Abstract
Transplantation of mesenchymal stem cells (MSCs) has been recently studied in animal models, and in clinical trials of patients with fulminant hepatic failure, end-stage liver diseases and inherited metabolic disorders. Modulatory cytokines produced by MSCs can inhibit immunocyte proliferation and migration to the liver, thereby attenuating inflammatory injury and reducing hepatocyte apoptosis. In addition, MSCs play an important role in regressing liver fibrosis and in supporting the function, proliferation and differentiation of endogenous hepatocytes under appropriate conditions. Although remarkable progress has been achieved in basic and clinical MSC studies, optimal therapeutic regimens for the clinical application of MSCs, such as optimal doses, transplantation routine and interval period for transplantation, need to be elucidated in detail. Furthermore, the long-term safety and therapeutic efficacy of MSC transplantation should be evaluated in future clinical trials. This review summarizes our current understanding of the immunomodulatory effects of MSC therapies on human liver diseases.
Keywords: immunomodulation, liver diseases, mesenchymal stem cells, transplantation
Introduction
Mesenchymal stem cells (MSCs) are adult stem cells that originate from the mesenchymal and connective tissue of the bone marrow, adipose tissue, placenta, umbilical cord, cord blood, peripheral blood, liver, etc. These cells show immunomodulation, self-renewal and multidirectional differentiation potential. In contrast to embryonic stem cells, MSCs are associated with fewer ethical concerns. More importantly, MSCs have greater expansion capability and exhibit faster growth in vitro. Many studies have shown that MSCs have the potential to differentiate into multiple cell lineages such as osteoblasts, chondrocytes, myoblasts, adipocytes, endothelial cells and neuron-like cells. Under appropriate conditions, MSCs can differentiate into hepatocyte-like cells that perform hepatic functions such as albumin production, glycogen storage, urea secretion, low-density lipoprotein uptake and phenobarbital-inducible cytochrome P450 activity.1 Owing to their capacity to engraft into the recipient after administration, MSCs have been used to treat children with severe osteogenesis imperfecta. The immunosuppressive effect of infused MSCs has been successfully employed in the treatment of acute severe graft-versus-host disease (GVHD). These findings indicate that MSC treatment is promising in the therapy of liver diseases. Currently, clinical trials are underway to assess the safety, feasibility and efficacy of MSC transplantation in liver diseases.
Therapeutic mechanism of MSC transplantation
Generally, MSCs can differentiate into target cells both in vivo and in vitro under appropriate conditions.2, 3 In particular, MSCs can differentiate into hepatocyte-like cells through a specific cell-signaling pathway upon stimulation with a combination of hepatocyte growth factor (HGF), fibroblast growth factor, epidermal growth factor and other cytokines.4, 5, 6 However, in a culture of human bone marrow-derived MSCs (BM-MSCs) and hepatocytes, BM-MSCs that undergo hepatic differentiation probably account for only a small percentage of the population,7 and any differentiation may not significantly change the outcome of the bone marrow stromal cell (BMSC) and hepatocyte coculture experiments.8
