Abstract
Aim:
To investigate the molecular mechanism and signaling pathway by which fenoterol, a β2-adrenergic receptor (β2-AR) agonist, produces anti-inflammatory effects.
Methods:
THP-1, a monocytic cell line, was used to explore the mechanism of β2-AR stimulation in LPS-induced secretion of inflammatory cytokines and changes of toll-like receptors (TLRs). We labeled TLR4 and CD14 using monoclonal anti-TLR4 PE-conjugated and anti-CD14 FITC-conjugated antibodies in THP-1 cells stimulated by β2-AR in the presence or absence of lipopolysaccharide (LPS) and small, interfering RNA (siRNA)-mediated knockdown of β-arrestin-2, and then analyzed their changes in distribution by flow cytometry, Western blotting and confocal analysis.
Results:
LPS-induced membrane-bound CD14, TLR4/CD14 complex levels and elevation of inflammatory cytokines were all significantly reduced by pre-incubation of fenoterol (P<0.05). However, the total level of CD14 and TLR4 was not significantly changed. Interestingly, confocal microscopy revealed redistribution of CD14 and TLR4/CD14 complex under β2-AR stimulation. Furthermore, siRNA-mediated knockdown of β-arrestin-2 eliminated the anti-inflammatory effects and redistribution of CD14 and TLR4/CD14 complex stimulated by β2-AR.
Conclusion:
β2-AR agonist exerts its anti-inflammatory effects by down-regulating TLR signaling in THP-1 cells, potentially resulting from β-arrestin-2 mediated redistribution of CD14 and TLR14/CD14 complex.
Keywords: β2-adrenoceptor, toll-like receptors, β-arrestin-2, fenoterol, confocal microscopy, lipopolysaccharide
Introduction
β2-adrenergic receptor (β2-AR) agonist is administered in a variety of clinical situations1, 2, 3, 4, 5 mostly for its bronchodilating effects. Furthermore, the regulation of β2-AR agonist on the production of inflammatory cytokines has been recognized. For example, it was shown that, salbutamol and albuterol, agonists of β2-AR, could inhibit tumor necrosis factor (TNF)-α production by human mononuclear cells6, 7, in addition, salbutamol exerts immunosuppressive effects through down-regulation of co-stimulatory molecules, inter-cellular adhesion molecule 1 (ICAM-1), CD40 and CD14 on monocytes7, endocytosis of the TLR4 complex was pertinent to anti-inflammatory effects8. Whether β2-AR stimulation mediated anti-inflammatory effects in monocytes depending on the endocytosis or redistribution of TLRs is not clear. Therefore, it is necessary to make clear the exact target of β2-AR stimulation during the process of anti-inflammation.
Upon agonist binding, β-arrestins1/2 is recruited to the plasma membrane and interacts directly with two structural components of clathrin-coated pits, clathrin and AP-2, which promote the endocytosis of β2-AR into early endosomes via clathrin-coated vesicles9, 10. Moreover, TLR4 was also endocytosed by a dynamin and clathrin dependent mechanism and colocalized with lipopolysaccharide (LPS) on early sorting endosomes11. Therefore, we hypothesized that β2-AR stimulation mediated β-arrestins' translocation was associated with redistribution of TLRs.
Lipopolysaccharide (LPS)-induced inflammation in THP-1 cells is a model to study TLRs12. As a receptor of LPS, TLRs play an important role during LPS-induced inflammation13. Recent studies have reported that β2-adrenergic agonist exert its “anti-inflammatory” effects in monocytic cells through the IκB/NF-κB pathway6. On the other hand, IκB/NF-κB is downstream signaling of TLR, which plays a pivotal role in regulating inflammatory gene expression and LPS-induced inflammation13. The exact relationship between β2-AR-mediated anti-inflammatory effects and TLR signaling pathway remained to be elucidated in monocytes.
In the present study, we aimed to explore the underlying mechanism of the anti-inflammatory effects mediated by β2-AR stimulation in THP-1 cells. We first investigated if LPS-induced cytokines could be suppressed by fenoterol via ELISA assay. To confirm fenoterol' anti-inflammatory effect, down-regulated LPS-induced membrane-bound TLR4/CD14 complex and CD14 level in THP-1 cells on stimulation of β2-AR were verified by flow cytometry. Then, we discovered that the total level of CD14 and TLR4 was not significantly changed by Western blotting, but interestingly, redistribution of CD14 and TLR4/CD14 complex mediated by β2-AR stimulation was found by confocal analysis. Lastly, anti-inflammatory effects and redistribution of CD14 and TLR4/CD14 complex mediated by β2-AR stimulation were abolished by siRNA-mediated knockdown of β-arrestin-2, which might play an important role in crosstalk of β2-AR and TLR14.
