Abstract
In this study, 40 biopsy samples collected from cervical cancer patients at the First Affiliated Hospital of Xi'an Jiaotong University, China, were retrospectively assessed using immunohistochemistry for CD4+ and CD8+ tumor-infiltrating lymphocytes (TILs) and were analyzed for the expression of FOXP3, OX40, granzyme B (GrB) and perforin (Prf). The proliferating index of the TILs was determined by assessing Ki67 expression. We determined the prognostic value of low and high numbers of TILs on survival by performing Kaplan–Meier analysis using median values as the cut-off points. Except for the number of CD4+FOXP3+ regulatory T cells (Tregs) and the CD4/CD8 ratio, none of the CD4+, CD8+, OX40+, GrB+ or Prf+ TILs were associated with the overall 5-year survival rate. The 5-year survival rate was significantly lower in patients who had a high percentage of Tregs as compared with the those who had a lower percentage (35.3% versus 88.9%, P=0.001), while the 5-year survival rate was significantly higher in patients with a high CD4/CD8 ratio as compared with patients who had a low CD4/CD8 ratio (82.4% versus 44.4%, P=0.029). When we considered the deaths and surviving cases as separate groups, we found that both the number of CD4+ T cells and the CD4/CD8 ratio were significantly lower in patients who died as compared with those who survived (26.33±11.80 versus 47.79±38.18, P=0.023 and 0.60±0.25 versus 1.17±1.02, P=0.019, respectively). In conclusion, decreased proportions of tumor-infiltrating CD4+ T cells with high percentages of Tregs and reversed CD4/CD8 ratios were significantly associated with the clinical outcome of patients with cervical carcinoma.
Keywords: CD4/CD8 ratio, cervical carcinoma, clinical outcome, tumor-infiltrating lymphocytes
Introduction
Tumor-infiltrating lymphocytes (TILs) are a part of the tumor surveillance system.1 They are frequently present in solid human tumors,2 and their role in different types of cancers has been controversial. The presence of TILs correlates with a better prognosis in several types of cancer, and each T-lymphocyte subset has a unique role in the antitumor response.3, 4, 5, 6, 7 The presence of tumor-infiltrating cytotoxic T lymphocytes (CTLs) has been linked to better patient survival in endometrial, ovarian, pancreatic and colorectal cancers.8, 9, 10 The role of CD4+ T cells is more controversial and is often considered to be a double-edged immunologic sword because CD4+ T cells play a central role in initiating and maintaining anticancer immune responses.11, 12, 13 CD4+ T-cell help is needed during the primary antigen-specific response to imprint CD8+ T cells with the ability to develop into long-lived functional memory cells.14 In antitumor immunity, CD4+ T cells have also been shown to be important in sustaining the functions of adoptively transferred CD8+ T cells.15 On the other hand, naturally occurring subsets of CD4+ T cells called regulatory T cells (Tregs) play an essential role in controlling immune responses to self- and non-self-antigens. Tumor-infiltrating Tregs have been reported to be associated with poor prognosis in some cancers because they suppress the proliferation of effector T lymphocytes (i.e., CTL), which prohibits an adequate tumor-specific immune response and enables tumor growth.16, 17, 18, 19, 20, 21 In contrast, tumor-infiltrating Tregs have also been reported to be either associated with a better prognosis or not associated with prognosis in some malignancies such as follicular lymphoma and squamous cell carcinoma.22, 23, 24
Antitumor immune responses are attributed mainly to cell-mediated immunity. The activation of both CD4+ and CD8+ T lymphocytes is needed for an efficient immune response that destroys tumor cells.11, 12, 13, 15 In this scenario, the infiltration of the tumor site with high numbers of CD8+ TILs would be desirable. The presence of CD4+ TILs would also be required because CD8+ T cells usually need CD4+ T cells to optimally function. The ratio of CD4+/CD8+ T cells is likely a key parameter for appropriate TIL function; this ratio may be different for different types of cancer.
Recently, the immunogenicity of malignancy has been reappraised according to the type of immune response provoked by the tumor. In our previous study, we observed that highly immunogenic tumor cells in syngeneic mice can induce type I immune responses, whereas poorly immunogenic tumor cells induce type II immune responses.25 Using an assay to determine the immune microenvironment of cervical cancer, others have shown a type II predominance.26, 27 Cervical cancer is one of the most common malignancies of married women globally and is the result of an uncontrolled persistent infection with a high-risk human papillomavirus (HPV) type, HPV16 and HPV18 in particular, which account for approximately two-thirds of these cancers.28, 29 The immune microenvironment of cervical cancer not only shares the nature of solid tumors in general, but also possesses the properties associated with its etiology. HPV may use different strategies to evade immune recognition. These characteristics make the microenvironment of cervical cancer more complicated.
In the present study, to examine the immune status of cervical carcinoma in situ by immunohistochemistry, we evaluated the status of CTLs and Tregs by characterizing their functional phenotypes (using granzyme B (GrB) and FOXP3) as markers of the immune response. This approach may contribute novel information to the field of cervical cancer immunology.
