Abstract
Cross-talk has been shown to occur between the immune system and bone metabolism pathways. In the present study, we investigated the impact of CD4+CD25+Foxp3+ regulatory T (Treg) cells on osteoclastogenesis and bone resorption. Treg cells that were isolated and purified from peripheral blood mononuclear cells (PBMCs) of healthy adults inhibited both the differentiation of osteoclasts (OCs) from human embryo bone marrow cells (BMCs) and the pit formation in a dose-dependent manner. In cell cocultures, the production levels of both interleukin-10 (IL-10) and transforming growth factor-beta 1 (TGF-β1) were proportionally upregulated as the ratio of Treg cells to BMCs was increased, and the inhibition of OC differentiation and bone resorption by Treg cells was completely reversed by anti-IL-10 and anti-TGF-β1 antibodies. Treatment of BMC and Treg cell cocultures with 17β-estradiol (E2) at concentrations between 10−7 and 10−9 mol/l suppressed OC differentiation and bone resorption more efficiently than it did in cultures of BMCs alone; this enhanced suppression occurred via the stimulation of Treg cell IL-10 and TGF-β1 expression. These data suggest that Treg cells suppress OC differentiation and bone resorption by secreting IL-10 and TGF-β1. E2 enhances the suppressive effects of Treg cells on OC differentiation and bone resorption by stimulating IL-10 and TGF-β1 secretion from these cells. Therefore, Treg cell-derived IL-10 and TGF-β1 are likely involved in the regulation of E2 on bone metabolism and represent potential therapeutic targets for the treatment of postmenopausal osteoporosis (PMO).
Keywords: bone resorption, osteoclast, PMO, regulatory T cells
Introduction
Bone remodeling is a dynamic process that is dependent on the balance between osteoblast (OB)-driven bone formation and osteoclast-mediated bone resorption.1, 2 A disruption of the balance between bone formation and resorption may lead to bone disease. Postmenopausal osteoporosis (PMO) is thought to result from estrogen deficiency that leads to increased OC formation, decreased levels of OC apoptosis and an increased capacity of mature OCs to resorb bone. Estrogen deficiency upregulates osteoclastogenesis through the production of the pro-osteoclastogenic cytokines tumor-necrosis factor (TNF)-α and receptor activator of nuclear factor kappa B ligand (RANKL). In addition, T lymphocytes play a fundamental role in postmenopausal bone loss and in the induction of osteoclastogenesis in humans.3
OCs are multinucleated cells that are derived from hematopoietic precursors of the monocyte/macrophage lineage in the presence of macrophage colony-stimulating factor (M-CSF) and RANKL.4, 5, 6, 7 Many other cytokines have been shown to affect bone metabolism and OC activity, including interleukin (IL)-1, IL-4, IL-7, IL-10, IL-13 and TNF.8, 9, 10 A previous study by our lab showed that both transforming growth factor-beta 1 (TGF-β1) and IL-10 suppress osteoclastogenesis and bone resorption in a dosage-dependent manner and exhibit synergistic effects.11 Moreover, in rheumatoid arthritis and inflammatory bowel disease, activated T cells were found to express RANKL and promote osteoclastogenesis, thus linking the activation of the immune system with osteoclastogenesis and bone resorption. The reciprocal communication between immune and skeletal systems is observed not only under normal physiological conditions but also in cases of autoimmune and other inflammatory diseases. This interrelationship led Arron and Choi to propose the concept of ‘osteoimmunology' to describe the interactions between the immune system and bone metabolism.12
Recently, immune cells have been linked to the etiology of PMO and bone loss caused by a variety of other endocrine disorders. Furthermore, PMO has been proposed to be a chronic inflammatory disease.13 Regulatory T (Treg) cells play a pivotal role in tolerance to self-antigens and tissue grafts and suppression of autoimmune reactions. However, little information is available regarding the role of Treg cells in osteoclastogenesis. Furthermore, no consensus regarding the inhibitory mechanism of Treg cells in cases of osteoclastogenesis has been established. Kim et al. found that Treg cells inhibit osteoclast differentiation from peripheral blood mononuclear cells (PBMCs) in a cytokine-dependent and cell-to-cell contact-independent manner and proposed that TGF-β and IL-4 may be the key cytokines for the suppressive function of Treg cells.14 Zaiss et al. concluded that Treg cells suppress osteoclast formation primarily through cell-to-cell contact via cytotoxic T lymphocyte antigen 4(CTLA-4), suggesting that IL-4 and IL-10 contributed to, but were not necessary for, the inhibitory effect.15 The goal of the present study was to elucidate the mechanisms underlying the modulation of OC differentiation and bone resorption by Treg cells, and to investigate the role of 17β-estradiol (E2) in this process.
Materials and methods
Subjects and reagents
All of the studies that were performed were approved by the ethics committee of the Hospital of Obstetrics and Gynecology, Fudan University (Shanghai, China), and have therefore been performed in accordance with the ethical standards. All volunteers were from the Hospital of Obstetrics and Gynecology and informed consent was obtained from each.