Materials and methods
Cell culture
The human monocytic cell line THP-1 (obtained from the cell center of Peking Union Medical College) was cultured in RPMI-1640 medium (Sigma Chemical Co, St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 g/mL streptomycin at 37 °C in 5% CO2 in a humidified incubator. Cells were centrifuged and resuspended with fresh medium at 106/mL and incubated for another 24 h before use. The cells were washed and distributed into sterile microtiter plates at 106/mL in RPMI-1640 medium containing 2% FBS stimulated with 0.1 μg/mL of Escherichia coli 0111:B4 LPS (Sigma) for 24 h (unless indicated otherwise) at 37 °C in the presence or absence of β2-AR agonists (fenoterol) and antagonists (ICI 118551) (both from Sigma).
Downregulation (siRNA) of the β-arrestin-2
Cells were split at least 24 h prior to transfection and transfected with siRNA designed against β-arrestin-2 or control siRNA using the Oligofectamine™ transfection reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the optimized procedure recommended by the producer as described elsewhere15. The siRNA sequence targeting β-arrestin-2 is 5′ AAGGACCGCAAAGUGUUUGUG 3′ (Shanghai GeneChem Co, Ltd, Shanghai,China). All assays were performed 72 h following transfection of siRNA. The inhibitory efficiency of siRNA probes was assessed by measuring knockdown of the β-arrestin-2 protein by Western blotting analysis.
ELISA assay
Concentrations of interleukin 8 (IL-8) and tumor necrosis factor α (TNF-α) from cell supernatants were determined by use of an ELISA system (R&D Systems, Minneapolis, MN) according to the manufacturer. The detection limits of ELISA for IL-8 and TNF-α were 10 pg/mL.
Flow cytometry
The expression of CD14 and TLR4/CD14 complex in THP-1 cells was determined by flow cytometry. After LPS stimulation in the presence or absence of fenoterol, the cells (106/sample) were washed once with PBS, then incubated at 4 °C for 30 min with a combination of anti-CD14 FITC-conjugated (clone 61D3, 10 g/mL; eBioscience) and anti-TLR4 PE-conjugated antibodies (clone HTA125, 10 g/mL; eBioscience). After washing, cells were analyzed by use of a FACS Calibur (Becton Dickinson Biosciences, San José, CA, USA), and data were analyzed by use of the CELL QUEST Program (Becton Dickinson).
Western blotting and immunoprecipitation
After treatment, THP-1 cells were lysed in 10 mmol/L HEPES, pH 7.9, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol, and 1 mmol/L phenylmethyl-sulfonylfuoride. Cell membrane proteins were prepared using the Plasma Membrane Protein Extraction Kit (Applygen Technologies Inc., Beijing, China). Cell membrane protein or cytoplasmic protein extracts, 60−90 μg were separated by 10% SDS-PAGE and electrotransferred onto anti-trocellulose membrane (Bio-Rad, Hercules, CA, USA). TLR4, CD14, and β-arrestin-2 were detected with use of mouse monoclonal anti-human TLR4, CD14, and β-arrestin-2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-mouse horseradish peroxidase-conjugated secondary antibody (Zhong Shan Jin Qiao Co, China), and enhanced chemiluminescence (Pierce Biotechnology). Band intensities were determined using computer program Image-J and were presented as the mean±SEM of the x-fold change over the respective control that was arbitrarily defined. For immunoprecipitation, 100 μg of membrane protein was incubated with 20 μL protein G plus-agarose (Santa Cruz Biotechnology, Santa Cruz, CA, USA) pre-equilibrated in lysis buffer and 10 μL of polyclonal antibodies for 4 h at 4 °C. Samples were then centrifuged for 10 s, and the pellets were washed three times with 1 mL of lysis buffer. Bound proteins were eluted by the addition of 15 μL of SDS sample buffer and boiling for 5 min and then analyzed by SDS-PAGE and immunoblotting.
