Abstract
Aim:
To investigate the proteome composition and function of human neonatal arterial umbilical cord.
Methods:
Serum proteomic analyses were performed on samples from both males and females by using a combination of techniques: (1) removal of six high-abundance proteins, (2) tryptic digestion of low-abundance proteins, (3) separation of peptide mixtures by reverse-phase high-performance liquid chromatography (RP-HPLC), and (4) peptide identification using electrospray ionization tandem mass spectrometry (ESI-MS/MS).
Results:
A total of 837 non-redundant proteins were identified, with 213 male-specific and 239 female-specific proteins. Among them, 319 proteins were identified by at least 2 distinct peptides. The subcellular localization, function, and pathway involvement for each of the identified proteins were analyzed. A comparison of this neonatal proteome to that of adult serum proteome revealed novel biomarkers, such as alpha-fetoprotein and periostin that were specific to newborn infants.
Conclusion:
These data will contribute to a better understanding of the composition of umbilical cord serum and aid the discovery of novel biomarkers for the prenatal diagnosis of fetal abnormalities.
Keywords: mass spectrometry, reference specimen, serum proteome, umbilical cord serum
Introduction
Blood serum contains thousands of small molecules, including salts, lipids, proteins, and sugars. Qualitative and quantitative changes in the levels of serum proteins can be related to physiological or pathological states1, 2, 3, 4, and serum markers have long been used for the clinical diagnosis and therapeutic monitoring of various diseases2 Proteomics is being extensively used to profile adult serum proteins, with a special focus on the identification of disease-related serum markers to facilitate the early diagnosis and treatment of disease2, 3, 5. Serum samples that are used in research are usually prepared from venal blood6. An equivalent comparison of the neonate proteome with the adult serum proteome poses significant challenges, since blood from newborn infants is very difficult to acquire by venipuncture. By contrast, umbilical cord blood (UCB) is easily obtained, with only a small risk to the donor. The protein profile and the levels of markers in UCB are related to the physiological or pathological state of the neonate. Many studies have detected novel biomarkers for fetal abnormalities from UCB7, 8. Low levels of α-2-HS glycoprotein/fetuin-A have been associated with intrauterine growth restriction (IUGR) cases8. These changes might be responsible for the impaired function of fetuin-A, which could lead to deficient fetal growth, especially with respect to osteogenesis, and/or to the development of complications that are frequently seen later in the lives of IUGR cases. To date, there has been no description of the protein profile of neonatal UCB by ESI-MS/MS. The focus of this study was to profile and analyze the proteome of UCB in order to investigate the neonate-specific composition and function of serum.
Materials and methods
Preparation of pooled male and pooled female serum specimens
All samples were provided by the Nanjing Maternity and Child Health Care Hospital and were prepared according to the BD protocol (the Human Proteome Organization Plasma Proteome Project pilot phase), with some modifications. This study was approved by the ethics committee of the Nanjing Maternity and Child Health Care Hospital, and UCB samples were collected after written informed consent was obtained from the parents.
The inclusion criteria of the subjects were as follows: (1) pregnant women who were aged between 23 and 30 years, were non-smokers, had no history of addiction to alcohol, and weighed between 40 and 70 kg; (2) no medications taken in the 7 days prior to delivery; (3) parents who were healthy and also tested negative for human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), and syphilis; (4) infants who were born via vaginal delivery with clear amniotic fluid; and (5) newborn infants who were healthy and also had Apgar scores of 10 at both 1 and 5 min, weighed between 2.5 and 4.0 kg, and had no abnormalities.
Approximately 20 mL of UCB was directly aspirated from a single umbilical cord artery without anticoagulant. The whole blood was allowed to clot for 2.5 h at 4°C, and the clotted material was removed by centrifugation at 2600×g for 15 min. The resulting serum was then centrifuged at 12 000×g for 5 min at 4°C to remove any remaining cells. A protease inhibitor cocktail (Pierce #Prod#78415, Rockford, USA) was added to the serum to prevent protein degradation. The pooled male and pooled female samples were created by the combination of 2.0-mL serum samples from each male or female donor, respectively. The pooled samples were then stored at -20 °C until use.