Fetal bovine serum (FBS), complete α-minimum essential medium (α-MEM) and α-MEM without phenol red were purchased from Gibco-Invitrogen (Karlsruhe, Germany). E2 and tartrate-resistant acid phosphatase (TRAP) kit were purchased from Sigma-Aldrich (St Louis, MO, USA). Penicillin, streptomycin, amphotericin B, M-CSF and RANKL were purchased from R&D Systems (Minneapolis, MN, USA). A Regulatory T Cell Isolation Kit and mouse anti-human CD25-PE were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Mouse anti-human CD3, anti-human CD4-FITC and anti-human Foxp3-PE monoclonal antibodies were purchased from eBioscience (San Diego, CA, USA). IL-10 and TGF-β1 ELISA kits were purchased from R&D Systems. Neutralizing anti-human IL-10 antibody and anti-human TGF-β1 antibodies were purchased from R&D Systems.
Induction of OCs
Osteoclastogenesis was induced in human bone marrow cells (BMCs) using the differentiation factors M-CSF and RANKL. The BMCs were collected from 13- to 16-week-post-gestation embryos following mifepristone- and misoprostol-induced abortion due to unexpected pregnancies. The pregnant women were all between 20 and 31 years of age (with a mean age of 26.5 years) and had not previously received any estrogen-like products. We isolated BMCs and induced osteoclastogenesis in vitro using the same previously described standard method that is used to culture murine OCs in vitro.16 In brief, the ends of the tibiae and femurs were removed, and each marrow cavity was flushed repeatedly by α-MEM containing 15% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and 25 µg/ml amphotericin B. Erythrocytes in the cell suspension were lysed by treatment with NH4Cl. The BMCs were then resuspended in α-MEM with 15% FBS and incubated with 50 ng/ml of M-CSF overnight at 37 °C in a humidified atmosphere containing 5% CO2. The non-adherent cells were subsequently harvested, washed and plated onto glass cover slips at a density of 1×106 cells/ml/well in 24-well plates and cultured with M-CSF (50 ng/ml) and RANKL (50 ng/ml). Every 3 days, half of the culture medium from each well was replaced with fresh medium containing M-CSF and RANKL. After being cultured for 1 week, the cells were subjected to TRAP staining; TRAP-positive multinucleated cells (i.e., those exhibiting three or more nuclei) were counted as OCs. TRAP staining and the resorption pit assay were used to determine OC differentiation and function.
TRAP staining
Cultured adherent cells were fixed on glass coverslips using a fixative and subsequently incubated for 50 min at 37 °C with TRAP-staining solution according to instructions provided with the TRAP kit. The cells were then analyzed using a microscope; TRAP-positive cells that contained three or more nuclei were counted as OCs.
Pit formation on dentine slices
The BMCs were plated on dentine slices (8 mm in diameter) at a density of 1×106 cells/ml/well in a 24-well plate and cultured with M-CSF (50 ng/ml) and RANKL (50 ng/ml) for 10 days. Half of the culture medium from each well was changed every 3 days. After being cultured for 10 days, the slices were fixed with 2.5% glutaraldehyde and rinsed three times in 25% ammonia while being subjected to ultrasonic cleaning to remove unattached cells. The slices were then washed in phosphate-buffered saline, gradient-dehydrated in 70, 95 and 100% ethanol and subsequently stained with 1% (w/v) toluidine blue. Images of pit formation were acquired using a light microscope and analyzed using Image Pro-Plus image analysis software.
Isolation and purification of CD4+CD25+ Treg cells
Treg cells were isolated and purified from PBMCs of healthy non-pregnant women between 25 and 35 years old (with a mean age of 29 years) by magnetic-activated cell sorting. PBMCs were obtained from blood samples of healthy volunteers by Ficoll-Hypaque gradient centrifugation. CD4+CD25+ Treg cells were isolated from PBMC populations by magnetic-activated cell sorting using a microbead-based regulatory T-cell isolation kit according to the manufacturer's instructions, including negative selection of total CD4+ T cells and positive selection of CD25+ T cells. To achieve higher purities, the positively selected CD25+ cell fractions were separated again over an LS column. The sorted Treg cells were incubated with mouse anti-human CD4-FITC and anti-human CD25-PE, and the purity level was assessed; subsequently, the intracellular levels of Foxp3, a highly specific marker of Treg cells, were analyzed by flow cytometry. The purities of both CD4+CD25+ and CD4+Foxp3+ T-cell populations were greater than 90%.
Coculture of CD4+CD25+ Treg cells with BMCs
The Treg cells were activated according to the method described by Coligan and colleagues in Current Protocols in Immunology; briefly, Treg cells were activated with an anti-CD3 antibody (5 µg/ml) and an anti-CD28 antibody (5 µg/ml) supplemented with IL-2 (100 U/ml) for 3 days before being cocultured with BMCs.17 In the cell contact wells, BMCs (1×106/well) and different numbers of activated Treg cells (2×104–2×105 cells/well) were cocultured in 24-well plates for either 7 or 10 days in the presence of M-CSF (50 ng/ml) and RANKL (50 ng/ml). In the no-contact transwell cocultures, the osteoclast precursors were loaded into the lower chambers and Treg cells were loaded into the upper chambers.
At 24 h of coculture, the supernatant was harvested, and IL-10 and TGF-β1 levels were measured using a quantitative sandwich ELISA according to the manufacturer's instructions with IL-10 and TGF-β1 ELISA kits. A total of 7 days later, the cells were cytochemically stained for TRAP, and TRAP-positive multinucleated cells that contained three or more nuclei were counted as OCs. A total of 10 days later, the resorption pits on dentine slices were stained with toluidine blue and examined using a confocal microscope. The mean area resorbed on each dentine slice was measured using Image Pro-Plus image analysis software.