Materials and methods
Samples
Under the approval of the institutional review board and ethics committee, biopsy samples from 40 cervical cancer patients were collected from the First Affiliated Hospital of the Medical College of Xi'an Jiaotong University. All cases had follow-up data for at least 5 years. No cases received radiation or chemotherapy prior to operation but were treated with radiotherapy after diagnosis and biopsy collection. All cases were histopathologically classified according to the International Federation of Obstetrics and Gynecology (FIGO) criteria. The tissue samples were fixed in formalin and embedded in paraffin. All cases were positive for HPV by PCR.30
Immunohistochemistry
Immunohistochemistry staining was conducted on 4- to 5-µm paraffin sections using horseradish peroxidase from the Envision Kit or the Catalyzed Signal Amplification Kit (Dako Corporation, Copenhagen, Denmark) for single staining, and alkaline phosphatase was used for double staining, as recommended by the manufacturer. The primary antibodies were mouse monoclonal anti-human anti-CD8 (DakoCytomation, Glostrup, Denmark; 1∶100 dilution), anti-GrB (Novocastra, Newcastle, UK; 1∶100), anti-Perforin (Prf; Novocastra; 1∶20), anti-CD4 (Novocastra; 1∶30), anti-OX40 (Novocastra; 1∶50), anti-FOXP3 (Abcam, Cambridge, UK; 1∶50), and anti-Ki67 (DakoCytomation; 1∶200). Briefly, the sections were dewaxed in xylene and rehydrated using graded concentrations of ethanol and distilled water. Endogenous peroxidase activity was blocked by submersion of the sections in a 0.5% H2O2/methanol solution for 10 min at room temperature (RT). Antigen was retrieved by heating the sections under high temperature and pressure in a stainless steel pressure cooker for 1 min and 30 s in unmasking solution (0.01 M citrate buffer, pH 6.0). After cooling the cooker to RT in running tap water, the sections were placed in TBS-Tween buffer for 5 min, blocked with 3% goat serum for 10 min at 37 °C, and incubated with primary antibody (at RT: CD8, 1 h; GrB, 2 h; Prf, 2 h; OX40, 1 h; Ki67, 1 h; and CD4 overnight at 4 °C). After the sections were rinsed three times with TBS-Tween buffer, they were subsequently incubated with Dako EnVision (or AP 1∶500 in the case of double staining) for 30 min or processed according to the instructions of the Catalyzed Signal Amplification Kit at RT. After the sections were washed with TBS-Tween buffer, the antigen–antibody reaction was visualized using 3,3′-diaminobenzidine in the case of horseradish peroxidase and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium in the case of AP, and the sections were counterstained with hematoxylin or methyl blue, respectively. Tonsil tissue was used as a positive control for all the antibodies. The numbers of labeled TILs were counted by the classical counting method with the light microscope using an ocular grid at ×200 magnification. Labeled cells were counted in the whole tissue section, and the total number of labeled cells was divided by the number of fields to calculate the mean value for each case. Counting was performed twice for each slide.
Statistical analysis
The non-parametric Wilcoxon signed-rank test was used to compare the mean number of TILs among the groups and subgroups. Follow-up time was calculated as the interval between the date of treatment and last follow-up or death. Survival rates were analyzed with the Kaplan–Meier method using the log-rank test to assess the difference between survival curves. A P value of less than 0.05 was considered statistically significant. Statistical calculations were performed using SPSS version 14.0 for Windows.
Results
Clinicopathological characteristics of patients
The clinicopathological characteristics of patients are provided in Table 1. The immunohistochemical variables are provided in Table 2. Among the 40 cases studied, five cases were not evaluable after staining, so they were excluded. The remaining 35 cases were evaluated. All data are shown as mean±SD.
Table 1. Clinical characteristics of 40 patients with squamous cell carcinoma of the cervix.
| Characteristics | No. (%) |
|---|---|
| Age | |
| Median | 47 |
| Range | 32–70 |
| Clinical stage (FIGO) | |
| IIB | 10 (25.0) |
| IIIB | 30 (75.0) |
| Lymph node (N) status | Unknown |
| Distant metastasis (M) status | |
| M0 | 38 (95.0) |
| M1 | 2 (5.0) |
| Progression | |
| No | 32 (80.0) |
| Yes | 8 (20.0) |
| Survival status | |
| Deceased | 15 (37.5) |
| Survivors | 25 (62.5) |
Table 2. Descriptive statistics of immunohistochemical variables.
| Variablea | Mean | SD | Median | Range |
|---|---|---|---|---|
| CD8+ TILs | 55.89 | 36.36 | 48.41 | 6.88–139.83 |
| Granzyme B+ TILs | 13.37 | 14.45 | 7.51 | 0.92–59.03 |
| Perforin+ TILs | 15.74 | 16.64 | 6.95 | 0.52–64.34 |
| CD8+FOXP3+ TILs | 3.32 | 4.05 | 1.89 | 0.10–19.70 |
| CD8+Ki67+ TILs | 0.96 | 1.22 | 0.43 | 0.00–5.44 |
| CD4+ TILs | 39.82 | 32.56 | 28.58 | 9.45–168.52 |
| OX40+ TILs | 3.66 | 3.91 | 2.1 | 0.00–12.65 |
| CD4+FOXP3+ TILs | 11.45 | 7.51 | 11.42 | 0.93–30.39 |
| CD4+Ki67+ TILs | 0.95 | 1.37 | 0.24 | 0.00–5.60 |
| CD4+/CD8+ ratio | 0.96 | 0.56 | 0.69 | 0.19–4.21 |
Abbreviation: TIL, tumor-infiltrating lymphocyte.
Number of TILs per field (×400).
Tumor-infiltrating CD8 T lymphocytes
CD8+ T lymphocytes (Figure 1) were found in both the stroma and tumor nest. Independent of whether the samples were from the deceased or surviving groups, the mean number of CD8+ T cells was lower in the stroma as compared with that in the tumor nest (21.06±16.58 versus 34.53±26.98, P=0.039 and 22.57±16.88 versus 33.51±27.79, P=0.050 in the deceased and surviving groups, respectively). Comparing the deceased and surviving groups, we observed no significant differences in either the stroma or the tumor nest, and we also observed no significant differences in the overall mean number of CD8+ T cells. When the patients were stratified by FIGO staging, the mean number of CD8+ T cells was higher in the tumor nest as compared with that in the stroma ( 20.19±14.42 versus 29.92±21.70, P=0.173; and 22.64±17.43 versus 35.22±29.01, P=0.018, in FIGO stage IIB and IIIB, respectively) (Table 3). The functional status of CD8+ T cells was evaluated using the expression of GrB and Prf. No significant differences were observed in the activated CTL either in the stroma or the tumor nest, nor overall in either group (decease or surviving, or FIGO IIB or IIIB groups; data not shown). As expected, the number of GrB+ and Prf+ TILs was consistent.