Depletion of the highly abundant serum proteins
The removal of high-abundance proteins from UCB was performed for two main reasons: (1) to prevent interference with the measurement of low-abundance proteins during MS and (2) to reduce the possibility that the MS-based sequence analysis of serum-derived peptides is complicated by regions of high-sequence variability found in the abundant serum immunoglobulins. Six high-abundance proteins, namely albumin, transferrin, haptoglobin, α-1-antitrypsin, IgA, and IgG, were selected for removal from the UCB serum samples using a multiple affinity removal column system (Agilent Technologies, Palo Alto, USA). Briefly, the crude serum was thawed, diluted five-fold with buffer A, pH 7.4 (product No 5185–5987; Agilent Technologies), and filtered through 0.22 μm filters (Agilent Technologies) by centrifugation at 16 000×g at room temperature for 1.5 min. The diluted serum samples were injected onto a Multiple Affinity Removal System HPLC column (Agilent Technologies) in buffer A at a flow rate of 0.25 mL/min for 9 min. The bound proteins were then eluted in buffer B at a flow rate of 1.0 mL/min for 3.5 min. All chromatographic fractionations were performed at room temperature on an HP1100 HPLC system with the automated sample injector set at 47 °C. The unbound (low-abundance) and bound (high-abundance) proteins were collected in Eppendorf tubes and stored at −20 °C for further analysis.
Protein concentration
The fractions that were collected from 6 males and 6 females were pooled, respectively, into two Microcon spin concentrators with a 5-kDa molecular weight cut-off (Millipore). The fractions were spun at 12 000×g at 47 °C for 2 h. Protein concentrations were estimated with a Bradford protein assay using bovine serum albumin (BSA) (BioRad, Hercules, CA, USA) as a protein standard. Each protein mixture was lyophilized for digestion.
One-dimensional SDS-PAGE and in-gel digestion
The extracted protein (100 mg) was dissolved in SDS-PAGE loading buffer, boiled for 5 min, and loaded onto a single lane of a 1-mm thick 10% polyacrylamide gel. After separation, the proteins were visualized by silver staining of the gel according to the published procedure9, with the minor modification of using a sensitizing solution that lacked glutardialdehyde. Follow-up analysis by Coomassie brilliant blue stain indicated that these six proteins were almost entirely removed (Figure 1).
Figure 1.
One-dimensional SDS-PAGE gel. F=low-abundance protein in females; FH=highly abundant protein in females; M=low-abundance protein in males; MH=highly abundant protein in males. Both A and B=other independent proteins.
The gel was then cut into 38 slices, which were subsequently cut into 1-mm3 gel particles and placed into 48 tubes for in-gel digestion. Briefly, the gel pieces were washed and destained in deionized water and then dehydrated. The gels were sequentially washed with 25 mmol/L ammonium bicarbonate and 50% acetonitrile (ACN) solution, followed by dehydration with 100% ACN and rehydration with 10 ng/μL trypsin (Promega, Madison, WI, USA) in 25 mmol/L ammonium bicarbonate. The gel pieces were incubated for 12 h at 37°C for protein digestion. The supernatants were transferred to fresh tubes, and the remaining peptides were extracted by incubating the gel pieces twice with 30% ACN in 3% trifluoroacetic acid (TFA), followed by dehydration with 100% ACN. The extracts were combined and lyophilized to dryness. The collected peptides were used for mass spectrometric analysis.
Online reversed-phase LC-MS/MS
For the capillary reversed-phase LC (cLC) and mass spectrometric (MS) analyses, the 48 fractions were sequentially loaded onto a Michrom peptide CapTrap (MW 0.5–50 kDa, 0.5×2 mm; Michrom BioResources, Auburn, USA) at a flow rate of 60 μL/min with buffer A (see below). The trap column effluent was then transferred to a reversed-phase microcapillary column (0.1×150 mm, packed with Magic C18, 5 μm, 100 Å Michrom Bioresources, Auburn, USA). The reversed-phase separation of peptides was performed using the following buffers: 5% ACN, 0.1% formic acid in 95% ACN (buffer A), and 0.1% formic acid (buffer B). The samples were run on a 73-min gradient, which consisted of a 5%–45% buffer B for 55 min, 90% buffer B for 5 min, and 5% buffer B for 13 min. The peptide analysis was performed using the Finnigan LTQ ORBitrap (ThermoFinnigan, San Jose, USA) coupled directly to an LC column. The MS survey scan was obtained for the m/z range 400–1800, and the MS/MS spectra were acquired from the survey scan for the 10 most intense ions as determined by the Xcaliber mass spectrometer software in real time. Dynamic mass exclusion windows of 60 s were used, and siloxane (m/z 445.120025) was used as an internal standard.