Neutralizing monoclonal antibodies against IL-10 (10 µg/ml) and TGF-β1 (10 µg/ml) were added to the cocultures at ratios of 10∶50 (i.e., BMCS 1×106/well and Treg cells 2×105/well) for 7 or 10 days in the presence of M-CSF (50 ng/ml) and RANKL (50 ng/ml) using an immunoglobulin isotype as a control.
Effect of E2 on OC function in the coculture with Treg cells
The cell cocultures containing a 10∶50 ratio of BMCs (1×106/well) and Treg cells (2×105/well) were treated with different doses of E2 (10−10, 10−9, 10−8 and 10−7 mol/l) for either 7 or 10 days, with cultures of BMCs or Treg cells alone treated with vehicle (ethanol) as controls. All of the cultures were grown in the presence of M-CSF (50 ng/ml) and RANKL (50 ng/ml) without phenol red to avoid its weak estrogen agonist effect with charcoalized FBS. After 24 h of treatment with E2, the supernatant was harvested, and IL-10 and TGF-β1 levels were measured using a quantitative sandwich ELISA. 7 day after treatment, the cells were cytochemically stained for TRAP, and 10 day after treatment, the resorption pits on the dentine slices were stained using toluidine blue.
Statistical analysis
All results are expressed as mean±standard deviation (SD). All data were analyzed using the SPSS database and one-way ANOVA analysis, and P values less than 0.05 were considered to represent statistical significance.
Results
Characterization of CD4+CD25+ Treg cells and OCs
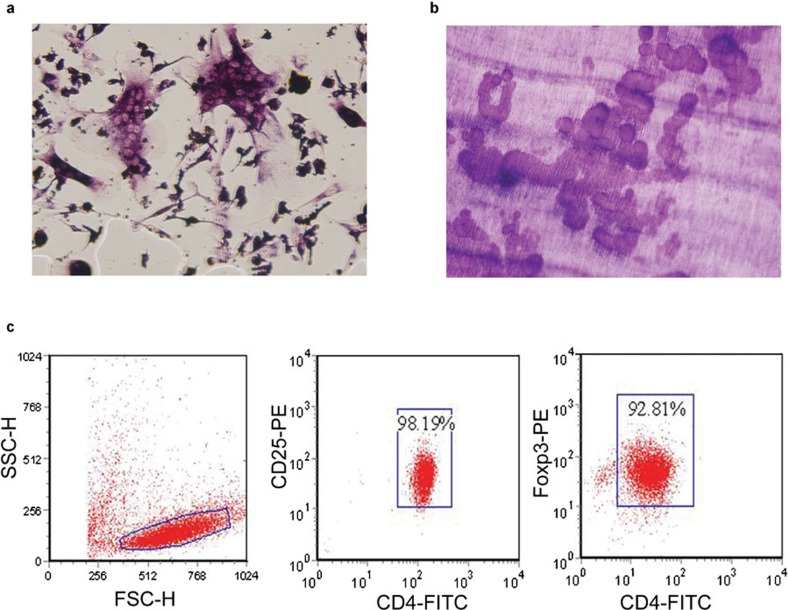
CD4+CD25+ Treg cells were isolated from PBMCs using magnetic-activated cell sorting. Flow cytometric analysis revealed a purity of 98.19% for the CD4+CD25+ T cells and 92.81% for the CD4+Foxp3+ T cells. OCs were induced by culturing the BMCs for 7 days in the presence of M-CSF (50 ng/ml) and RANKL (50 ng/ml). TRAP-positive multinuclear cells began to form on the fifth day of culture. On the seventh day, the OC precursors began to differentiate by making contacting and fusing together, forming larger cells with more than three nuclei. The OC is a polykaryon that is unusually large and contains TRAP-positive granules. The numbers of cells per unit of surface area was counted using a light microscope. On the dentine slices, rounded, elliptic and sausage-like bone lacunae were observed on the tenth day. Functional evidence of the existence of OCs was obtained by measuring the resorption area on the dentine slices (Figure 1).
Figure 1.
Osteoclasts stained with TRAP (a, ×200), bone resorption lacuna stained with toluidine blue (b, ×200) and the characteristics of peripheral human CD4+CD25+Foxp3+ Treg cells (c). TRAP, tartrate-resistant acid phosphatase.