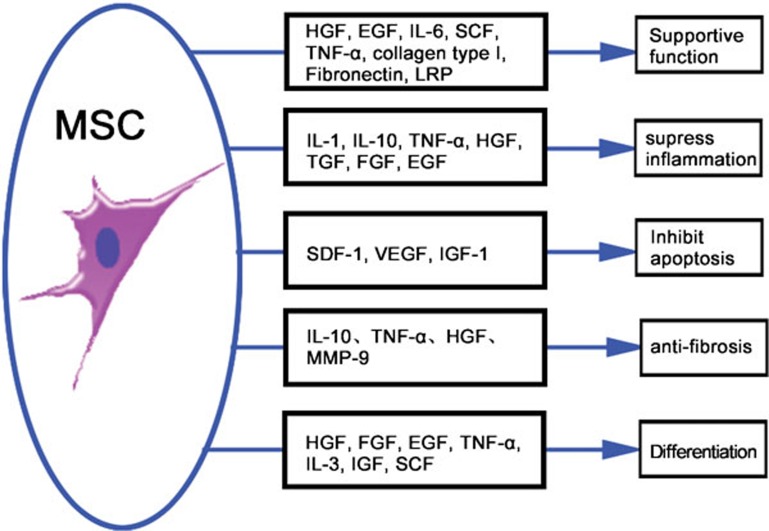
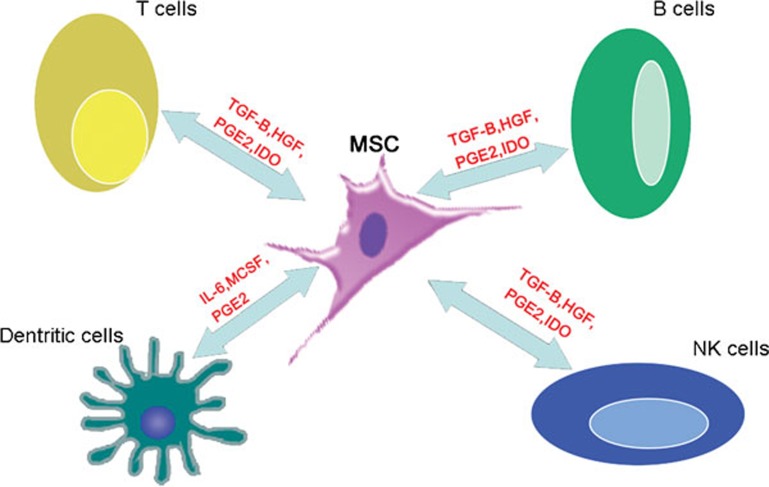
MSCs have recently emerged as promising candidates for cell-based immunotherapy because these cells can modulate the immune responses in various ways. MSCs can produce a series of growth factors, cytokines and signal molecules that can potentially suppress inflammatory responses, reduce hepatocyte apoptosis, regress liver fibrosis, enhance hepatocyte functionality and stimulate the proliferation of endogenous hepatocytes9 (Figure 1). The interaction between MSCs and immunocytes plays a crucial role in immunoregulation. Because immunocyte infiltration is an essential step leading to liver injury, resolution of inflammatory cell infiltration is critical for preventing immunocyte-mediated chronic damage. Several studies have indicated that MSCs can interfere with the functioning of antigen-presenting cells, block B-cell differentiation and suppress the immune response of T cells and natural killer cells via the secretion of soluble cytokines and direct cell-to-cell contacts (Figure 2). In addition, since cytokines are important components of the machinery that mediates inflammatory responses, prevention of the inflammatory effects of proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1 are critical for preventing tissue damage. In models of lung or other organ injury, MSC therapy can downregulate local and systemic proinflammatory responses by secreting IL-1 receptor antagonists, and it can upregulate anti-inflammatory cytokines such as IL-10.10 Furthermore, because of the lack of human leukocyte antigen (HLA) class II and costimulatory molecules such as CD80, CD86, CD40 or CD40L, MSC transplantation does not result in host-versus-graft responses.11
Figure 1.
Trophic mechanisms of MSC therapy. Mediator-specific effects of MSC-derived factors on pathophysiological processes in liver injury. EGF, epidermal growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; MMP, matrix metalloproteinase; MSC, mesenchymal stem cell; LRP, liver-regulating protein; TGF-β, transforming growth factor-β SCF, stem cell factor; SDF-1, stromal cell-derived factor-1; TNF-α, tumor necrosis factor-α VEGF, vascular endothelial growth factor.
Figure 2.
Immunomodulatory properties of MSCs. MSCs modulate the functions of the immune system by interacting with a wide range of immune cells, including T lymphocytes, B lymphocytes, natural killer cells and dendritic cells, by secreting dissoluble cytokines and by direct cell–cell contacts. MSCs inhibit T-cell proliferation in a dose-dependent manner: the greater the number of MSCs, the greater the inhibition of T-cell proliferation. In contrast, the greater the number of MSCs, the lower the inhibition of B-cell proliferation. HGF, hepatocyte growth factor; IDO, indoleamine 2,3-dioxygenase; IL-6, interleukin-6; MCSF, macrophage colony-stimulating factor; MSCs, mesenchymal stem cells; PGE2, prostaglandin E2; TGF-β, transforming growth factor-β.
