enrichment Kit from Stem Cell Technologies (Vancouver, BC, Canada). Newborn RAG2−/−γc−/− pups were sublethally irradiated and injected intrahepatically with 1×106–2×106 CD34-enriched hematopoietic stem cells. Reconstitution of human immune cells in mice was evaluated by the expression of human lymphocyte markers on cells in the liver, spleen, bone marrow, lymph nodes and thymus by flow cytometry using human-specific antibodies.
Salmonella strain, culturing and in vivo mouse injection
The bacterial strain Salmonella enterica serovar Typhi (BKC 3233) was obtained from Dr Brian Coombes (McMaster University) and was grown in antibiotic-free Luria–Bertani (LB) agar or LB broth (Invitrogen, Burlington, ON, Canada). Bacterial inocula were prepared with late exponential phase bacteria in phosphate-buffered saline (PBS) after several washes and were injected intravenously (2×106 colony-forming unit (CFU) per animal) into reconstituted (humanized) RAG2−/−γc−/− mice, non-reconstituted (irradiated control) RAG2−/−γc−/− mice and background control mice (Balb/c). Mice were then monitored for body weight, neurological symptoms and survival. Animals were killed at the end of the experiment or day 2 or 9 post-infection. Liver, spleen, blood and bone marrow samples were collected separately in sterile PBS and homogenized (MixerMill 400; Retsch, Haan, Germany). Homogenates were then serially diluted with PBS and plated on LB agar. After incubation at 37 °C for 16–20 h, bacterial CFUs were counted and analyzed.
Immunohistochemistry and flow cytometry
Formalin-fixed, paraffin-embedded liver sections were dewaxed in xylene, and antigen retrieval was performed in steaming citrate buffer. A primary mouse anti-human CD45 antibody (Dako, Burlington, ON, Canada), a mouse monoclonal antibody (clone AC04) to S. typhi (Abcam, Cambridge, MA, USA) and a biotinylated secondary antibody (Envision Detection Kit; Dako , Burlington, ON, Canada) were used. The sections were developed with 3-amino-9-ethylcarbazole chromogen solution and counterstained with Meyer's hematoxylin. For flow cytometry, single-cell suspensions were prepared with liver cells and surface stained with an anti-human CD45 antibody (HI30 Pacific Blue-conjugated) or isotype-matched controls (Biolegend, San Diego, CA, USA) according to the manufacturer's instructions. Cells were then run on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA, USA), and the data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis.