Suppressive effect of Treg cells on the formation and function of OCs
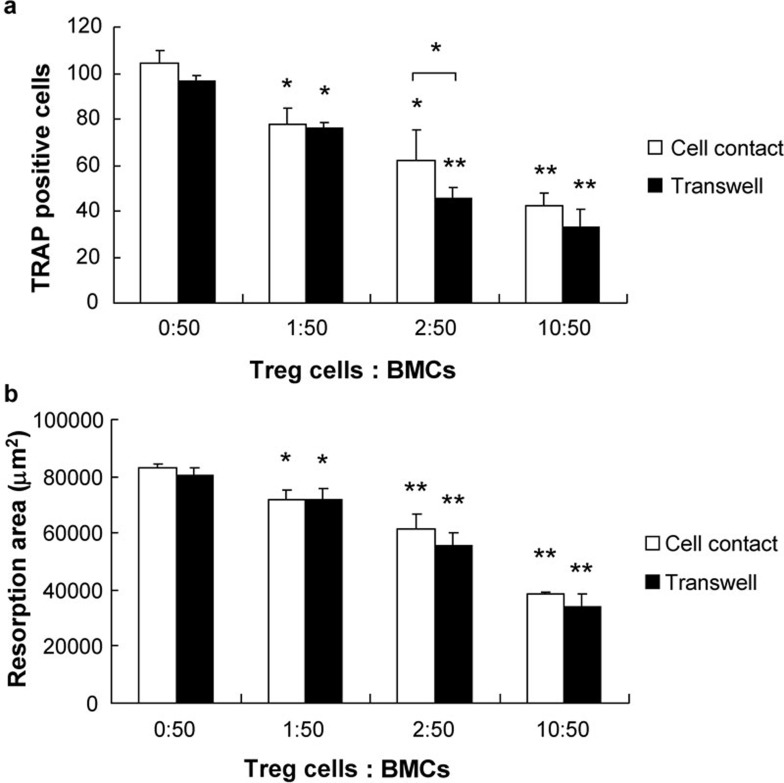
To investigate whether Treg cells affect the differentiation of OCs from BMCs, we used BMCs that were cocultured with anti-CD3- and anti-CD28-activated Treg cells; we found that the number of TRAP-positive multinucleated OCs was significantly lower in the coculture with Treg cells, indicating that Treg cells inhibit the formation of OCs. To assess the effect of Treg cells on OC bone-resorbing ability, we performed the same coculture on dentine slices and observed a significant dose-dependent reduction in the area of lacuna in the coculture with Treg cells. This suggests that Treg cells inhibit M-CSF and RANKL-induced OC formation and bone resorption in a dose-dependent manner (Figure 2).
Figure 2.
Inhibitory actions of activated Treg cells on OC formation and bone resorption. BMCs and activated Treg cells were cocultured in either cell contact and non-contact transwells in the presence of 50 ng/ml M-CSF and 50 ng/ml RANKL for either 7 or 10 days. Treg cells were activated with a soluble anti-CD3 and cnti-CD28 monoclonal antibody (5 µg/ml) with IL-2 (100 µg/ml) for 3=d prior to being added to the coculture. Treg cells inhibited OC formation and functional bone resorption in both of the cocultures. No significant difference in the suppressive effect of Treg cells between the two cocultures was found. The data are expressed as mean±SD (n=5). *P<0.05, **P<0.01, compared with controls. BMC, bone marrow cell; OC, osteoclast; RANKL, receptor activator of nuclear factor kappa B ligand; TRAP, tartrate-resistant acid phosphatase; Treg, regulatory T.
To determine whether the suppressive effect of Treg cells depends on cell contact, BMCs and Treg cells were cocultured in transwells under conditions that either allowed for or prohibited cell contact. No significant difference in the suppressive effects of Treg cells on OC formation and functional bone resorption between the cocultures was observed, suggesting that Treg cells inhibit the formation and function of OCs via a mechanism that involves soluble molecules.
Suppression of Treg cells on OC formation and function by secretion of TGF-β1 and IL-10
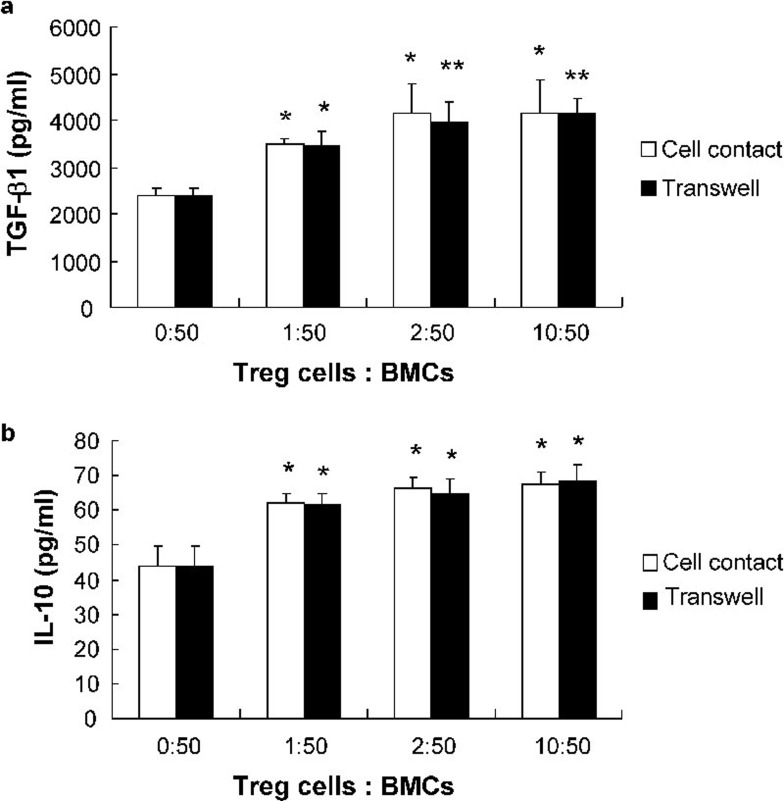