MSCs can also produce some anti-apoptotic cytokines such as stromal cell-derived factor-1 and vascular endothelial growth factor, which efficiently reduce the apoptosis of recipient cells via the stromal cell-derived factor-1/CX chemokine receptor-4 axis. The antiapoptotic effects of MSCs have been observed in liver injury models. In rat models of acute liver injury, human BMSCs or MSC-conditioned medium transplantation significantly reduced rat mortality; this was found to be correlated with a decrease in the number of apoptotic hepatocytes.8, 12 Furthermore, MSCs can also secrete several cytokines such as HGF, epidermal growth factor, IL-6 and TNF-α to stimulate hepatocyte proliferation and maintain hepatocyte function, as indicated by the high levels of albumin and urea secretion.13 These secreted components of MSCs can enhance liver regeneration in a fulminant hepatic failure (FHF) model.14
Several animal and clinical trials have demonstrated that MSCs have the potential to reverse the fibrotic process by inhibiting collagen deposition and transforming growth factor-β1 production and by their capacity to differentiate into hepatocytes.15, 16, 17 The antifibrotic mechanism of MSC transplantation can be mainly attributed to the high levels of matrix metalloproteinase-9 expressed by MSCs, which may directly degrade the extracellular matrix18 and indirectly regulate hepatic stellate cells to secrete cytokines. In addition, MSC-derived IL-10 and TNF-α can also inhibit hepatic stellate cell regeneration and matrix synthesis.19 However, there are still some doubts regarding the antifibrotic effect of MSCs because MSCs have the potential to differentiate into fibrotic cells.20, 21
Efficiency of MSC transplantation
Recent experimental studies have shown the successful application of MSC transplantation in the treatment of FHF, end-stage liver disease (ESLD) and inherited metabolic disorders (IMDs). These studies have shown that MSC transplantation can partially restore the liver function, ameliorate the symptoms and enhance the survival rates.12, 22
In liver tissues from patients with liver cirrhosis, the formation of pseudolobules has a negative effect on duct construction and can impede the interchange between hepatocytes and serum. MSC transplantation should ideally facilitate duct construction. In animal experiments, MSC transplantation was shown to regress liver fibrosis and restore liver function. In a clinical trial from Iran, autologous BM-MSCs were transplanted in patients with decompensated cirrhosis. All these patients with ESLD showed good tolerance and decreased Model for end-stage liver disease (MELD) scores. These patients also showed an improvement in albumin production and liver function after 6 months of follow-up.23 However, these findings need to be confirmed in a larger cohort of patients. The long-term efficiency and safety of MSC transplantation also needs to be determined by tracer studies.
FHF is another severe liver disease that is accompanied by massive hepatocyte necrosis and discompensatory liver regeneration. The efficiency of MSC treatment in FHF patients was evidenced by the fact that FHF was rescued 4 weeks after MSC transplantation.24 The rapid therapeutic effect indicates that the paracrine secretions of MSCs play a more important role than differentiation in MSC-mediated restoration of liver function.25
IMDs including Crigler–Najjar syndrome26 and primary systemic amyloidosis27 have been successfully treated by stem cell transplantation in clinical trials. A combination of cellular transplantation and gene therapy is currently a popular prospective therapeutic option for IMDs and is being tested in animal models.28 In these experiments, exogenous genes were transferred into MSCs, and gene-related cytokines such as HGF, IL-3, interferon-β and somatotrophic hormone did not influence the characteristics of the MSCs. However, gene modification of MSCs is associated with some problems such as the low efficiency of transfection, safety of the virus vehicle and possibility of oncogenicity.29 Overall, the current data indicate that although the therapeutic effect of MSCs requires further investigation, these cells can be considered for therapeutic purposes in liver diseases.
Quality parameters for the application of MSCs
The use of MSC-conditioned medium transplantation and MSC bioreactors has been successful in preclinical studies and initial clinical trials on MSC transplantation. However, some important issues related to the optimal delivery method for MSC therapy remain to be resolved. In comparison with MSC-conditioned medium, transplanted MSCs have demonstrated the potential to be able to home in to the site of injury and ensure continued delivery of tropic signal molecules.25 However, long-term engraftment levels are low, and invasive methods for the local delivery of MSCs are often necessary to circumvent the lodging of these cells in the pulmonary flow.30 Furthermore, direct delivery of the effector molecules secreted by MSCs has the potential to become a promising clinical therapy. Efforts have been made to identify specific mediators responsible for the therapeutic effects of MSCs, and the roles of several growth factors, cytokines, soluble receptors and cytokine antagonists have been revealed; however, many other mechanisms remain unknown. A better understanding of the mediators secreted by MSCs will not only facilitate the understanding of the mechanism of action of MSC therapy, but also eventually lead to the development of an optimized cocktail of crucial compounds responsible for the therapeutic activity of MSCs. The environment in which MSCs are engrafted is very important for the development, differentiation and paracrine secretion of the cells and for the long-term prognosis of MSC transplantation. The new strategy is to cotransplant MSCs with biomaterials, growth factors or cytokines; one example is the use of bioreactor-integrated mixtures of MSC with extracellular matrix or collagen biomaterials to reduce the loss of nutrients in order to improve the survival of MSCs.31 The problem of concurrent immunosuppression is another factor that can influence the efficiency of MSC delivery, such as calcineurin inhibition after experimental solid organ transplantation, which abrogates the immunosuppressive effect of MSC therapy. Thus, a clinical protocol that involves immune-inhibitor drugs is still to be determined.