Settings for peak list file generation and database searches
SEQUEST analysis software (Bioworks version 3.3; ThermoFinnigan) was used for the identification of peptide sequences10. DTA files (Bioworks version 3.3) for each MS/MS spectrum, with a minimum ion count of 8, were generated from the raw data for the peptide mass range of 400–8000. The International Protein Index (IPI) human database (ipi.HUMAN.v3.31; downloaded from ftp.ebi.ac.uk/pub/databases/IPI), which contains 67,511 entries was used in the SEQUEST analysis. The SEQUEST parameters were set as follows: peptide (parent ion) tolerance of 10 ppm, fragment ion tolerance of 1 Da, missed cleavages of 2 allowed, fixed modification of carbamidomethylation on Cys (+57 Da), and a differential modification of oxidation on Met (+16 Da). The criteria used for filtering peptides with low confidence scores were as follows: cross-correlation values (Xcorr) greater than 2.1 and 2.5 for doubly-charged ions and triply or higher charged ions, respectively; ΔCn values, representing the difference in Xcorr from the next highest value, of less than 0.1 were removed from the matched sequences; removal of the infrequent singly-charged ions. The false-positive error rate was calculated by analyzing all files with the same method, except against a “sequence-reversed” IPI Human database. The false-positive rate (FPR) was calculated as follows: FPR=# of false peptides/(# of true peptides+# of false peptides). The confidence score of the protein was calculated by applying the Peptide Prophet algorithm using the Scaffold software (v01_07_00; Proteome Software)11.
Bioinformatics analysis
Functional annotations were performed using the 2008 DAVID software program (http://david.abcc.ncifcrf.gov/). DAVID is a web-based and client/server application that allows users to access a relational database of functional annotations for proteins. Entrez Gene IDs were entered into DAVID to determine whether there was any enrichment of gene ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways12
Results
Identification of neonatal umbilical cord serum proteins
The resulting peptides that were extracted from each gel slice were analyzed by automated reversed phase LC (RP-LC) coupled with MS/MS. From 48 LC-MS/MS runs, a total of 104,234 MS/MS spectra were acquired and searched against the IPI Human database v3.31. The resulting lists of proteins from each run were combined into a collective list of proteins with the Scaffold program. To experimentally test the FPR in our dataset, all output files were searched against the reversed sequence database. A peptide FPR of 5% and an 80% protein identification probability13 were used as the cut-offs for Scaffold in order to generate a statistically valid list of proteins.
Since multiple unique peptide sequences can include identical protein sequences, determining the identities of proteins based upon sequenced peptides can occasionally be difficult unless specific peptides are identified. Therefore, shared information among the identified peptides was further analyzed. Proteins with shared peptides were organized into a single group, which was referred to as a protein group, in Scaffold. If a protein group comprised only isoforms or overlapping database entries that were indistinguishable by MS/MS analysis, then all of the proteins in this group were counted as a single protein. If these proteins were the products of distinct genes, then all the proteins in the group were discarded. As a result, the final number of identified proteins was lower than the actual value. These analyses resulted in the identification of 837 proteins that corresponded to 815 distinct genes.