Confocal analysis
A standard immunocytoplasmic staining protocol was used16. Briefly, after LPS stimulation in the presence or absence of fenoterol and siRNA-mediated knockdown of β-arrestin-2, THP-1 cells were cultured in a chamber slide (Zhong Shan Jin Qiao Co, China) for 20 min, then fixed with ice-cold acetone for 20 min and stained with PE-conjugated monoclonal antibodies for mouse anti-human TLR4 (HTA125) and FITC-conjugated monoclonal antibodies for mouse anti-human CD14 (61D3) for 24 h at room temperature, then washed with PBS twice and stained with Hoechst-33342 (Sigma-Aldrich) for 15 min to visualize the nuclei, washed with PBS twice, then mounted with use of Antifadent Mountant Solutions (Zhong Shan Jin Qiao Co, China) and viewed under a confocal laser scanning microscope (LSM 510 META, Zeiss, Germany).
Statistical analysis
Experiments were repeated at least three times. Data are presented as mean±SEM. The statistical significance of the differences between the means of the groups was determined by one-way ANOVA followed by Bonferroni post-hoc test. P values of <0.05 were considered statistically significant.
Results
Fenoterol inhibits LPS-stimulated IL-8, TNF-α release from THP-1 cells
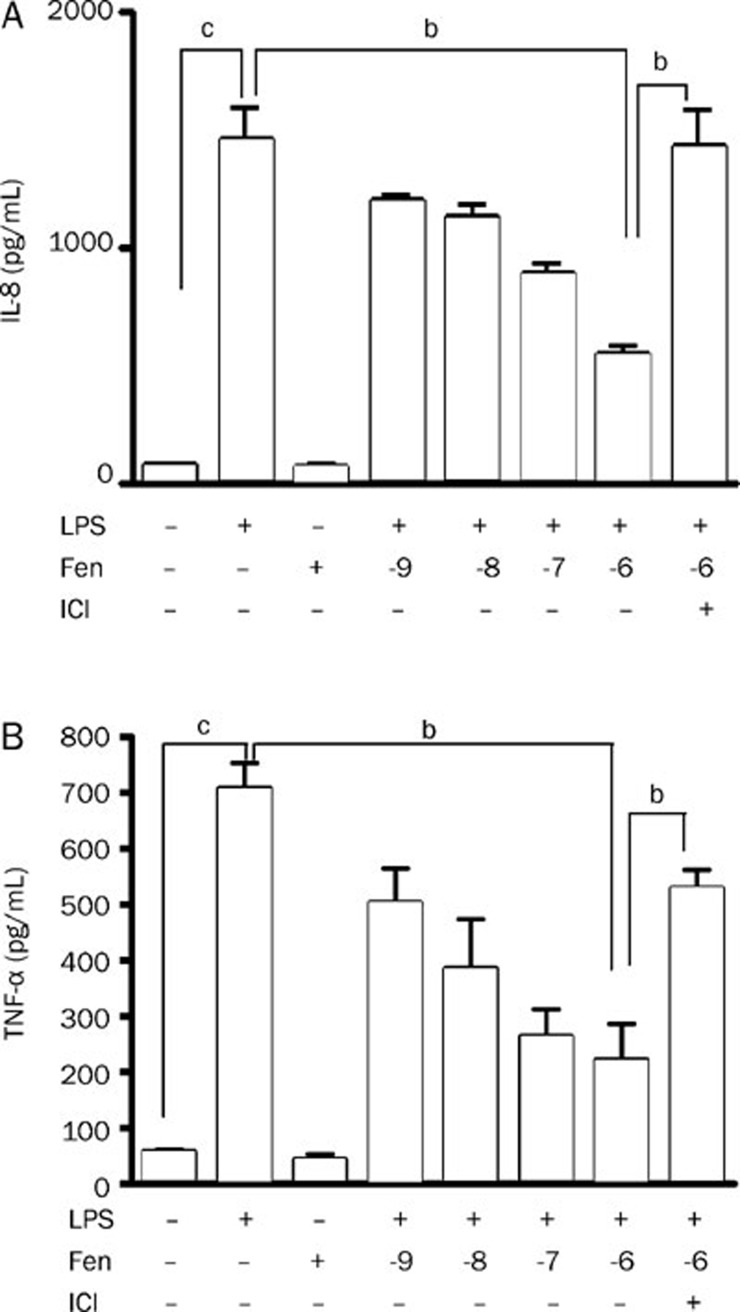
The concentration of IL-8 increased about 20-fold on stimulation with LPS (0.1 μg/mL) in THP-1 cells. The elevated concentration of IL-8 was significantly decreased by pre-incubation with up to 10−6 mol/L fenoterol. Furthermore, this effect was largely attenuated in the presence of 10−6 mol/L ICI118551, the antagonist of β2-AR (Figure 1A). Similar results were found for TNF-α (Figure 1B).
Figure 1.