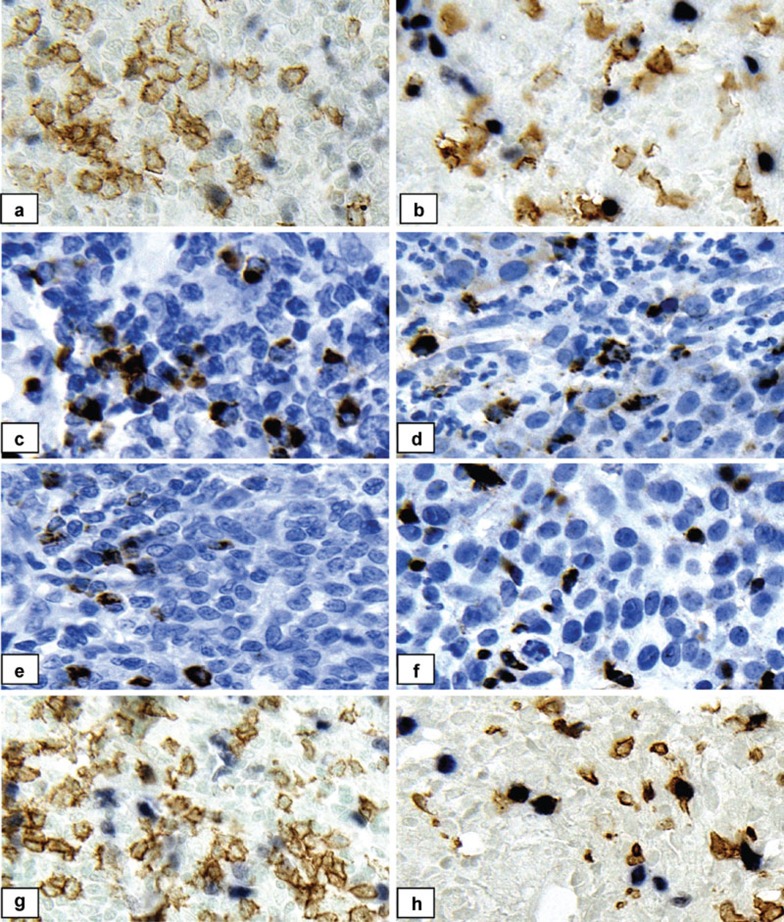
Figure 1.
Representative immunohistochemically stained specimens. The left panel shows positive controls (lymph nodes); the right panel shows cervical cancer tissues. (a, b) Double staining for CD8 (brown) and FOXP3 (pink). (c, d) Single staining for Granzyme B (brown). (e, f) Single staining for Perforin (brown). (g, h) Double staining for CD8 (brown) and Ki67 (pink). Counterstaining: hematoxylin for single immunostaining and methyl green for double immunostaining; magnification: ×400.
Table 3. Mean numbers of tumor-infiltrating CD8+ T cellsa.
| CD8+ TILs | Stroma | Tumor nest | Total |
|---|---|---|---|
| Survivors (22) | 22.57±16.88 | 33.51±27.79 | 56.07±36.05 |
| Deceased (13) | 21.06±16.58b* | 34.53±26.98 | 55.60±38.39 |
| FIGO IIB (9) | 20.19±14.42 | 29.92±21.70 | 50.07±29.34 |
| FIGO IIIB (24) | 22.64±17.43b* | 35.22±29.01 | 57.91±38.82 |
Abbreviation: TIL, tumor-infiltrating lymphocyte.
Data shown as mean±SD, *P<0.05.
Stroma versus tumor nest.
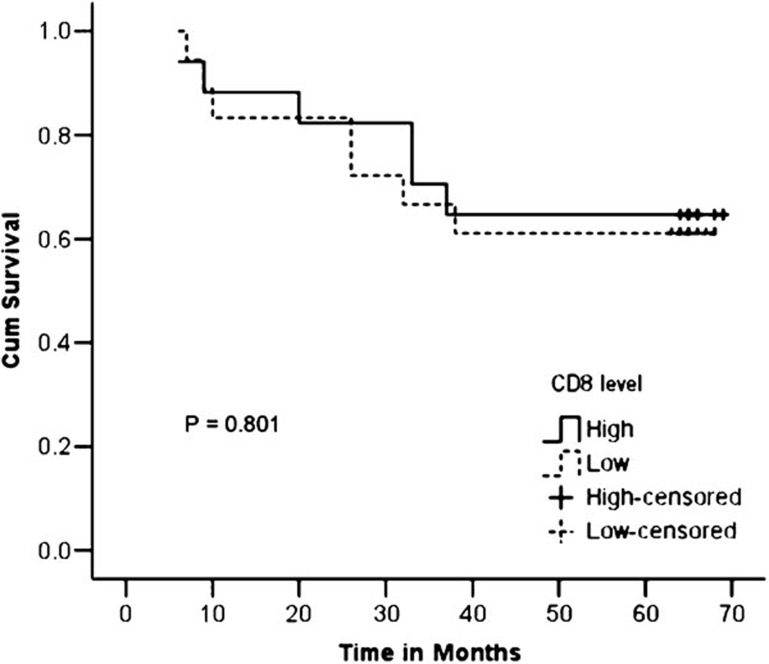
When the survival rate was determined using the Kaplan–Meier method and when the patients were divided into two groups (low and high levels of CD8+ T cells) based on the median value, no significant difference was observed in the 5-year survival rate between the low- and high-level groups (64.7% versus 61.1%, P=0.801) (Figure 2).
Figure 2.
Kaplan–Meier curves showing the 5-year survival rate with respect to the level of CD8+ T cells.