Gene ontology (GO) analysis of the identified proteins
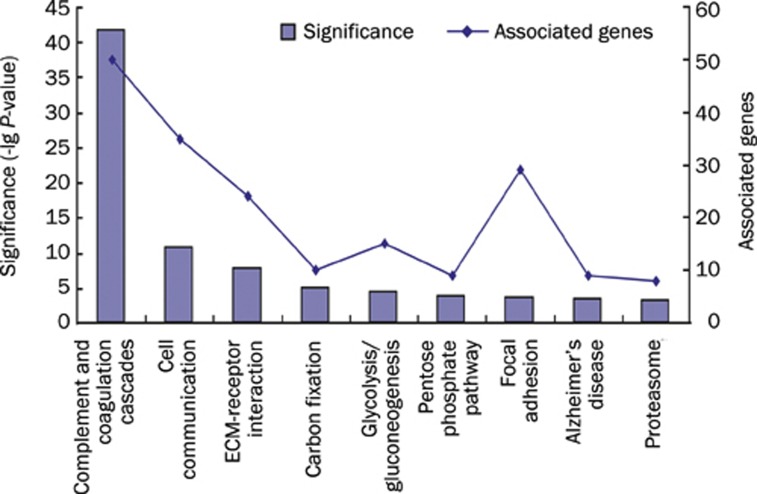
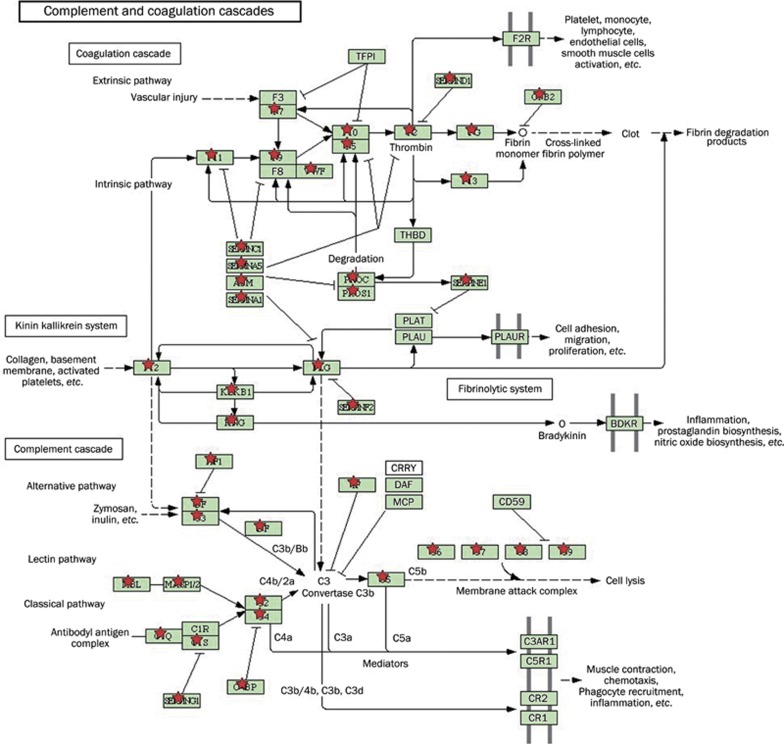
The proteins that were identified in this study were examined by GO analysis and categorized according to their cellular localization. The most abundant proteins were derived from the cytoplasm (29%), followed by membrane proteins (21%), extracellular region proteins (20%), unknown or unclassified proteins (15%), and nuclear proteins (12%) (Figure 2). In addition to cellular localization, the UCB proteins were also analyzed at the genome level (Figure 3). In addition, the UCB was enriched with proteins that were involved in cell communication pathways as well as in the complement and coagulation cascades (Figure 4)14.
Figure 2.
Classification of UCB proteins according to subcellular localization. The pie chart described the subcellular localizations of UCB proteins from the proteome. Subcellular localizations were assigned according to gene ontology annotations. When one protein was known to be localized in more than one cellular compartment, all of the localizations were counted non-exclusively.
Figure 3.
UCB protein-associated genes. Proteins in UCB were found to be rich in many KEGG pathways. The numbers of proteins and significance of enrichment calculated by DAVID 2008 in each pathway were shown.
Figure 4.
UCB proteins belonging to the complement and coagulation cascade pathways. Many of the proteins detected in UCB participate in the complement and coagulation cascade pathways according to KEGG pathway database. The proteins identified in UCB was marked by asterisks.
Comparison of male and female umbilical cord sera
The number of unique peptides is generally accepted as a semiquantitative measure of protein abundance15, 16. The proteins in male and female UCB were sorted into separate databases that included the unique peptides that were identified in each. The ten proteins with the most abundant number of unique peptides in males and in females are listed in Table 1. Apolipoprotein B, complement C3, and alpha-2-macroglobulin had the highest number of unique peptides, which indicated that the earlier depletion of the six most abundant proteins was efficient. The two datasets were compared in order to investigate the similarities and differences between male and female UCB. Differentially expressed proteins were determined using the following criteria: (1) identification only in one dataset by at least two different peptides or (2) identification in one dataset by more than three times as many peptides in the other dataset16. There were 53 proteins that were differentially expressed between male and female UCB. The differential expression of these proteins could be a result of the distinct metabolic statuses between male and female fetuses.
Table 1. The ten most abundant proteins in UCB. The proteins from male and female UCB are sorted according to the unique peptides that were identified.