Data were analyzed using GraphPad Prism software (version 3.06; GraphPad, San Diego, CA, USA). Survival plots were analyzed using the log-rank (Mantel-Cox) test. P values of <0.05 were considered significant.
Results
Humanized mice are susceptible to S. typhi infection
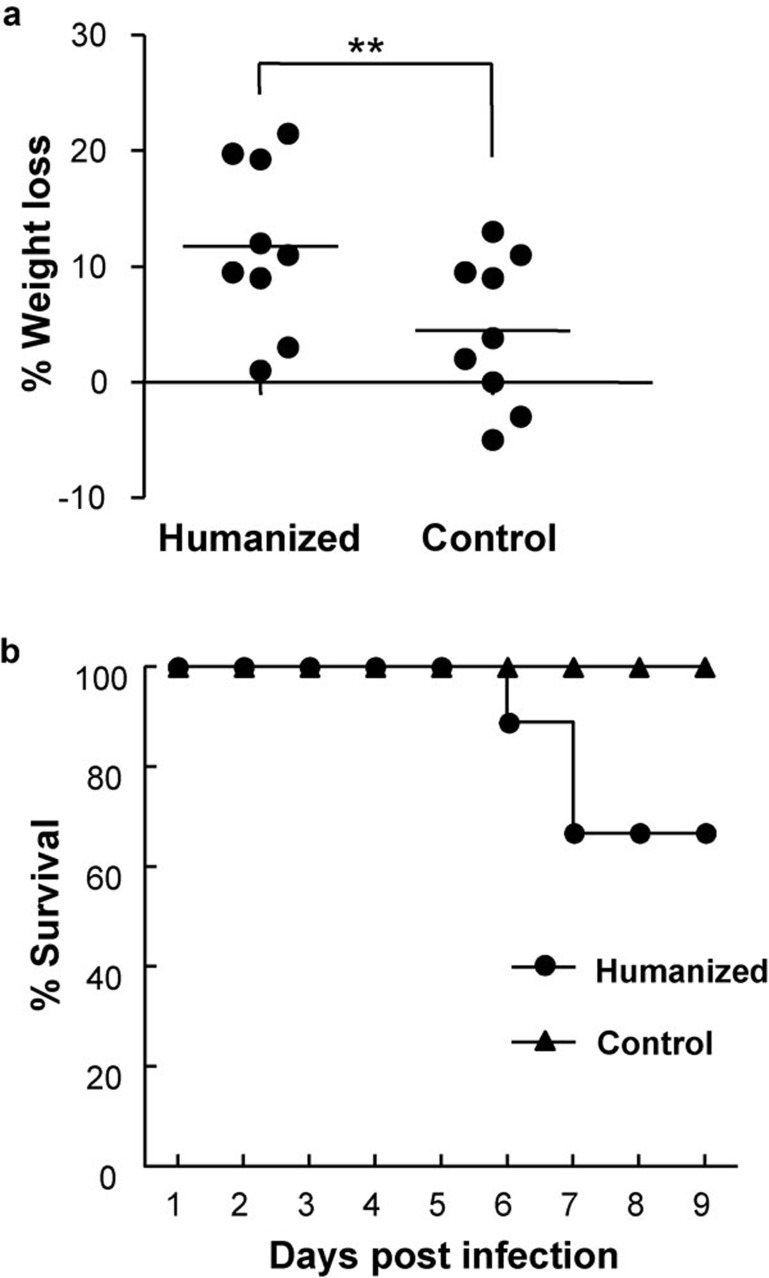
S. typhi infection remains a constant threat to human health due to the emergence of new antibiotic-resistant strains. There has been no animal model to study the pathogenesis of S. typhi or to use in preclinical therapeutic evaluations. Here we investigated whether S. typhi can establish an infection in humanized mice. We recently reported a high degree of human leukocyte engraftment in this model.13, 15 In this study, we confirmed the reconstitution of human cells in the mice used for S. typhi challenge experiments (data not shown). We intravenously inoculated humanized mice with S. typhi, and these mice showed a marked decrease in body weight compared to irradiated control and background control mice (Figure 1a). The inoculated humanized mice displayed an overall decline in natural feeding, movement and other physical conditions. Interestingly, some of the infected humanized mice developed critical neurological symptoms such as spinning and loss of coordination. We recorded the survival rate among the groups of mice inoculated with S. typhi up to day 9 post-infection. As depicted in Figure 1b, three of the humanized mice succumbed to infection on days 6 and 7. In contrast, the survival rate for both groups of control mice was 100% during the study period.
Figure 1.
Body weight and survival of humanized and control mice following S. typhi infection. (a) Humanized and irradiated control mice were inoculated intravenously with S. typhi (2×106 CFU per mouse) in sterile PBS, and body weights were recorded daily until day 9 post-infection. (b) Mice were monitored for any visible clinical signs, body condition, body weight and survival. Nine or ten mice were inoculated from each group. Data are means with standard errors. *P<0.05, **P<0.01. CFU, colony-forming unit; PBS, phosphate-buffered saline; S. typhi, Salmonella enterica serovar Typhi.