Tumor-infiltrating CD4+FOXP3+ Tregs
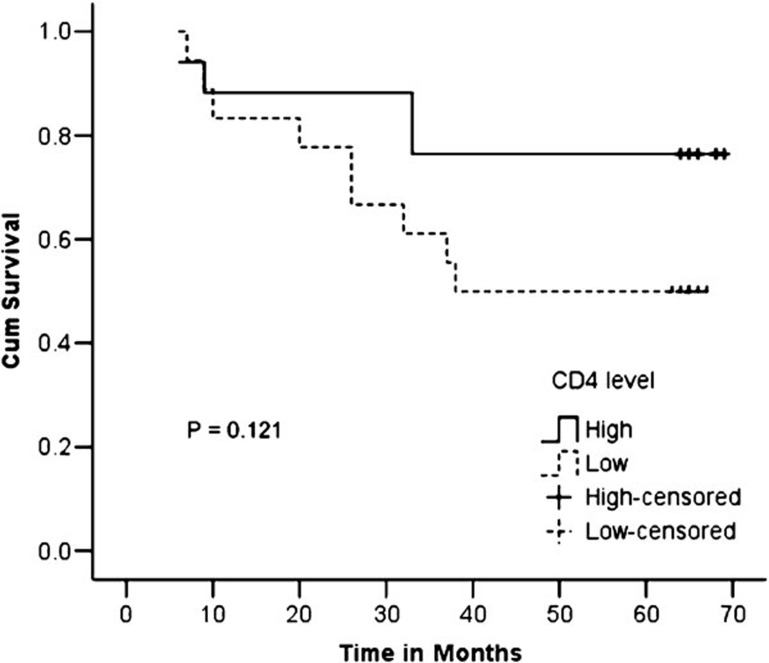
The mean number of CD4+ T cells (Figure 3) was almost the same in the stroma and tumor nest in the surviving group (23.79±23.97 versus 24.03±17.68, P=0.950), while the mean number was slightly higher in the stroma than in the tumor nest in the deceased group (15.98±9.73 versus 10.45±4.44, P=0.075). Comparing the deceased and surviving groups, we observed that the overall mean number of CD4+ T cells was significantly lower in the deceased group as compared with the mean number in the surviving group (26.33±11.80 versus 47.79±38.18, P=0.023); the same was observed when we compared the stroma and tumor nest separately (15.98±9.73 versus 23.79±23.97, P=0.039 and 10.45±4.44 versus 24.03±17.68, P=0.023, stroma and tumor nest, respectively). No significant differences were observed in the mean numbers of CD4+ T cells between the tumors-designated FIGO stage IIB and IIIB; no significant differences were observed between the groups or within the groups when the CD4+ T cells were counted overall or separately in the stroma and tumor nest (Table 4). However, although the number of CD4+ TILs was significantly lower in the deceased group as compared with those in the surviving group, we found no statistically significant difference in the 5-year survival rate between the groups with high or low levels of CD4+ TILs (76.5% versus 50.0%, P=0.121) (Figure 4).
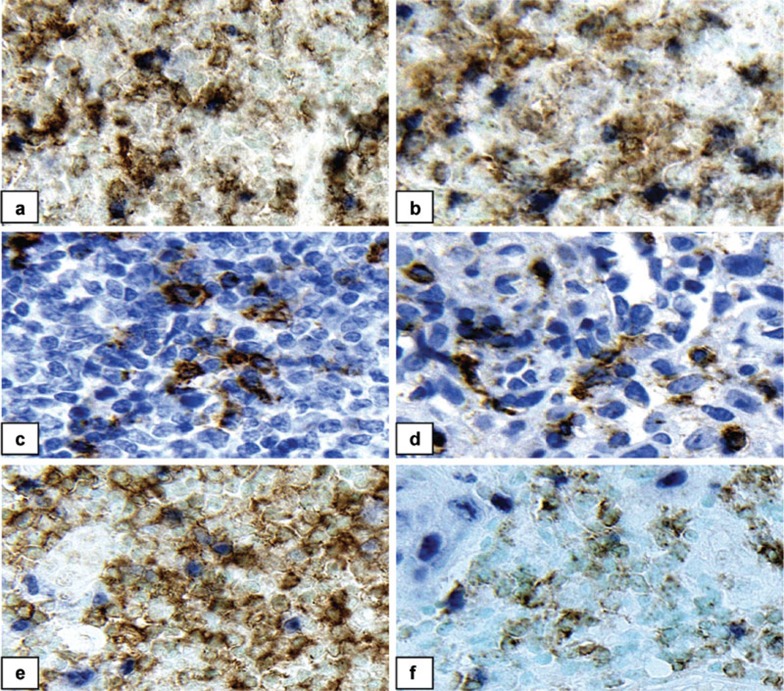
Figure 3.
Representative immunohistochemically stained specimens. The left panel shows positive controls (lymph nodes); the right panel shows cervical cancer tissues. (a, b) Double staining for CD4 (brown) and FOXP3 (pink). (c, d) Single staining for OX40 (pink). (e, f) Double staining for CD4 (brown) and Ki67 (pink). Counterstaining: hematoxylin for single immunostaining and methyl green for double immunostaining; magnification, ×400.
Table 4. Mean numbers of tumor-infiltrating CD4+ T cellsa.
| CD4+ TILs | Stroma | Tumor nest | Total |
|---|---|---|---|
| Survivors (22) | 23.79±23.97 | 24.03±17.68 | 47.79±38.18 |
| Deceased (13) | 15.98±9.73b* | 10.45±4.44c* | 26.33±11.80d* |
| FIGO IIB (9) | 22.54±11.87 | 16.34±11.21 | 38.88±21.45 |
| FIGO IIIB (24) | 20.32±22.40 | 19.90±16.99 | 40.14±35.97 |
Abbreviation: TIL, tumor-infiltrating lymphocyte.
Data shown as mean±SD, *P<0.05.
Comparison between stromas.
Comparisons between tumor nests.
Comparison between total.
Figure 4.
Kaplan–Meier curves showing the 5-year survival rate with respect to the level of CD4+ T cells.
To further differentiate the subgroups of CD4+ T cells, we evaluated the Tregs. The number of CD4+FOXP3+ T cells (Tregs) was significantly higher in the deceased group than in the surviving group in the stroma and tumor nest (47.64±20.41 versus 22.83±18.97, P=0.003 and 68.98±20.76 versus 27.96±21.27, P=0.005, respectively) and also overall (54.47±17.84 versus 23.53±15.59, P=0.002). Interestingly, the mean number of Tregs was significantly higher in the tumor nest than in the stroma in the deceased group (68.98±20.76 versus 47.64±20.41, P=0.023) and in the FIGO stage IIIB group (46.44±28.07 versus 35.16±6.15, P=0.046), while no significant differences were observed between the tumor nest or stroma in the surviving group (27.96±21.27 versus 22.83±18.97, P=0.465) or in the FIGO stage IIB group (33.83±30.94 versus 23.05±18.18, P=0.441) (Table 5).