| Representative accession numbers | Protein name | Gender (Top 10) | Number of unique peptides (Female) | Number of unique peptides (Male) |
|---|---|---|---|---|
| IPI00478003 | Alpha-2-macroglobulin precusor | Male, Female | 75 | 69 |
| IPI00745872 | Isoform 1 of serum albumin precursor | Male, Female | 48 | 52 |
| IPI00022229 | Apolipoprotein B-100 precursor | Male, Female | 169 | 155 |
| IPI00783987 | Complement C3 precursor (Fragment) | Male, Female | 98 | 83 |
| IPI00032291 | Complement C5 precursor | Male, Female | 45 | 42 |
| IPI00029739 | Isoform 1 of Complement factor H precursor | Male, Female | 45 | 45 |
| IPI00017601 | Ceruloplasmin precursor | Male, Female | 39 | 39 |
| IPI00019568 | Prothrombin precursor (Fragment) | Male, Female | 29 | 28 |
| IPI00022418 | Isoform 1 of Fibronectin precursor | Male, Female | 57 | 49 |
| IPI00292530 | Inter-alpha-trypsin inhibitor heavy chain H1 precursor | Male, Female | 32 | 27 |
| IPI00022463 | Serotransferrin precursor | Male, Female | 39 | 39 |
Comparison of neonatal umbilical cord serum and adult serum proteomes
To further investigate the highly expressed proteins in UCB and identify proteins that were specific to newborn infants, our protein dataset was compared with the adult plasma proteome. A total of 61 proteins, which were identified by two different peptides and were present in at least one gender, were present in the neonatal UCB serum proteome and not in the adult serum proteome (Table 2). Some of these proteins that are uniquely found in the UCB may be essential for fetal development.
Table 2. The 61 neonate-specific proteins in UCB. A total of 61 proteins were identified in the UCB that were not present in the adult plasma proteome. Each neonate-specific protein was identified by two different peptides in at least one gender.
| Accession numbers | Protein name | Number of unique peptides (Female) | Number of unique peptides (Male) |
|---|---|---|---|
| IPI00410241 | POSTN Periostin, osteoblast specific factor | 16 | |
| IPI00430808 | IGKC IGKC protein | 11 | 13 |
| IPI00293665 | KRT6B Keratin, type II cytoskeletal 6B | 7 | 4 |
| IPI00156171 | ENPP2 Isoform 1 of Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 precursor | 6 | 3 |
| IPI00031008 | |||
| IPI00220213 | TNC Isoform 1 of Tenascin precursor | 5 | 7 |
| IPI00399007 | IGHG2 Hypothetical protein DKFZp686I04196 (Fragment) | 5 | 5 |
| IPI00021263 | |||
| IPI00789337 | YWHAZ 14-3-3 protein zeta/delta | 5 | 3 |
| IPI00303482 | FGL1 Fibrinogen-like 1 precursor | 4 | 4 |
| IPI00299485 | CD93 Complement component C1q receptor precursor | 4 | 3 |
| IPI00014592 | CHAD Chondroadherin precursor | 4 | 3 |
| IPI00009276 | PROCR Endothelial protein C receptor precursor | 4 | 3 |
| IPI00220301 | PRDX6 Peroxiredoxin-6 | 4 | 2 |
| IPI00550363 | |||
| IPI00644531 | |||
| IPI00647915 | TAGLN2 Transgelin-2 | 4 | 2 |
| IPI00386879 | IGHA1 CDNA FLJ14473 fis, clone MAMMA1001080, highly similar to Homo sapiens SNC73 protein (SNC73) mRNA | 4 | 2 |
| IPI00215914 | |||
| IPI00215917 | ARF1 ADP-ribosylation factor 1 | 4 | |
| IPI00032190 | IHH Indian hedgehog protein precursor | 3 | 4 |
| IPI00845354 | IGKC IGKC protein | 3 | 4 |
| IPI00002732 | EXTL2 EXTL2 protein (Fragment) | 3 | 4 |
| IPI00019038 | LYZ Lysozyme C precursor | 3 | 3 |
| IPI00291922 | PSMA5 Proteasome subunit alpha type 5 | 3 | 1 |
| IPI00009851 | PCDH12 PCDH12 protein | 3 | 1 |
| IPI00291005 | MDH1 Malate dehydrogenase, cytoplasmic | 3 | 1 |
| IPI00020990 | OMD Osteomodulin precursor | 3 | 1 |
| IPI00745745 | FUCA1 fucosidase, alpha-L-1, tissue | ||
| IPI00843910 | 3 | 1 | |