Concentration of LPS-stimulated IL-8 and TNF-α in cell supernatants determined by ELISA in the presence or absence of fenoterol (lg mol/L) and ICI118551 (10−6 mol/L). (A) Inhibitory effect of fenoterol on IL-8 production from THP-1 cells stimulated for 24 h with LPS (0.1 μg/mL). (B) Inhibitory effect of fenoterol on TNF-α production from THP-1 cells stimulated for 24 h with LPS (0.1 μg/mL). Data are presented as mean±SEM. bP<0.05, cP<0.01.
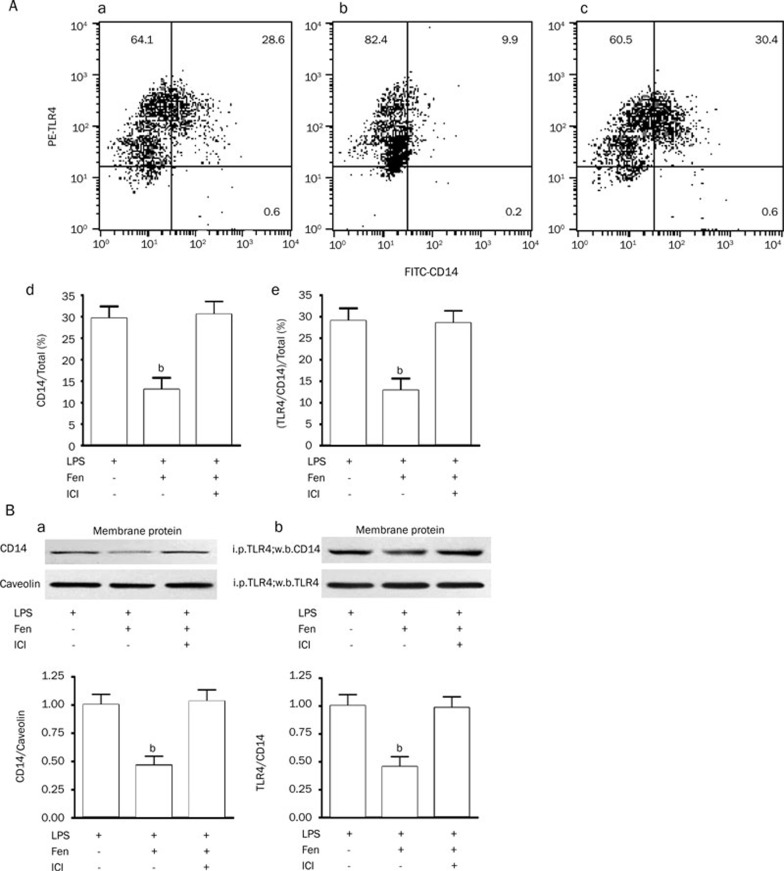
Fenoterol down-regulates membrane-bound TLR4/CD14 complex and CD14 in THP-1 cells
After LPS (0.1 μg/mL) stimulation for 24 h, the effect of fenoterol (10−6 mol/L) on change of the membrane-bound TLR4/CD14 complex and CD14 levels in THP-1 cells was examined by flow cytometry. Although LPS-induced TLR4 expression was not significantly changed with β2-AR stimulation (data not shown), the membrane-bound TLR4/CD14 complex and CD14 levels in THP-1 cells were significantly decreased on incubation with fenoterol, pre-incubation with ICI118551 for 30 min abolished the effect of down-regulation of TLR4/CD14 complex and CD14 mediated by fenoterol (Figure 2A). Similar results were found by Western blotting (Figure 2B).
Figure 2.