Table 5. Percentage of CD4+FOXP3+ Tregsa.
| CD4+FOXP3+ %Tregs | Stroma | Tumor Nest | Total |
|---|---|---|---|
| Survivors (22) | 22.83±18.97 | 27.96±21.27 | 23.53±15.59 |
| Deceased (13) | 47.64±20.41b*c** | 68.98±20.76d** | 54.47±17.84e** |
| FIGO IIB (9) | 23.05±18.18 | 33.83±30.94 | 26.32±19.77 |
| FIGO IIIB (24) | 35.16±6.15b* | 46.44±28.07 | 38.03±22.54 |
Abbreviation: Treg, regulatory T cell.
Data shown as mean±SD, *P<0.05, **P<0.01.
Stroma versus tumor nest.
Comparison between stromas.
Comparisons between tumor nests.
Comparison between totals.
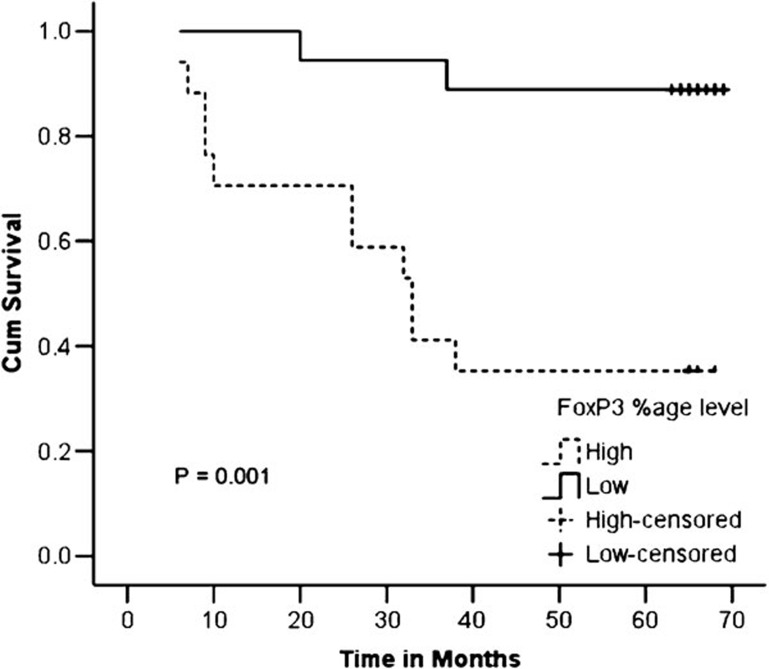
A significantly lower survival rate was observed in patients with high levels of CD4+FOXP3+ Tregs as compared with those with low levels of these cells (35.3% versus 88.9%, P=0.001) (Figure 5).
Figure 5.
Kaplan–Meier curves showing the 5-year survival rate with respect to the percentage CD4+FOXP3+ T cells.
Comparative numbers of CD4+ and CD8+ T lymphocytes and the CD4/CD8 ratio
The overall mean number of CD4+ T cells was significantly lower than the mean number of CD8+ T cells in the deceased group (26.33±11.80 versus 55.60±38.39, P=0.001), but the difference was not significant in the surviving group (47.79±38.18 versus 56.07±36.05, P=0.322). Similarly, there was no significant difference in the number of CD4+ T cells as compared with the number of CD8+ T cells in the stroma (15.98±9.73 versus 21.06±16.58, P=0.221), but it was significantly lower in the tumor nest (10.45±4.44 versus 34.53±26.98, P=0.001) of the deceased group. However, no significant differences were observed in the number of CD4+ T cells as compared with the number of CD8+ T cells either in the stroma or in the tumor nest in the surviving group.
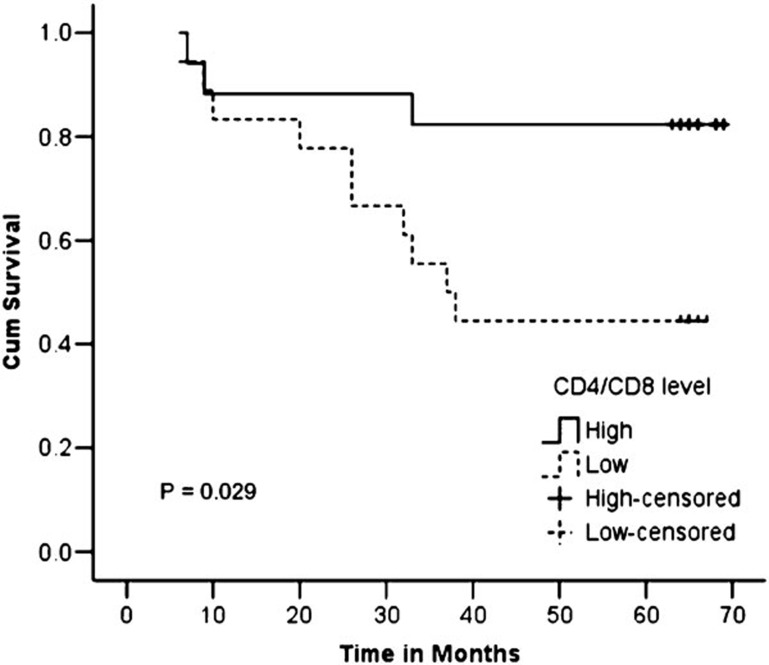
The ratio of CD4+/CD8+ T cells was significantly lower in the deceased group than in the surviving group (0.60±0.25 versus 1.17±1.02, P=0.019). In comparing the stroma and tumor nest of the two groups, we observed no significant difference in the ratios of CD4+/CD8+ T cells between the stroma of the deceased and surviving groups (0.99±0.66 versus 1.58±1.43, P=0.075), but the ratio was significantly lower in the tumor nest of the deceased group as compared with that of the surviving group (0.41±0.17 versus 1.16±1.13, P=0.006). In the within-group comparison, we found that the CD4+/CD8+ T-cell ratio was significantly lower in the tumor nest than in the stroma in the deceased group (0.41±0.17 versus 0.99±0.66, P=0.003), while we found no significant difference in the CD4+/CD8+ T-cell ratio between the tumor nest and stroma of the surviving group (1.16±1.13 versus 1.58±1.43, P=0.131). In comparing the FIGO stage IIB with IIIB groups, we observed no significant differences in the CD4+/CD8+ T-cell ratio between the two groups, either overall or separately in the tumor nest and stroma. The ratio of CD4+/CD8+ T cells was significantly lower in the tumor nest than in the stroma of the FIGO stage IIB group (0.70±0.43 versus 1.68±1.29, P=0.028), while this difference was non-significant in the FIGO stage IIIB group (0.94±1.09 versus 1.25±1.21, P=0.069) (Table 6). A significant difference was observed between the survival rates of patients with high and low CD4+/CD8+ T-cell ratios (82.4% versus 44.4%, P=0.029) (Figure 6).