| IPI00430847 | IGKC IGKC protein | 2 | 3 |
| IPI00291136 | COL6A1 Collagen alpha-1(VI) chain precursor | 2 | 3 |
| IPI00011194 | FGFBP2 Fibroblast growth factor-binding protein 2 precursor | 2 | 2 |
| IPI00643041 | |||
| IPI00792352 | |||
| IPI0079567 | RAN GTP-binding nuclear protein | ||
| IPI00796462 | Ran | 2 | 2 |
| IPI00017672 | NP CDNA FLJ25678 fis, clone TST04067, highly similar to PURINE NUCLEOSIDE PHOSPHORYLASE | 2 | 2 |
| IPI00784430 | IGKV3D-11 Similar to Ig kappa chain V-III region VG precursor | 2 | 2 |
| IPI00328703 | OAF Out at first protein homolog precursor | 2 | 2 |
| IPI00031708 | FAH Fumarylacetoacetase | 2 | 2 |
| IPI00014424 | EEF1A2 Elongation factor 1-alpha 2 | 2 | 2 |
| IPI00170867 | DAXX Isoform 1 of Death domain-associated protein 6 | 2 | 1 |
| IPI00220885 | |||
| IPI00736885 | |||
| IPI00827773 | LOC440786 Ig kappa chain V-II region TEW | 2 | 1 |
| IPI00003802 | MAN2A1 Alpha-mannosidase 2 | 2 | 1 |
| IPI00009950 | LMAN2 Vesicular integral-membrane protein VIP36 precursor | 2 | 1 |
| IPI00215767 | B4GALT1 Isoform Long of Beta-1,4-galactosyltransferase 1 | 2 | |
| IPI00759755 | 1 | ||
| IPI00218493 | HPRT1 Hypoxanthine-guanine phosphoribosyltransferase | 2 | 1 |
| IPI00306311 | |||
| IPI00742980 | PLEK Pleckstrin | 2 | |
| IPI00030205 | IGKV3-20 Ig kappa chain V-III region HAH precursor | 2 | |
| IPI00736041 | SERPINA11 similar to Serpin A11 precursor | 2 | |
| IPI00028908 | NID2 Nidogen-2 precursor | 2 | |
| IPI00442073 | CSRP1 Cysteine and glycine-rich protein 1 | 2 | |
| IPI00303476 | |||
| IPI00789157 | ATP5B ATP synthase subunit beta, mitochondrial precursor | 1 | 3 |
| IPI00791052 | |||
| IPI00746963 | IGKC IGKC protein | 1 | 2 |
| IPI00382606 | F7 Factor VII active site mutant immunoconjugate | 1 | 2 |
| IPI00295414 | COL15A1 Collagen alpha-1(XV) chain precursor | 1 | 2 |
| IPI00784142 | |||
| IPI00010896 | |||
| IPI00554767 | CLIC1 Chloride intracellular channel protein 1 | 1 | 2 |
| IPI00334715 | |||
| IPI00718985 | GRLF1 Isoform 1 of Glucocorticoid receptor DNA-binding factor 1 | 1 | 2 |
| IPI00007960 | POSTN Isoform 1 of Periostin precursor | 14 | |
| IPI00641231 | |||
| IPI00418413 | FLJ32569 hypothetical protein LOC148811 | 4 | |
| IPI00186460 | |||
| IPI00383762 | |||
| IPI00395679 | COL2A1 Isoform 1 of Collagen alpha-1(II) chain precursor | 3 | |
| IPI00219525 | |||
| IPI00747533 | PGD 6-phosphogluconate dehydrogenase, decarboxylating | 2 | |
| IPI00550488 | |||
| IPI00744692 | TALDO1 similar to transaldolase 1 | 2 | |
| IPI00019176 | RARRES2 Retinoic acid receptor responder protein 2 precursor | 2 | |
| IPI00219446 | PEBP1 Phosphatidylethanolamine-binding protein 1 | 2 | |
| IPI00003815 | |||
| IPI00793767 | |||
| IPI00794402 | ARHGDIA Rho GDP-dissociation inhibitor 1 | 2 | |
| IPI00796541 | |||
| IPI00472961 | IGKC IGKC protein | 2 | |
| IPI00019954 | |||
| IPI00788184 | CST6 Cystatin-M precursor | 2 | |
| IPI00410402 | TUBA3E Tubulin alpha-3E chain | 2 |
Discussion
The UCB proteome contains many high-abundance proteins that have various housekeeping roles and a number of secreted or shed low-abundance proteins that are critical for signaling cascades and regulatory events. During fetal development, regulatory factors can influence the fetus via the UCB. Meanwhile, metabolic products from the fetus may be released into UCB. Given that these components are present in UCB, a proteomic approach to identify biomarkers for these processes is highly merited. This study revealed that there were differences between the proteomic profiles of male and female fetuses and between those of the fetal and adult serums.