Bacterial load and distribution in humanized and control mice
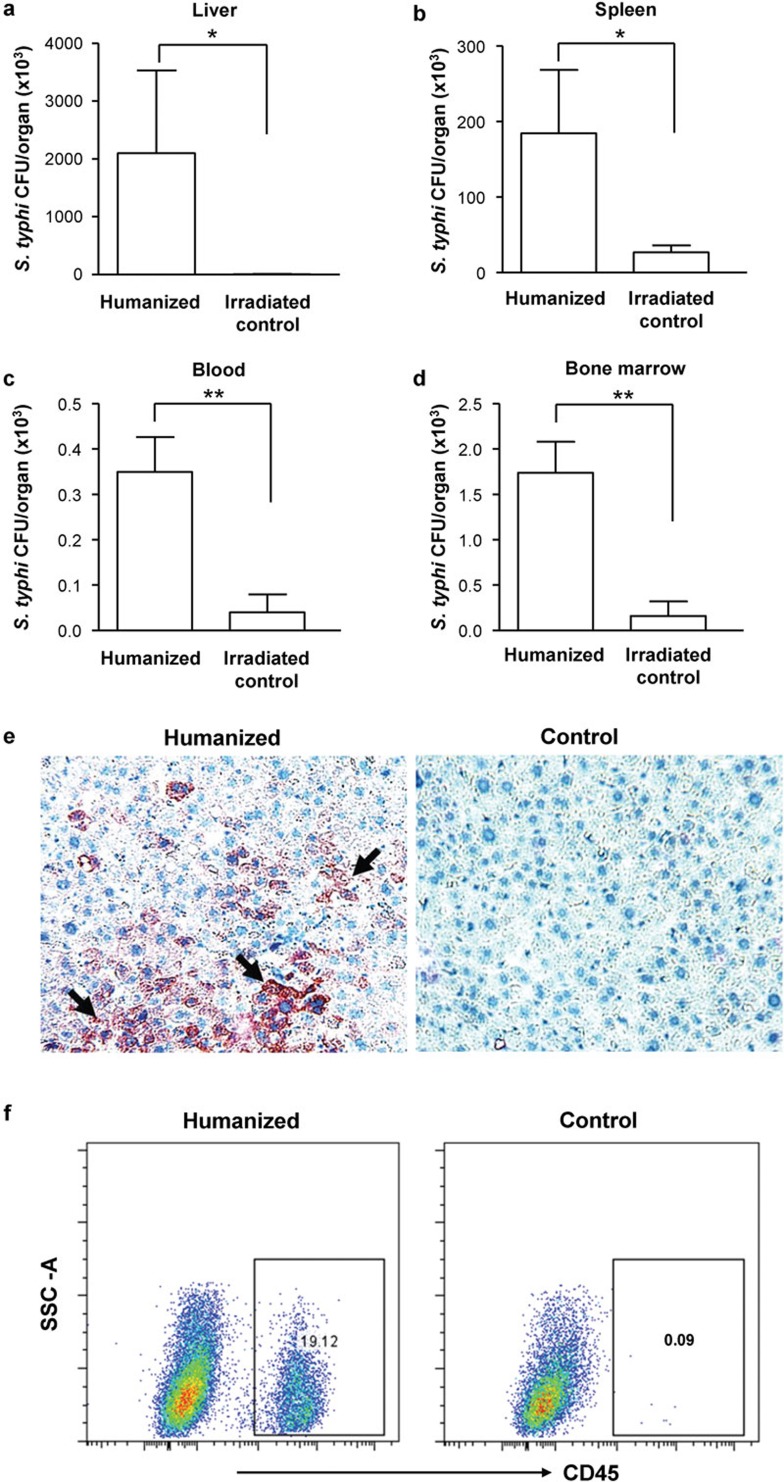
We then analyzed the bacterial load in different organs in the different groups of mice inoculated with S. typhi. Liver, spleen, blood and bone marrow homogenates from inoculated mice were cultured on LB agar plates, and bacterial colonies were enumerated. While humanized mice had very high bacteria counts in the organs, non-reconstituted mice showed no or little bacterial loads (Figure 2a–d). Regarding the distribution of bacteria in the different organs, the liver had the highest level of S. typhi CFUs, followed by the spleen and the bone marrow. To analyze the immune cell types infected by S. typhi, we examined liver cells both from infected humanized mice and non-humanized control mice utilizing immunohistochemistry and flow cytometry. As depicted in Figure 2e, humanized, but not control, mice displayed pronounced S. typhi infection (arrowhead) in the liver sections. Importantly, flow cytometry analysis of liver cells revealed the presence of human CD45+ cells only in infected livers, suggesting that the cells likely infected by S. typhi are predominantly CD45+ human leukocytes, which were absent in control mice (Figure 2f).
Figure 2.