Table 6. Ratio of CD4+/CD8+ T cellsa.
| CD4+/CD8+ TILs | Stroma | Tumor nest | Total |
|---|---|---|---|
| Survivors (22) | 1.58±1.43 | 1.16±1.13 | 1.17±1.02 |
| Deceased (13) | 0.99±0.66b* | 0.41±0.17c** | 0.60±0.25d* |
| FIGO IIB (9) | 1.68±1.29b* | 0.70±0.43 | 1.02±0.44 |
| FIGO IIIB (24) | 1.25±1.21 | 0.94±1.09 | 0.94±0.92 |
Abbreviation: TIL, tumor-infiltrating lymphocyte.
Data shown as mean±SD, *P<0.05, **P<0.01.
Stroma versus tumor nest.
Comparison between tumor nests.
Comparison between totals.
Figure 6.
Kaplan–Meier curves showing the 5-year survival rate with respect to the CD4+/CD8+ T-cell ratio.
Discussion
In this study, we tried to assess the number and functional status of TILs in situ in relation to the 5-year survival of 35 patients with cervical cancer in FIGO stage IIB and IIIB. With regard to the number of CD8+ TILs, we did not find any significant difference between the deceased and surviving groups or between the FIGO stage IIB and IIIB. Similarly, no differences were observed for the number of CD8+FOXP3+ T cells or proliferating CD8+Ki67+ T cells between the deceased and surviving groups and the FIGO stage IIB and IIIB groups (data not shown). Also, we observed no association between the number of CD8+ TILs and survival. This result is in contrast with those from a number of studies that describe CD8+ T lymphocytes as a favorable or non-favorable prognostic factor for survival. Piersma et al.31 observed that the presence of intratumoral CD8+ T cells in cervical cancer was correlated with the lack of pelvic lymph node spread and was, therefore, correlated indirectly with prognosis. A high number of tumor-infiltrating CD8+ T lymphocytes were observed as a favorable prognostic factor for survival in some studies of human cancers, including endometrial cancer,32 ovarian cancer,8 colorectal cancer,33 esophageal cancer34 and urothelial carcinoma.35 Other studies on non-small cell lung cancer,36 anal squamous cell carcinoma24 and renal cell carcinoma37 reported that CD8+ T lymphocytes were a negative prognostic factor.
The CD8+ T-cell subpopulation has been considered to be the effector in the antitumor response; however, when the status of activation was explored, most of the cells were underprimed (CD45RO+)38 or inactivated (in this study, the GrB/CD8 ratio was 7/48), regardless of the effector/target ratio within the tumor tissue (data not shown). It is unlikely that such a weak functional population could eliminate the tumor burden. The similarity in the numbers of CD8 TILs between the two groups suggests that the recruitment of CD8+ T cells to the tumor site occurs under similar conditions. More importantly, the similarity of the two cytotoxic markers (GrB and Prf) reflects that the cytotoxic machinery of the infiltrates was intact, suggesting that the dysfunctional status of the effector cells was due to the influence of the microenvironment39 rather than to the physical dysfunction of signal transduction in the T cells.40 Thus, the low expression of the T-cell receptor-ζ chain, the T cell-signaling molecule, observed in CD8+ TILs of cervical cancer patients, may not be associated with chronic viral infection.39 Interestingly, these results differ from our previous data regarding breast cancer, which revealed a significant discrepancy in the numbers of GrB- and Prf-positive cells.41 The orchestration of these two molecules is known to be a prerequisite for CD8+ T cells to exert their killing function. This indicates that the status of CTL in different malignant microenvironments requires further exploration.
The main mechanism by which TILs control tumor growth is postulated to be via a cytotoxic mechanism. In this scenario, the infiltration of the tumor site with high numbers of activated CD8+ TILs would be desirable. CD4+ TILs would also be required because to function optimally, CD8+ T cells usually require CD4+ T cells (Th1 cells are assumed to play a key role in the antitumor milieu). Therefore, we next examined the number of CD4+ TILs in both the stroma and tumor nest. A significantly lower number of CD4+ TILs was observed in the deceased group than in the surviving group (26.33±11.80 versus 47.79±38.18, P=0.023). Similarly, the number of CD4+ TILs was significantly lower in the deceased group than in the surviving group in both the stroma and tumor nest (15.98±9.73 versus 23.79±23.97, P=0.039 and 10.45±4.44 versus 24.03±17.68, P=0.023, respectively). No differences were observed in the numbers of CD4+ TILs between the FIGO stage IIB and IIIB groups. Although the number of CD4+ TILs was significantly lower in the deceased group than in the surviving group, we observed no statistically significant difference in the 5-year survival rates between the groups with high and low levels of CD4+ TILs (76.5% versus 50.0%, P=0.121). However, when the CD4+ TILs were separately evaluated as helper and Tregs based on their positivity for the FOXP3 marker, we found that the percentage of Tregs was significantly higher in the deceased group than in the surviving group both in the stroma and tumor nest (47.64±20.41 versus 22.83±18.97, P=0.003 and 68.98±20.76 versus 27.96±21.27, P=0.005, respectively) and overall (54.47±17.84 versus 23.53±15.59, P=0.002). In addition, we observed a significantly lower survival rate in patients with high levels of Tregs than in those with low levels (35.3% versus 88.9%, P=0.001).