The proteins that belong to the UCB serum proteome are produced and secreted by either the fetus or the placenta. Many of the proteins that are present in UCB are of great interest, since their expression levels can reflect certain physiological and pathological conditions of the fetus and/or the status of the pregnancy. One protein of interest is alpha-fetoprotein (AFP), since its elevation in maternal serum correlates with several fetal abnormalities, including spina bifida, Down's syndrome, trisomy 13, and trisomy 187. Our proteomic analysis identified several putative or currently used biomarkers for the status of the fetus: glyceraldehyde 3-phosphate dehydrogenase, collagen alpha 1 (I), collagen alpha 1 (III), and HSPG2, which are used to test for Down's syndrome14; AFP, which is used for trisomy 13 and trisomy 18; fibronectin and plasminogen activator inhibitor, which are used for pre-eclampsia; and fibronectin15, 17, 18, 19 and apolipoprotein A-I, which are used for cell membrane rupture7. Our results strongly indicate that a quantitative shotgun proteomic analysis of UCB is a feasible and effective method to screen for multiple pathologies.
A major challenge and bottleneck in proteomics lies between protein identification and target validation. Proteins with potential as biomarkers need to undergo further comparative analysis using samples from unaffected and affected pregnancies. In addition, markers that are currently used individually often have insufficient detection rates and specificities, which therefore requires the use of multiple markers. As an example, the prenatal screen for Down's syndrome currently includes three to five different markers. An integrated test or a fingerprinting assay is currently thought to potentially reflect the health status of a fetus better than a test involving a single marker. The MS-derived, systematic expression profiling of proteins from the UCB may serve as one such effective screening tool.
Our analysis identified 53 proteins that are differently expressed between male and female UCB. Of these, 28 proteins were higher in the female UCB, while 25 were higher in the male. Haptoglobin (Hp), which is a hemoglobin(Hb)-binding plasma protein, was identified by seven unique peptides in female UCB but not at all in male UCB. Hp is an acute-phase protein that responds to interleukin 6-type cytokines and is synthesized in the liver and, to some extent, in fat tissue19. There are two major allelic variants of Hp, which are Hp1 and Hp220. Hp1 has higher Hb binding21 and antioxidant capacities than Hp222, 23, 24. Individuals carrying the Hp1 allele exhibit a lower incidence of angiopathies25. Gestational diabetes mellitus (GDM), which is a type of diabetes that is usually confined to the time of gestation, can be an early manifestation of type II diabetes since it increases the risk of developing type II diabetes later in life. Therefore, the high expression level of Hp in female UCB is of great interest, and, although our analysis could not discriminate between Hp1 and Hp2, further efforts should be made to examine this observation.
Proteins that are identified in the UCB serum proteome that are also absent from the adult serum proteome may reflect the specific metabolic status of the fetus and correlate with potentially important regulatory factors for the fetus. Periostin is one of these neonatal specific proteins that has a high expression level in UCB. Periostin, which was originally named osteoblast-specific factor-2 (Osf2), was first identified in bone and was shown to regulate the adhesion and differentiation of osteoblasts26, 27. Recently, periostin has been reported to be frequently over-expressed in various human cancers, since it stimulates metastatic growth by promoting cancer cell survival, invasion, and angiogenesis28. The expression of periostin in UCB is consistent with the marked rate of growth and development of the fetus.
Author contribution
Hong-juan SONG, Ping ZHANG and Xue-jiang GUO designed and performed the research; Xue-jiang GUO, Jia-hao SHA, Zuo-min ZHOU analyzed the data; Hong-juan SONG, Ping ZHANG, Xue-jiang GUO, Lian-ming LIAO, Yu-gui CUI, Hui JI, and Jia-yin LIU wrote the manuscript.
Acknowledgments
This study was supported by grants from the National Basic Research Program of China (2006CB944005 and 2006CB701503), the National 863 High Technology Foundation of China (No 2006AA02Z4A4), the Project for Creative Teams approved by the Ministry of Education (IRT0631), and a Grant from the Department of Education of Jiangsu Province, China (No JHB06-13).