Distribution and CFU counts of S. typhi in inoculated humanized and control mice. Liver (a), spleen (b), blood (c) and bone marrow (d) were isolated from infected humanized mice and non-humanized irradiated control mice at day 2. The organs were homogenized separately in sterile PBS, serially diluted (10-fold) in PBS and then plated on LB agar plates. The plates were incubated at 37 °C, and bacterial CFUs were enumerated after 16–20 h (n=9 or 10 per group). Data are means with standard errors from two individual experiments. *P<0.05, **P<0.01. (e) Representative micrograph showing immunohistochemical detection of S. typhi antigens (black arrowhead) in liver sections of humanized mice using a mouse monoclonal antibody to S. typhi; control mice had no reactivity. (f) Representative flow cytometry analysis showing human CD45+ cell populations from the livers of humanized and non-humanized control mice. CFU, colony-forming unit; LB, Luria–Bertani; PBS, phosphate-buffered saline; S. typhi, Salmonella enterica serovar Typhi.
Finally, we confirmed that the intravenous route of inoculation with S. typhi in RAG2−/−γc−/− and Balb/c (the background control) mice could establish an infection. As expected, control Balb/c mice cleared the bacteria by 48 h post-infection (Supplementary Figure 1). Immunocompromised RAG2−/−γc−/− mice had bacterial loads in the liver at day 2 post-infection; however, the bacteria were cleared by day 9 post-infection (Supplementary Figure 1).
Discussion
Animal models for human-restricted bacterial pathogens often fail to reproduce the key features of the infection process.16 Salmonella enterica serovar Typhi is a host-specific pathogen for humans and is unable to cause infection or disease in mice.17 Here we provide novel experimental evidence that S. typhi can establish infection in humanized mice. This infection resulted in marked weight loss in humanized mice but not in control mice. In humans, S. typhi infection has been associated with meningitis and cerebrospinal fluid pleocytosis resulting from Salmonella sepsis.18 Recently, S. typhi infections in children have been reported to present symptoms of meningitis.19 Interestingly, in our model of S. typhi infection, some of the infected humanized mice displayed imbalance or circling movement, a manifestation of meningitis, and these mice eventually succumbed to infection on day 6. Meningitis could be caused by the release of bacterial endotoxin into the bloodstream or an inflammation that leads to an increased permeability of the blood-brain barrier and subsequent bacterial invasion of the central nervous system. The control mice did not display any clinical symptoms or weight loss following intravenous inoculation with S. typhi.
In the present study, S. typhi infections spread into various organs of the humanized mice inoculated intravenously. In particular, the liver and spleen had an approximately 20-fold increase in bacterial CFUs compared to irradiated control or Balb/c mice. This suggests clear replication of S. typhi in human cells and not just survival of the initial inoculates. Although the initial number of bacteria was 2×106, we recovered between 1×106 and 3×106 CFUs from only one organ, the liver. Although humanized and control mice displayed very low bacterial counts both in circulation and the bone marrow, infected humanized mice clearly had significantly higher CFUs than control mice. The greater bacterial localization in the liver and spleen compared to the blood or bone marrow may be due to the inherent tropism of S. typhi bacteria to these specified organs in the natural human host. Our immunohistochemical data clearly reveal pronounced S. typhi infection in the liver immune cells of humanized mice but not in control mice. CD45 immunostaining also showed some reactivity in humanized mouse livers; however, we were unable to overcome the strong background, as a mouse monoclonal anti-human CD45 antibody was used in mouse liver tissue sections (data not shown). Finally, using flow cytometry analysis, we ascertained that the major immune cell populations in the humanized mouse liver were CD45+ human leukocytes, which might be the target of S. typhi infection. The organ-specific localization of S. typhi in this study showed a similar trend to that of S. typhimurium colonization, likely because splenic and liver macrophages are the major cells in which the bacteria reside.20 Because the main goal of this study was to examine the establishment of S. typhi infection in humanized mice, we delivered the bacterial inocula via the intravenous route. Obviously, this is not the natural route of infection for S. typhi. A recent study was unsuccessful in establishing S. typhi infection in vivo via the intraperitoneal route in Balb/c mice.14 Now - we have shown that humanized mice are susceptible to S. typhi infection, it would be of interest to determine if oral delivery of S. typhi also results in the productive infection of humanized mice. Taken together, our data support the use of this novel humanized mouse model of S. typhi infection to better understand S. typhi pathogenesis and to offer future preclinical validation of novel antimicrobial therapies.