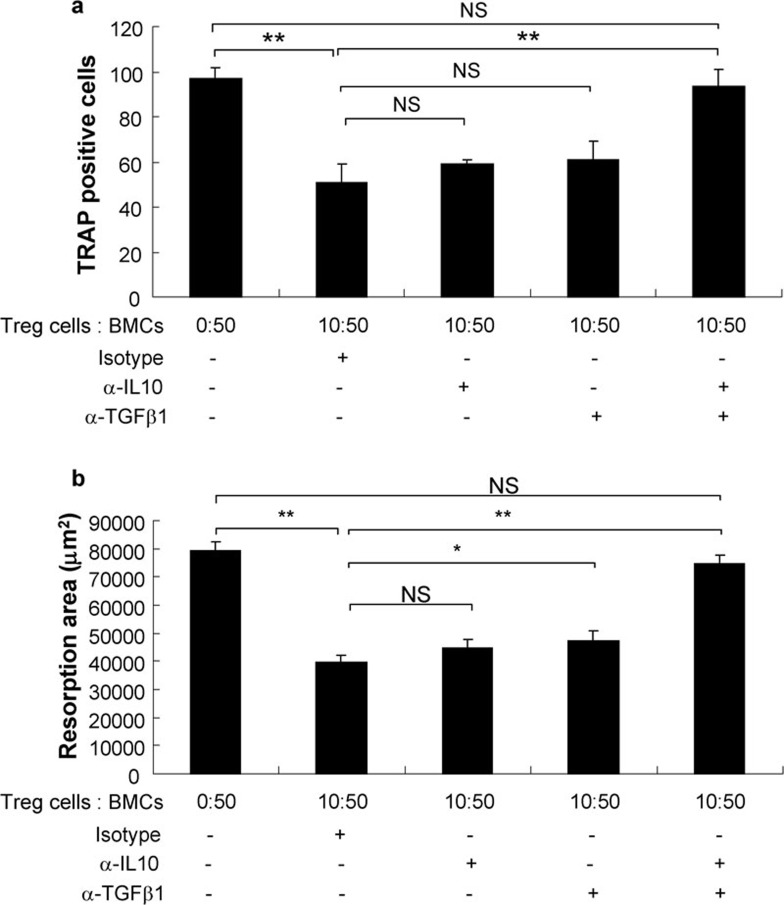
A previous study by our lab showed that TGF-β1 and IL-10 both suppress osteoclastogenesis and bone resorption in a dosage-dependent manner and that these two compounds exhibit a synergistic effect.11 Therefore, we measured the production of TGF-β1 and IL-10 in the coculture supernatants using ELISA. The levels of TGF-β1 and IL-10 in the cocultures were significantly higher than those observed in cultures of BMCs alone and increased with an increase in the ratio of Treg cells to BMCs. No significant difference was observed between supernatants from cell contact and non-contact transwell cocultures. Moreover, a combination of anti-IL10 and anti-TGF-β1 neutralizing antibodies completely abolished the suppressive effects of Treg cells, although neither anti-IL10 nor anti-TGF-β1 neutralizing antibodies alone could (Figures 3 and 4).
Figure 3.
IL-10 and TGF-β1 levels in BMCs and Treg cell coculture supernatants. The levels of IL-10 and TGF-β1 in BMC and Treg cell coculture supernatants were measured by ELISA. The data are expressed as mean±SD (n=5). *P<0.05, **P<0.01, compared with controls. BMC, bone marrow cell; TGF, transforming growth factor; Treg, regulatory T.
Figure 4.
Treg cells inhibit OC formation and bone resorption via the secretion of IL-10 and TGF-β1. The formation of OCs (a) and the surface area of bone resorption lacuna (b) were evaluated after neutralization with antibodies against IL-10 and TGF-β1 in BMC and Treg cell cocultures. The data are expressed as mean±SD (n=5) *P<0.05, **P<0.01, compared with each other. BMC, bone marrow cell; NS, not significant; OC, osteoclast; TGF, transforming growth factor; Treg, regulatory T.
The effect of estrogen on the differentiation and function of OCs in cocultures with CD4+CD25+ Treg cells
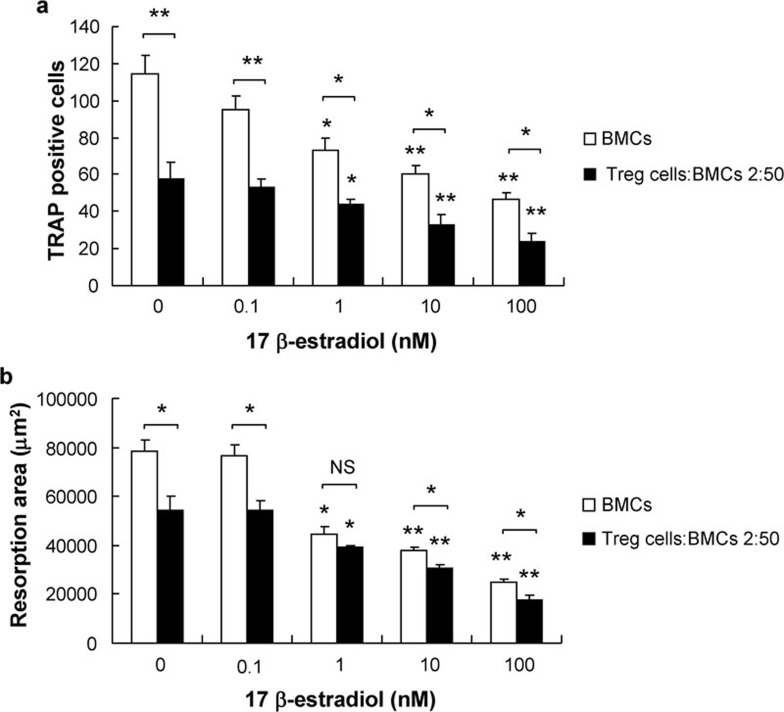
To elucidate the involvement of Treg cells in the effects of estrogen on bone metabolism, Treg cells and BMCs were cocultured at ratios of 10∶50, 2∶50 and 1∶50; these cocultures were then treated with different concentrations of E2. Osteoclastogenesis rarely occurred when the ratio of Treg cells to BMCs approached 1∶5; therefore, we chose a 2∶50 coculture ratio of Treg cells to BMCs for further research.