References
- Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, et al. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics. 2002;1:947–55. doi: 10.1074/mcp.m200066-mcp200. [DOI] [PubMed] [Google Scholar]
- Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–67. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572–7. doi: 10.1016/S0140-6736(02)07746-2. [DOI] [PubMed] [Google Scholar]
- Pieper R, Gatlin CL, Makusky AJ, Russo PS, Schatz CR, Miller SS, et al. The human serum proteome: display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics. 2003;3:1345–64. doi: 10.1002/pmic.200300449. [DOI] [PubMed] [Google Scholar]
- Aivado M, Spentzos D, Germing U, Alterovitz G, Meng XY, Grall F, et al. Serum proteome profiling detects myelodysplastic syndromes and identifies CXC chemokine ligands 4 and 7 as markers for advanced disease. Proc Natl Acad Sci USA. 2007;104:1307–12. doi: 10.1073/pnas.0610330104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, He HZ, Dai J, Wang Y, Sheng QH, Zhou LP, et al. The human plasma proteome: analysis of Chinese serum using shotgun strategy. Proteomics. 2005;5:3442–53. doi: 10.1002/pmic.200401301. [DOI] [PubMed] [Google Scholar]
- Vuadens F, Benay C, Crettaz D, Gallot D, Sapin V, Schneider P, et al. Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics. 2003;3:1521–5. doi: 10.1002/pmic.200300455. [DOI] [PubMed] [Google Scholar]
- Karamessinis PM, Malamitsi-Puchner A, Boutsikou T, Makridakis M, Vougas K, Fountoulakis M, et al. Marked defects in the expression and glycosylation of alpha2-HS glycoprotein/fetuin-A in plasma from neonates with intrauterine growth restriction: proteomics screening and potential clinical implications. Mol Cell Proteomics. 2008;7:591–9. doi: 10.1074/mcp.M700422-MCP200. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–8. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Yates JR, 3rd, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem. 1995;67:1426–36. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–4. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsangaris GT, Karamessinis P, Kolialexi A, Garbis SD, Antsaklis A, Mavrou A, et al. Proteomic analysis of amniotic fluid in pregnancies with Down syndrome. Proteomics. 2006;6:4410–9. doi: 10.1002/pmic.200600085. [DOI] [PubMed] [Google Scholar]
- Van Hoof D, Passier R, Ward-Van Oostwaard D, Pinkse MW, Heck AJ, Mummery CL, et al. A quest for human and mouse embryonic stem cell-specific proteins. Mol Cell Proteomics. 2006;5:1261–73. doi: 10.1074/mcp.M500405-MCP200. [DOI] [PubMed] [Google Scholar]
- Stubbs TM, Lazarchick J, Horger EO., 3rd Plasma fibronectin levels in preeclampsia: a possible biochemical marker for vascular endothelial damage. Am J Obstet Gynecol. 1984;150:885–7. doi: 10.1016/0002-9378(84)90468-x. [DOI] [PubMed] [Google Scholar]
- Gao M, Nakabayashi M, Sakura M, Takeda Y. The imbalance of plasminogen activators and inhibitor in preeclampsia. J Obstet Gynaecol Res. 1996;22:9–16. doi: 10.1111/j.1447-0756.1996.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman BH, Kurosky A. Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet. 1982;12:189–261. 453–4. doi: 10.1007/978-1-4615-8315-8_3. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Nagai T. Difference in hemoglobin-binding ability of polymers among haptoglobin phenotypes. Clin Chem. 1997;43:2012–3. [PubMed] [Google Scholar]
- Langlois MR, Delanghe JR, De Buyzere ML, Bernard DR, Ouyang J. Effect of haptoglobin on the metabolism of vitamin C. Am J Clin Nutr. 1997;66:606–10. doi: 10.1093/ajcn/66.3.606. [DOI] [PubMed] [Google Scholar]
- Melamed-Frank M, Lache O, Enav BI, Szafranek T, Levy NS, Ricklis RM, et al. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001;98:3693–8. doi: 10.1182/blood.v98.13.3693. [DOI] [PubMed] [Google Scholar]
- Bernard D, Christophe A, Delanghe J, Langlois M, De Buyzere M, Comhaire F. The effect of supplementation with an antioxidant preparation on LDL-oxidation is determined by haptoglobin polymorphism. Redox Rep. 2003;8:41–6. doi: 10.1179/135100003125001233. [DOI] [PubMed] [Google Scholar]
- Delanghe J, Cambier B, Langlois M, De Buyzere M, Neels H, De Bacquer D, et al. Haptoglobin polymorphism, a genetic risk factor in coronary artery bypass surgery. Atherosclerosis. 1997;132:215–9. doi: 10.1016/s0021-9150(97)00089-0. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–49. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Litvin J, Selim AH, Montgomery MO, Lehmann K, Rico MC, Devlin H, et al. Expression and function of periostin-isoforms in bone. J Cell Biochem. 2004;92:1044–61. doi: 10.1002/jcb.20115. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Siriwardena BS, Hatano H, Ogawa I, Takata T. Periostin: novel diagnostic and therapeutic target for cancer. Histol Histopathol. 2007;22:1167–74. doi: 10.14670/HH-22.1167. [DOI] [PubMed] [Google Scholar]