MSCs are usually infused into the injured liver through a portal vein, peripheral artery, or by other approaches such as the intrahepatic, intrasplenic and intraperitoneal routes; however, the ideal route is chosen according to the type of disease and the treatment goals. In the application of MSC transplantation, local injuries can be managed by point injection, while diffuse injuries can be treated by vascular injection. Experiments in a mouse model of FHF showed that MSC transplantation through the veins had a better effect than intrasplenic transplantation.23 IMDs or FHF can be successfully treated by MSC transplantation through the portal vein, although temporary portal hypertension has been observed in some animal studies. However, in ESLD, distorted architecture, point injection and portal infusion may result in pulmonary embolism and long-term portal hypertension.32 One clinical trial showed that the infusion of hematopoietic stem cells through the hepatic artery in decompensated cirrhosis could marginally improve liver function in some patients; however, it could also cause major adverse effects such as radio contrast nephropathy and hepatorenal syndrome.33
The quantity of MSCs may influence the therapeutic efficiency of MSC transplantation. Although MSCs can suppress the proliferation of activated lymphocytes in vitro in a dose-dependent manner, there are no reliable results to show that the treatment efficiency is dose-dependent. In more than half of the patients with GVHD, a single dose produced a response, whereas in a few patients with partial response or with recurrent GVHD, several doses were required to induce a lasting response. Theoretically, although not many MSCs are required to restore liver function, promote hepatocyte generation and regress liver fibrosis, the quantity of transplanted MSCs must be higher than the effective amount in clinical trials. In animal studies, the quantity of transplanted stem cells is usually about 106–107. In two of the clinical studies mentioned above, four patients with decompensated cirrhosis15 and eight cirrhosis patients with ESLD received 3.2×107 and 3–5×107 autologous BM-MSCs, respectively, through the peripheral or portal vein.21 In both trials, all the patients tolerated the transplantation well during and until the end of the follow-up period. An animal trial showed that the effect of MSC transplantation was directly related to the number of homing cells.34 However, this number did not show any obvious dependence on the number of transplanted MSCs.35 It is difficult to obtain a large quantity of high-purity MSCs in a short time under the existing conditions used for culture. Therefore, instead of using large quantities of MSCs in transplantation, the therapeutic efficiency of MSC transplantation may be improved by an effective method that enhances the homing efficiency of MSCs to the injured liver.
Therapeutic safety in clinical trials of MSC transplantation
Finally, an issue of crucial importance is the safety of injected MSCs. Although their use in most hemato-oncological conditions has been considered safe, their long-term effects on immune function and tumorigenic risk remain unknown. Theoretically, MSCs can directly facilitate tumor growth via transformation, suppression of the antitumor immune response and direct trophic action on tumor cells. However, potent antitumor effects of MSCs have also been observed. Although the study showed that the balance between the naive stem state and the differentiated state is highly dependent on the stem cell niche,36 more evidence is required to understand how MSCs positively or negatively modulate carcinogenesis and to evaluate the safety of MSC transplantation. High-risk patients may not be suitable candidates for MSC therapy, and the possibility of spontaneous tumor formation in such patients should be considered.37 However, it is generally accepted that the rapid migration of MSCs to the damaged organ is concomitant with their subsequent clearance. At present, the persistence of MSCs in vivo is not known. Another key issue that needs to be addressed for clinical purposes concerns the immunogenicity of MSCs. However, allogeneic and xenogeneic MSCs have been effectively used in many clinical trials. Third-party MSCs were also found to be as effective as HLA-identical MSCs. MSCs can nonspecifically inhibit allogeneic lymphocyte proliferation,38 thereby indicating their potential clinical applications in the prevention and treatment of therapy-resistant acute GVHD and autoimmune diseases.39 However, the test revealed that of the nine GVHD patients who had undergone MSC infusion, three developed viral infections. Therefore, concerns were raised that MSC infusion may increase the susceptibility to human herpesvirus infection and immunosuppression and reduce the immunosurveillance of pathogens. These concerns were supported by in vitro observations that lymphocyte proliferation induced by herpesviruses is suppressed by MSCs.40 A better understanding of the safety of MSCs will allow us to apply our basic knowledge of MSC biology to the design of clinical therapies. Further clinical trials to this end should be considered.