The number of TRAP-positive multinucleated OCs and the area of pit formation were both efficiently suppressed by E2 at concentrations between 10−7 and 10−9 M in either cultures of BMCs alone or in cocultures containing both BMCs and Treg cells. The direct inhibitory effect of E2 on OC differentiation and bone resorption was dose-dependent; when the concentration of E2 was reduced to 10−10 M, it had no significant effect. Compared with cultures of BMCs alone, the number of OCs and the area of bone resorption were more efficiently suppressed by E2 in cocultures with Treg cells, demonstrating that the inhibitory effect of E2 is enhanced by the presence of Treg cells in cocultures. When the concentration of E2 was reduced to 10−10 M, this inhibitory effect was no longer observed in the cocultures (Figure 5).
Figure 5.
The effect of E2 on OC formation and bone resorption in BMC and Treg cell cocultures. BMCs were cocultured with Treg cells in cell-contact transwells at a ratio of 2∶50 on slides (a) and dentine slices (b), with BMC and Treg cell monocultures as controls. E2 was added at the indicated concentrations, with solvent alone included as a control. The data are expressed as mean±SD (n=5). *P<0.05, **P<0.01, compared with controls. BMC, bone marrow cell; E2, 17β-estradiol; OC, osteoclast; TGF, transforming growth factor; TRAP, tartrate-resistant acid phosphatase; Treg, regulatory T.
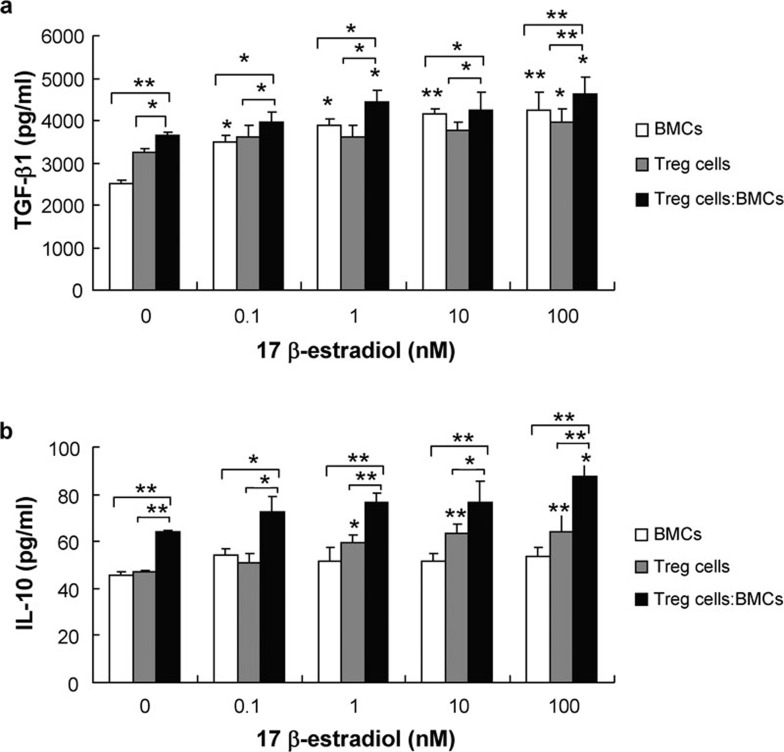
We found that the levels of IL-10 and TGF-β1 in the cocultures treated with E2 were higher than those observed in cultures of BMCs or Treg cells alone. When the concentration of E2 reached 10−7 M, the levels of both IL-10 and TGF-β1 in the supernatants of BMC and Treg cell cocultures were significantly increased (Figure 6).
Figure 6.
The effect of E2 on the secretion of IL-10 and TGF-β1 in BMC and Treg cell cocultures. A total of 24 h after BMCs were cocultured with Treg cells at a ratio of 2∶50 with E2 at the indicated concentrations; the levels of IL-10 and TGF-β1 in the supernatant were measured by ELISA. The data are expressed as mean±SD (n=5). *P<0.05, **P<0.01, compared with controls. BMC, bone marrow cell; E2, 17β-estradiol; TGF, transforming growth factor; Treg, regulatory T.