Acknowledgments
We are thankful to Dr Brain Coombes, McMaster University, Canada, for providing us with Salmonella typhi and for helpful discussions. This study was supported by a grant from CIHR to Ali A Ashkar. AAA is a recipient of a Career Award in Health Sciences from Rx&D/CIHR.
Footnotes
Note: Supplementary information is available on the Cellular & Molecular Immunology website (http://www.nature.com/cmi/).
Supplementary Information
References
- Retamal P, Castillo-Ruiz M, Mora GC. Characterization of MgtC, a virulence factor of Salmonella enterica Serovar Typhi. PLoS One. 2009;4:e5551. doi: 10.1371/journal.pone.0005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang T, Levine MM, Ivanoff B, Wain J, Finlay BB. Typhoid fever—important issues still remain. Trends Microbiol. 1998;6:131–133. doi: 10.1016/s0966-842x(98)01236-0. [DOI] [PubMed] [Google Scholar]
- Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- Lynch MF, Blanton EM, Bulens S, Polyak C, Vojdani J, Stevenson J, et al. Typhoid fever in the United States, 1999–2006. JAMA. 2009;302:859–865. doi: 10.1001/jama.2009.1229. [DOI] [PubMed] [Google Scholar]
- Matheson N, Kingsley RA, Sturgess K, Aliyu SH, Wain J, Dougan G, et al. Ten years experience of Salmonella infections in Cambridge, UK. J Infect. 2010;60:21–25. doi: 10.1016/j.jinf.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Legrand N, Ploss A, Balling R, Becker PD, Borsotti C, Brezillon N, et al. Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host Microbe. 2009;6:5–9. doi: 10.1016/j.chom.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- Muchmore EA. Chimpanzee models for human disease and immunobiology. Immunol Rev. 2001;183:86–93. doi: 10.1034/j.1600-065x.2001.1830107.x. [DOI] [PubMed] [Google Scholar]
- Baenziger S, Tussiwand R, Schlaepfer E, Mazzucchelli L, Heikenwalder M, Kurrer MO, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−gammac−/− mice. Proc Natl Acad Sci USA. 2006;103:15951–15956. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol. 2005;79:13797–13799. doi: 10.1128/JVI.79.21.13797-13799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota J, Rico-Hesse R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J Virol. 2009;83:8638–8645. doi: 10.1128/JVI.00581-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrini P, Sasso R, Germoni S, Marcucci I, Celluci A, Di Marco A, et al. Development of humanized mice for the study of hepatitis C virus infection. Transplant Proc. 2006;38:1181–1184. doi: 10.1016/j.transproceed.2006.02.149. [DOI] [PubMed] [Google Scholar]
- Kwant-Mitchell A, Ashkar AA, Rosenthal KL. Mucosal innate and adaptive immune responses against herpes simplex virus type 2 in a humanized mouse model. J Virol. 2009;83:10664–10676. doi: 10.1128/JVI.02584-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Maloy S, McGuire KL. Macrophages influence Salmonella host-specificity in vivo. . Microb Pathog. 2009;47:212–222. doi: 10.1016/j.micpath.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Kwant-Mitchell A, Pek EA, Rosenthal KL, Ashkar AA. Development of functional human NK cells in an immunodeficient mouse model with the ability to provide protection against tumor challenge. PLoS One. 2009;4:e8379. doi: 10.1371/journal.pone.0008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramuzzino DA, McNiff JM, Bessen DE. Humanized in vivo model for streptococcal impetigo. Infect Immun. 2000;68:2880–2887. doi: 10.1128/iai.68.5.2880-2887.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien AD. Innate resistance of mice to Salmonella typhi infection. Infect Immun. 1982;38:948–952. doi: 10.1128/iai.38.3.948-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim M, Islam N. Salmonella meningitis: report of three cases in adults and literature review. Infection. 2002;30:104–108. doi: 10.1007/s15010-002-2071-8. [DOI] [PubMed] [Google Scholar]
- Mittal S, Saxena A, Garg P. Unusual presentations of Salmonella Typhi infections in children. Trop Doct. 2009;39:27–28. doi: 10.1258/td.2008.070449. [DOI] [PubMed] [Google Scholar]
- Leung KY, Finlay BB. Intracellular replication is essential for the virulence of Salmonella typhimurium. . Proc Natl Acad Sci USA. 1991;88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.