Conclusion
MSC transplantation for the treatment of human liver diseases is at least partly based on the differentiation and repopulation of MSCs and their immunomodulatory effects. Despite significant recent progress, some concerns with MSC transplantation have to be addressed before their use in intensive clinical treatment. First, the mechanism underlying MSC differentiation and immunomodulation should be elucidated. Second, the dynamics of transplanted MSCs in recipients in vivo needs to be analyzed by tracer methods. Third, the optimal transfusion quantity and route of MSC transplantation need to be determined. Finally, additional multicenter, randomized, placebo-controlled, clinical trials should be conducted to prove the therapeutic efficiency of this approach and to determine the adverse effects and complications that could arise in clinical situations.
Acknowledgments
This work was supported by grants from the National Grand Program on Key Infectious Disease (No. 2009ZX10004-309, No. 2008ZX10002-007 and No. 2008ZX10002-005-6) and the National Key Basic Research Program of China (No. 2007CB512805 and No. 2007CB512804).
References
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells drived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Deng X, Chen YX, Zhang X, Zhang JP, Yin C, Yue HY, et al. Hepatic stellate cells modulate the differentiation of bone marrow mesenchymal stem cells into hepatocyte-like cells. J Cell Physiol. 2008;217:138–144. doi: 10.1002/jcp.21481. [DOI] [PubMed] [Google Scholar]
- Okuyama H, Krishnamachary B, Zhou YF, Nagasawa H, Bosch-Marce M, Semenza GL. Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor. J Biol Chem. 2006;281:15554–15563. doi: 10.1074/jbc.M602003200. [DOI] [PubMed] [Google Scholar]
- Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. . Gut. 2009;58:570–581. doi: 10.1136/gut.2008.154880. [DOI] [PubMed] [Google Scholar]
- Bonora-Centelles A, Jover R, Mirabet V, Lahoz A, Bonora-Centelles A, Carbonell F, et al. Sequential hepatogenic trans-differentiation of adipose tissue-derived stem cells: relevance of different extracellular signaling molecules, transcription factors involved and expression of new key marker genes. Cell Transplant. 2009;18:1319–1340. doi: 10.3727/096368909X12483162197321. [DOI] [PubMed] [Google Scholar]
- Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833–848. doi: 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, et al. Liver-specific gene expression in mesenchymal stem cells is induced by liver cells. World J Gastroenterol. 2005;11:4497–4504. doi: 10.3748/wjg.v11.i29.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. . Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- Zhou P, Hohm S, Olusanya Y, Hess DA, Nolta J. Human progenitor cells with high aldehyde dehydrogenase activity efficiently engraft into damaged liver in a novel model. Hepatology. 2009;49:1992–2000. doi: 10.1002/hep.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hematti P. Role of mesenchymal stromal cells in solid organ transplantation. Transplant Rev. 2008;22:262–273. doi: 10.1016/j.trre.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J, Urbán VS, Dudics V, Vas V, Uher F. Mesenchymal stem cells and the immune system–immunosuppression without drugs. Orv Hetil. 2008;149:339–346. doi: 10.1556/OH.2008.28291. [DOI] [PubMed] [Google Scholar]
- Yagi K, Kojima M, Oyagi S, Ikeda E, Hirose M, Isoda K, et al. Application of mesenchymal stem cells to liver regenerative medicine. Yakugaku Zasshi. 2008;128:3–9. doi: 10.1248/yakushi.128.3. [DOI] [PubMed] [Google Scholar]
- Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008;26:2705–2712. doi: 10.1634/stemcells.2008-0034. [DOI] [PubMed] [Google Scholar]
- Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Mohyeddin-Bonab M, Bagheri M, Bashtar M, et al. Phase I trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated cirrhosis. Arch Iran Med. 2007;10:459–466. [PubMed] [Google Scholar]
- Tsai PC, Fu TW, Chen YM, Ko TL, Chen TH, Shih YH, et al. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009;15:484–495. doi: 10.1002/lt.21715. [DOI] [PubMed] [Google Scholar]
- Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, et al. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem. Biophys Res Commun. 2007;363:247–252. doi: 10.1016/j.bbrc.2007.05.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AB, Quintanilha LF, Dias JV, Paredes BD, Mannheimer EG, Carvalho FG, et al. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells. 2008;26:1307–1314. doi: 10.1634/stemcells.2007-0941. [DOI] [PubMed] [Google Scholar]
- di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, et al. Human MSCs as a two-edged sword in hepatic regenerative medicine-engraftment and hepatocytes differentiation versus profibrogenic potential. Gut. 2008;57:223–231. doi: 10.1136/gut.2006.111617. [DOI] [PubMed] [Google Scholar]
- Agnieszka B, Takumi T, Yusuke Y, Tokuhara M, Takeshita F, Osaki M, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70–77. doi: 10.1111/j.1440-1746.2008.05496.x. [DOI] [PubMed] [Google Scholar]
- Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I–II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Parekkadan B, Suganuma K, Soto-Gutierrez A, Tompkins R, Tilles A, et al. Long term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng Part A. 2009;15:3377–3388. doi: 10.1089/ten.tea.2008.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A, Ravindraprakash HR, Venkateswarlu J, et al. Treatment of Crigler–Najjar Syndrome type 1 by hepatic progenitor cell transplantation: a simple procedure for management of hyperbilirubinemia. Transplant Proc. 2008;40:1148–1150. doi: 10.1016/j.transproceed.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Frossard V, Ketterer N, Rosselet A, Meier P, Cairoli A, Duchosal MA, et al. Early intensification and autologous stem cell transplantation in patients with systemic AL amyloidosis: a single-centre experience. Ann Hematol. 2009;88:681–685. doi: 10.1007/s00277-008-0652-z. [DOI] [PubMed] [Google Scholar]
- Miranda PS, Bosma PJ. Towards liver-directed gene therapy for Crigler–Najjar syndrome. Curr Gene Ther. 2009;9:72–82. doi: 10.2174/156652309787909508. [DOI] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- McBride C, Gaupp D, Phinney DG. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by real-time PCR. Cytotherapy. 2003;5:7–18. doi: 10.1080/14653240310000038. [DOI] [PubMed] [Google Scholar]
- Takeda M, Yamamoto M, Isoda K, Higashiyama S, Hirose M, Ohgushi H, et al. Availability of bone marrow stromal cells in three-dimensional coculture with hepatocytes and transplantation into liver-damaged mice. J Biosci Bioeng. 2005;100:77–81. doi: 10.1263/jbb.100.77. [DOI] [PubMed] [Google Scholar]
- Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Mohamadnejad M, Namiri M, Bagheri M, Hashemi SM, Ghanaati H, Zare Mehrjardi N, et al. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007;13:3359–3363. doi: 10.3748/wjg.v13.i24.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang GA, Zhang GQ, Fang CH, Gao P, Chen KY. A preliminary study of the homing capacity of allograft mesenchymal stem cells to rat liver. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:994–997. [PubMed] [Google Scholar]
- Hashemi S, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, et al. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur Heart J. 2008;29:251–259. doi: 10.1093/eurheartj/ehm559. [DOI] [PubMed] [Google Scholar]
- Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2010;222:268–277. doi: 10.1002/jcp.21940. [DOI] [PubMed] [Google Scholar]
- Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit. Stem cells. 2008;26:1387–1394. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Su J, Zhang L, Zhao X, Ling W, L'huillie A, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann NY Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- Sundin M, Orvell C, Rasmusson I, Sundberg B, Ringdén O, Le Blanc K. Mesenchymal stem cells are susceptible to human herpesviruses, but viral DNA cannot be detected in the healthy seropositive individual. Bone Marrow Transplant. 2006;37:1051–1059. doi: 10.1038/sj.bmt.1705368. [DOI] [PubMed] [Google Scholar]