Discussion
OC differentiation and function are regulated by T and B lymphocytes, marrow stromal cells and OCs in the local bone microenvironment. All of these cell types produce cytokines, chemokines and hormones that can either stimulate or inhibit osteoclastogenesis and resorption activity.18 Previous experimental evidence obtained both in vivo and in vitro suggested that Treg cells play a role in bone resorption in cases of rheumatoid arthritis19, 20 and periodontal disease.21 Ernst et al. found that the frequency of forkhead box P3 (FoxP3)/CD25 double-positive cells was strikingly reduced in bone resorption lesions occurring in cases of periodontal disease, with a corresponding increase in RANKL+ T cells.22 Kelchtermans et al. found that activated CD4+CD25+ Treg cells improve the clinical symptoms of collagen induced arthritis, regulate cytokine production and inhibit osteoclastogenesis both in vitro and in vivo.23
The naturally occurring CD4+CD25+Foxp3+ T cells (nTreg cells) that develop in the thymus and constitute approximately 5–10% of peripheral CD4+ T cells play a pivotal role in the maintenance of immunological self-tolerance and the modulation of immune responses. The nTreg cells constitutively expressed the α-chain of IL-2 receptor (CD25), intracellular cytotoxic T lymphocyte antigen, glucocorticoid-induced TNF receptor and forkhead box P3, a key transcription factor that is required for their development, maintenance and function.24, 25 In our study, the purified cells showed purity levels of 98.19% for CD4+CD25+ and of 92.81% for CD4+Foxp3+, both characteristic surface markers of CD4+CD25+ Treg cells. Because the purities of levels of both were greater than 90%, we used the cells as CD4+CD25+Foxp3+ T cells in subsequent experiments. Currently, there is no consensus regarding the mechanism of Treg cell action in inhibiting immune responses. Treg cells modulate the immune response through three primary mechanisms: (i) the direct killing of cytotoxic cells through cell-to-cell contact; (ii) the inhibition of cytokine production by cytotoxic cells, in particular IL-2; and (iii) the direct secretion of immunomodulatory cytokines, such as TGF-β and IL-10.26
In this study, we investigated whether Treg cells affect OC formation and functional bone resorption and how such effects may occur. Our results showed that Treg cells inhibit the differentiation of OCs from BMCs in addition to the bone resorption of mature OCs. Furthermore, we found that this suppression is cytokine-dependent and does not require direct cell-to-cell contact. We previously demonstrated that both TGF-β1 and IL-10 suppress osteoclastogenesis and bone resorption in a dose-dependent manner and together exhibit synergistic effects.11 Here, we found that the levels of both TGF-β1 and IL-10 are higher in the supernatants of BMC and Treg cell cocultures than in the supernatants of the respective monocultures. The combined administration of anti-IL-10 and anti-TGF-β1 neutralizing antibodies completely abolished the suppressive effects of Treg cells, although administration of neutralizing antibodies against just one of the cytokines alone had no effect. Our data show that Treg cells suppress osteoclastogenesis and bone resorption in a TGF-β1 and IL-10 cytokine-dependent manner; therefore, TGF-β1 and IL-10 both play pivotal roles in the coculture system.
In recent years, immune cells have been linked to the etiology of both postmenopausal and senile osteoporosis in addition to bone loss caused by a variety of endocrine conditions. D'Amelio et al. suggested that estrogen deficiency stimulates OC formation by increasing the production of both TNF-α and RANKL, in turn increasing the number of OC precursors. Women with postmenopausal osteoporosis exhibit higher T-cell activities than healthy postmenopausal subjects; thus, T cells contribute to the bone loss caused by estrogen deficiency in humans and mice.3 Estrogen plays a pivotal role as an inhibitor of bone resorption by acting directly on OCs to decrease their resorption activity and increase their rate of apoptosis.27 In our study, both OC differentiation and bone resorption were efficiently suppressed by E2 at concentrations that fall within the ranging from 10−7 to 10−9 M; importantly, these concentrations fall within those that occur under physiological conditions. In BMCs and Treg cell cocultures that were treated with E2, both OC differentiation and bone resorption were suppressed more efficiently than in cultures that contained BMCs alone. Furthermore, osteoclastogenesis in the presence of E2 is rarely observed when the ratio of Treg cells to BMCs approaches 10∶50. Therefore, Treg cells enhance the inhibition of E2 on OC differentiation and function. Tai et al. found that E2 injection increases the number of CD4+CD25+ T cells in different lymphoid tissues (such as spleen and inguinal lymph nodes) in addition to enhancing the expression of Foxp3 in Treg cells and inducing secretion of more IL-10 but not TGF-β from these cells.28 IL-10, an immune suppressive cytokine that is secreted by Treg cells, plays a critical role in the regulation of osteoclastogenesis and bone resorption. In this study, we found that E2 at a concentration of 10−7 M increases the production of both IL-10 and TGF-β1 in the supernatant of BMCs and Treg cell cocultures in comparison to either monocultures or untreated cocultures, therefore demonstrating that E2 can enhance the secretion of both IL-10 and TGF-β1. After the addition of E2, the concentrations of IL-10 and TGF-β1 in both cocultures and Treg cell monocultures increased in comparison with BMC monocultures, thus indicating that E2 enhances the suppressive effects on OC differentiation and bone resorption by stimulating IL-10 and TGF-β1 secretion from Treg cells. The specific mechanisms responsible for the actions of IL-10 and TGF-β1 on osteoclastogenesis and bone resorption remain to be investigated. Mohamed et al. found that IL-10 inhibited RANKL-induced osteoclastogenesis primarily through the inhibition of NFATc1 expression at both mRNA and protein levels in RAW264.7 cells and mouse bone marrow cells. In addition, IL-10 inhibited the expression of both c-Fos and c-Jun in RAW264.7 cells and mouse bone marrow cells. Both c-Fos and c-Jun were reported to be critical for transcriptional activation of NFATc1 in RANKL-induced osteoclastogenesis. IL-10 also decreased the RANKL-induced expression of NF-κB p50 and phosphorylation of c-Jun N-terminal kinase.29 Evans and Fox reported that IL-10 inhibited the RANKL-induced expression and translocation of NFATc1 by disrupting Ca2+ mobilization in RAW264.7 cells.30 Park-Min et al. found that IL-10 inhibited RANK-induced osteoclastogenesis and selectively inhibited calcium signaling downstream of RANK by inhibiting transcription of TREM-2. Downregulation of TREM-2 expression resulted in diminished RANKL-induced activation of the CaMK–MEK–ERK pathway and decreased the expression levels of NFATc1, the master regulator of osteoclastogenesis.31 Fox et al. demonstrated that TGF-β primed monocytes for osteoclast formation within 24 h by directly inducing cytoplasmic NFATc1 expression, although TGF-β was unable to stimulate NFATc1 nuclear translocation. TGF-β enables NFATc1 expression during osteoclastogenesis.32 Further investigation is required to determine how these mechanisms relate to the findings of this study.