A low density of CD3+, CD4+ and CD8+ cells is associated with an increased risk of relapse in squamous cell cervical cancer, while a high density of all three cell types is associated with a decreased risk of relapse.42 High numbers of CD4+ TILs have also been observed as a favorable prognostic marker in other human cancers such as non-small cell lung cancer36 and head and neck cancer.43 The characteristics of CD4+ Tregs and the mechanisms leading to their biological effects remain the subject of extensive investigation.44, 45 In principle, Tregs may downregulate the antitumor immune response. Thus, increased Tregs within the primary tumor may predict a poor prognosis. Several studies in mice have shown that Tregs inhibit the antitumor immune response46, 47, 48 and that the depletion of Tregs can enhance effector T-cell antitumor responses.49, 50 HPV-specific Tregs can be detected in both the draining lymph nodes and the tumors of women infected with HPV, and they seem to be another means to downregulate host immune responses. van der Burg et al.51 isolated HPV-specific CD4+ T cells from lymph node biopsies of cervical cancer patients and found that they had the ability to suppress proliferation and cytokine (IFN-γ and IL-2) production by responder T cells. The capacity of HPV-specific CD4+ T cells to exert this suppressive effect depended on their activation by cognate HPV antigen and on close-range interactions with responder T cells. HPV-specific CD4+ Tregs were also retrieved from cervical cancer biopsies, suggesting that they may interfere with the antitumor immune responses on both the induction and effector levels. Notably, the relationship between Tregs and cervical cancer is unknown, but many of these data support a role for Tregs in disease progression.
An earlier study by Sheu et al.52 showed that reversed CD4/CD8 ratios of TILs are correlated with the progression of human cervical carcinoma; the study found that the CD4/CD8 ratios of TILs were reversed in both cervical squamous cell carcinoma and cervical adenocarcinoma. The proportion of CD4+ T cells was significantly lower in tumors from patients with lymph node metastases than in those from patients without lymph node metastases, as was the reversed CD4/CD8 ratio. Similarly, Piersma et al.31 found a significantly stronger CD8+ T-cell tumor infiltration, a higher CD8+/CD4+ T-cell ratio, and a higher CD8+/Tregs ratio in patients with tumors that failed to metastasize to the tumor-draining lymph node. Loddenkemper et al.,27 in a comparison study of high-grade CIN (CIN III) and cervical carcinoma with colon cancer, skin melanoma and bronchial carcinoma, revealed that HPV-derived lesions have a significantly higher number of infiltrating lymphocytes and FOXP3+ Tregs as compared with three other common tumor types. Similar to the above studies, in our study, we observed a reversed CD4/CD8 ratio in cervical cancer. The overall mean number of CD4+ T cells was significantly lower than the mean number of CD8+ T cells in the deceased group (26.33±11.80 versus 55.60±38.39, P=0.001), but this difference was not significant in the surviving group (47.79±38.18 versus 56.07±36.05, P=0.322); indeed, the ratio of CD4+/CD8+ T cells was significantly lower in the deceased group than in the surviving group (0.60±0.25 versus 1.17±1.02, P=0.019). The survival rate was significantly higher in patients with high CD4+/CD8+ T-cell ratios than in patients with low CD4+/CD8+ T-cell ratios.
The generation of a specific cytotoxic T-cell response is known to depend on sufficient help from activated Th1-type CD4+ T cells.53, 54 The inability of the host to reject a tumor may be due to the insufficient generation of tumor-specific CD4+ T cells.55, 56 The relatively low number of Th1-type CD4+ T cells with reversed CD4/CD8 ratios in the deceased group in our study supports this hypothesis. Unitt et al.57 observed that a high CD4+/CD8+ T-cell ratio is associated with a reduced risk of tumor recurrence after liver transplantation in hepatocellular carcinoma. Our results obviously reflect the cancerous environment per se and demonstrate that the analysis of the functional phenotypes of TILs ‘in situ' may more precisely describe the tumor milieu. However, the mechanism by which Tregs influence the functional maturation of CD8+ T cells remains to be explored because there were no differences in the numbers of activated CD8+ T cells among all of the studied groups.
Acknowledgments
The authors thank the Department of Radiation Oncology of the First Affiliated Hospital of the Medical College of Xi'an Jiaotong University for providing tissue samples and clinical data. This study was supported by the Higher Education Commission of Pakistan and the Foundation of Bureau of Health (Grant No. 04D13) of Shaanxi Province, China.