In the present study, we demonstrated that CD4+CD25+Foxp3+ Treg cells suppress OC differentiation and bone resorption via the secretion of IL-10 and TGF-β1. Furthermore, we found that 10−7 M E2 enhances both IL-10 and TGF-β1 secretion from Treg cells, thus further inhibiting OC differentiation and bone resorption. Therefore, Treg cell-derived IL-10 and TGF-β1 are likely involved in the E2-mediated regulation of bone metabolism via their activities against OCs. These findings provide new insight into mechanisms and therapeutic strategies for PMO.
Acknowledgments
We thank the volunteers for generously participating in this study. This work was supported by the National Key Research Program of China (No. 2006CB944007; to DJL), the National Natural Science Foundation of China (No. 30801502; to LW), the Shanghai Leading Academic Discipline Project B117 (to DJL) and the Program for Outstanding Medical Academic Leaders (to DJL).
References
- Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- D'Amelio P, Grimaldi A, Di Bella S, Tamone C, Brianza SZ, Ravazzoli MG, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER, et al. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993;91:257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theill LE, Boyle WJ, Penninger JM. RANKL and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2005;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL–RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Lorenzo J. Cytokines regulating osteoclast formation and function. Curr Opin Rheumatol. 2006;18:411–418. doi: 10.1097/01.bor.0000231911.42666.78. [DOI] [PubMed] [Google Scholar]
- Luo CY, Wang L, Sun C, Li DJ. Impact of IL-10 and TGF-beta1 from CD4+CD25+ regulatory T cells on the osteoclast differentiation and bone resorption. Chin J Endocrinol Metab. 2009;25:434–437. [Google Scholar]
- Arron JR, Choi Y. Osteoimmunology: bone versus immune system. Nature. 2000;408:535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Kim YG, Lee CK, Nah SS, Mun SH, Yoo B, Moon HB. Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2007;357:1046–1052. doi: 10.1016/j.bbrc.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Zaiss MM, Axmann R, Zwerina J, Polzer K, Guckel E, Skapenko A, et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arhritis Rheum. 2007;56:4104–4112. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim T, Jeong D, Kim N, Choi Y. The tec family tyrosine kinase Btk regulates RANKL-induced osteoclast maturation. J Biol Chem. 2008;283:11526–11534. doi: 10.1074/jbc.M708935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays for T cell function. Curr Protoc Immunol. 2004;Chapter 3:3.12.11–3.12.15. doi: 10.1002/0471142735.im0312s60. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Regulation of osteoclast differentiation. Ann NY Acad Sci. 2006;1068:100–109. doi: 10.1196/annals.1346.013. [DOI] [PubMed] [Google Scholar]
- van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4+CD25+ regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335–R346. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T, Ueki-Maruyama K, Oda T, Ohsawa Y, Ito H, Seymour GJ, et al. Regulatory T-cells infiltrate periodontal disease tissues. J Dent Res. 2005;84:639–643. doi: 10.1177/154405910508400711. [DOI] [PubMed] [Google Scholar]
- Ernst CW, Lee JE, Nakanishi T, Karimbux NY, Rezende TM, Stashenko P, et al. Diminished forkhead box P3/CD25 double-positive T regulatory cells are associated with the increased nuclear factor-kappaB ligand (RANKL+) T cells in bone resorption lesion of periodontal disease. Clin Exp Immunol. 2007;148:271–280. doi: 10.1111/j.1365-2249.2006.03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68:744–750. doi: 10.1136/ard.2007.086066. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev. 2008;7:370–375. doi: 10.1016/j.autrev.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Riggs BL. The mechanism of estrogen regulation of bone resorption. J Clin Invest. 2006;106:1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- Mohamed SG, Sugiyama E, Shinoda K, Taki H, Hounoki H, Abdel-Aziz HO, et al. Interleukin-10 inhibits RANKL-mediated expression of NFATc1 in part via suppression of c-Fos and c-Jun in RAW264.7 cells and mouse bone marrow cells. Bone. 2007;41:592–602. doi: 10.1016/j.bone.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Evans KE, Fox SW. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007;8:1–9. doi: 10.1186/1471-2121-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Min KH, Ji JD, Antoniv T, Reid AC, Silver RB, Humphrey MB, et al. IL-10 suppresses calcium-mediated costimulation of receptor activator NF-kappa B signaling during human osteoclast differentiation by inhibiting TREM-2 expression. J Immunol. 2009;183:2444–2455. doi: 10.4049/jimmunol.0804165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SW, Evans KE, Lovibond AC. Transforming growth factor-β enables NFATc1 expression during osteoclastogenesis. Biochem Biophys Res Commun. 2008;366:123–128. doi: 10.1016/j.bbrc.2007.11.120. [DOI] [PMC free article] [PubMed] [Google Scholar]