References
- Umansky V, Schirrmacher V, Rocha M. New insights into tumor-host interactions in lymphoma metastasis. J Mol Med. 1996;74:353–363. doi: 10.1007/BF00210630. [DOI] [PubMed] [Google Scholar]
- Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373:673–683. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini E, Asioli S, Filosso PL, Lyberis P, Bruna MC, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–371. doi: 10.1016/j.athoracsur.2008.10.067. [DOI] [PubMed] [Google Scholar]
- Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415–420. doi: 10.1016/j.ygyno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Dunn IF, Curry WT. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007;7:12. [PMC free article] [PubMed] [Google Scholar]
- Couzin J. Cancer. T cells a boon for colon cancer prognosis. Science. 2006;313:1868–1869. doi: 10.1126/science.313.5795.1868b. [DOI] [PubMed] [Google Scholar]
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall F, Duhrkop T, Weirich V, Ostwald C, Lenz P, Nizze H, et al. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808–816. doi: 10.1016/j.humpath.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:26–31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8+ T cells: it may get by with a little help from its friends. J Clin Invest. 2002;110:1415–1417. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;19:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani I, Le Gouvello S. Critical role for CD8+FoxP3+ regulatory T cells in colon cancer immune response in humans. Gut. 2009;58:743–744. doi: 10.1136/gut.2008.175521. [DOI] [PubMed] [Google Scholar]
- Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- Gjerdrum LM, Woetmann A, Odum N, Burton CM, Rossen K, Skovgaard GL, et al. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007;21:2512–2518. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes. Lab Invest. 2006;86:231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Tanaka F, Mimori K, Inoue H, Kai S, Shibata K, et al. Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2008;34:173–179. doi: 10.1016/j.ejso.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Miyamoto M, Cho Y, Ishikawa K, Tsuchikawa T, Kadoya M, et al. Infiltrating regulatory T cell numbers is not a factor to predict patient's survival in oesophageal squamous cell carcinoma. Br J Cancer. 2008;98:1258–1263. doi: 10.1038/sj.bjc.6604294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NR, Song EK, Jang KY, Choi HN, Moon WS, Kwon K, et al. Prognostic impact of tumor infiltrating FOXP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk Lymphoma. 2008;49:247–256. doi: 10.1080/10428190701824536. [DOI] [PubMed] [Google Scholar]
- Grabenbauer GG, Lahmer G, Distel L, Niedobitek G. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res. 2006;12:3355–3360. doi: 10.1158/1078-0432.CCR-05-2434. [DOI] [PubMed] [Google Scholar]
- Yan W, Le Z, Hongyan W, Juxiang X, Lusheng S, Yili W. The ex vivo microenvironments in MLTC of poorly immunogenic tumor cells facilitate polarization of CD4+CD25+ regulatory T cells. Cell Mol Immunol. 2006;3:123–129. [PubMed] [Google Scholar]
- Patel S, Chiplunkar S. Host immune responses to cervical cancer. Curr Opin Obstet Gyneco. 2009;21:54–59. doi: 10.1097/GCO.0b013e32831a9890. [DOI] [PubMed] [Google Scholar]
- Loddenkemper C, Hoffmann C, Stanke J, Nagorsen D, Baron U, Olek S, et al. Regulatory (FOXP3+) T cells as target for immune therapy of cervical intraepithelial neoplasia and cervical cancer. Cancer Sci. 2009;100:1112–1117. doi: 10.1111/j.1349-7006.2009.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsaguû X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Shah W, Hongwei C, Jin Z, Lifang D, Jun Y, Yili W. The prevalence of human papillomavirus type 58 in Chinese patients with cervical carcinoma and its influence on survival. Clin Oncol (R Coll Radiol) 2009;21:768–774. doi: 10.1016/j.clon.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, et al. High Number of Intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Resnick. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, et al. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8+ T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–1009. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, et al. Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- Monnier-Benoit S, Mauny F, Riethmuller D, Guerrini JS, Căpîlna M, Félix S, et al. Immunohistochemical analysis of CD4+ and CD8+ T-cell subsets in high risk human papillomavirus-associated pre-malignant and malignant lesions of the uterine cervix. Gynecol Oncol. 2006;102:22–31. doi: 10.1016/j.ygyno.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Santin AD, Bellone S, Palmieri M, Bossini B, Roman JJ, Cannon MJ, et al. Induction of tumor-specific cytotoxicity in tumor infiltrating lymphocytes by HPV16 and HPV18 E7-pulsed autologous dendritic cells in patients with cancer of the uterine cervix. Gynecol Oncol. 2003;89:271–280. doi: 10.1016/s0090-8258(03)00083-0. [DOI] [PubMed] [Google Scholar]
- Baniyash M. TCR ζ-chain down regulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol. 2004;4:675–687. doi: 10.1038/nri1434. [DOI] [PubMed] [Google Scholar]
- Wang HY, He JJ, Shi QF, Lai BC, Ding HY, Zheng J, et al. Clinicopathological significance of cytotoxic lymphocytes in breast cancer and draining lymph nodes Zhonghua Bing Li Xue Za Zhi 200938384–388.Chinese. [PubMed] [Google Scholar]
- Nedergaard BS, Ladekarl M, Thomsen HF, Nyengaard JR, Nielsen K. Low density of CD3+, CD4+ and CD8+ cells is associated with increased risk of relapse in squamous cell cervical cancer. Br J Cancer. 2007;97:1135–1138. doi: 10.1038/sj.bjc.6604001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunityby removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- Nishikawa H, Kato T, Tawara I, Takemitsu T, Saito K, Wang L, et al. Accelerated chemically induced tumor development mediated by CD4+CD25+ regulatory T cells in wild-type hosts. Proc Natl Acad Sci USA. 2005;102:9253–9257. doi: 10.1073/pnas.0503852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Tanaka J, Kjaergaard J, Shu S. Depletion of CD4+CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J Immunother. 2002;25:207–217. doi: 10.1097/00002371-200205000-00003. [DOI] [PubMed] [Google Scholar]
- van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci USA. 2007;104:12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu BC, Hsu SM, Ho HN, Lin RH, Torng PL, Huang SC. Reversed CD4/CD8 ratios of tumor-infiltrating lymphocytes are correlated with the progression of human cervical carcinoma. Cancer. 1999;86:1537–1543. doi: 10.1002/(sici)1097-0142(19991015)86:8<1537::aid-cncr21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Chen L, Linsley PS, Hellström KE. Costimulation of T cells for tumor immunity. Immunol Today. 1993;14:483–486. doi: 10.1016/0167-5699(93)90262-J. [DOI] [PubMed] [Google Scholar]
- Cheng TY, Wu JT, Lin RH. Induction of tumor-specific T cell response by cognating tumor cells with foreign antigen-primed Th cells. Int Immunol. 1998;10:1397–1406. doi: 10.1093/intimm/10.10.1397. [DOI] [PubMed] [Google Scholar]
- Greenberg PD. Adoptive T cells therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- Baskar S, Glimcher L, Nabavi N, Jones RT, Ostrand-Rosenberg S. Major histocompatibility complex II1B7211 tumor cells are potent vaccines for stimulating tumor rejection on tumor-bearing mice. J Exp Med. 1995;181:619–629. doi: 10.1084/jem.181.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler S, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45:246–253. doi: 10.1016/j.jhep.2005.12.027. [DOI] [PubMed] [Google Scholar]