Expression of membrane-bound TLR4, CD14, and TLR4/CD14 complex in THP-1 cells by two-staining flow cytometry method (PE-TLR4 and FITC-CD14) and immunoprecipitation and immunoblotting. (A) (a) Expression of TLR4, CD14 and TLR4/CD14 complex in THP-1 cells stimulated by LPS (0.1 μg/mL) for 24 h (representative experiment). (b) Fenoterol (10−6 mol/L) for 24 h down-regulates LPS-stimulated membrane-bound CD14 and TLR4/CD14 complex in THP-1 cells (representative experiment). (c) Pre-incubation of ICI118551 for 30 min abolished fenoterol-induced down-regulation of membrane-bound CD14 and TLR4/CD14 complex (representative experiment). (d) Down-regulating effect of fenoterol (10−6 mol/L) for 24 h on LPS-stimulated membrane-bound CD14 in THP-1 cells. Data are presented as mean±SEM. bP<0.05 vs LPS or LPS+Fen+ICI118551 group. (e) Down-regulating effect of 24 h fenoterol (10−6 moL/L) on LPS-stimulated membrane-bound TLR4/CD14 complex in THP-1 cells. Data are presented as mean±SEM. bP<0.05 vs LPS or LPS+Fen+ICI118551 group. (B) (a) Down-regulating effect of fenoterol (10−6 moL/L) for 24 h on LPS-stimulated membrane-bound CD14 in THP-1 cells by Western blotting. Data are presented as mean±SEM. bP<0.05 vs LPS or LPS+Fen+ICI118551 group. (b) Down-regulating effect of fenoterol (10−6 mol/L) for 24 h on LPS-stimulated membrane-bound TLR4/CD14 complex in THP-1 cells by immunoprecipitation and immunoblotting. Data are presented as mean±SEM. bP<0.05 vs LPS or LPS+Fen+ICI118551 group.
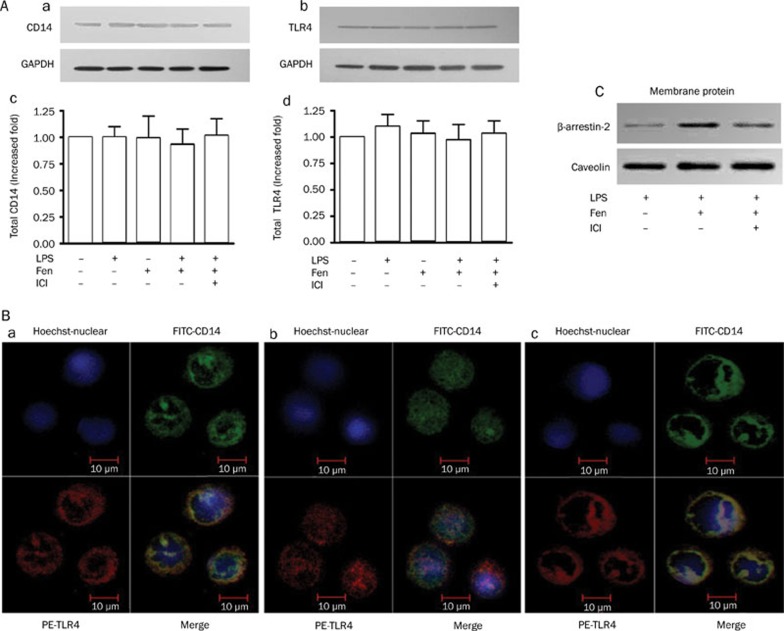
Fenoterol enhances redistribution of LPS-stimulated TLR4/CD14 complex and increases membrane-bound β-arrestin-2 expression in THP-1 cells
The total protein expression of CD14 and TLR4 in THP-1 cells was not significantly changed by treatment with fenoterol (10−6 mol/L) or LPS (0.1 μg/mL) (Figure 3Aa, 3Ad). However, confocal microscopy revealed that the membrane-bound TLR4/CD14 complex was reduced in level with pre-incubation of fenoterol (10−6 mol/L) (Figure 3Bb) in LPS-stimulated THP-1 cells (Figure 3Ba) and redistribution of TLR4/CD14 complex under stimulation with β2-AR was abolished with pre-incubation of ICI118551 for 30 min (Figure 3Bc). Meanwhile, membrane-bound β-arrestin-2 was increased by treatment with fenoterol (10−6 mol/L) for 3 min (Figure 3C).
Figure 3.
Expression of CD14, TLR4, and membrane-bound β-arrestin-2 in the presence or absence of fenoterol by Western blotting. Distribution of LPS-stimulated TLR4/CD14 complex on stimulation with β2-AR examined on confocal analysis. (A) (a,c) Representative Western blotting and analysis of CD14 and GAPDH protein expression; (A) (b,d) Representative Western blotting and analysis of TLR4 and GAPDH expression. GAPDH was used as an internal loading control. (B) (a) Confocal analysis of LPS-stimulated TLR4/CD14 complex in THP-1 cells; (b) Confocal analysis of redistribution of LPS-stimulated TLR4/CD14 complex from THP-1 cells under stimulation with β2-AR. (c) Redistribution of LPS-stimulated TLR4/CD14 complex under stimulation of β2-AR with pre-incubation of ICI118551 in THP-1 cells. (C) Expression of membrane-bound β-arrestin-2 in the presence or absence of fenoterol for 3 min, LPS and ICI118551 by Western blotting analysis (representative experiment).
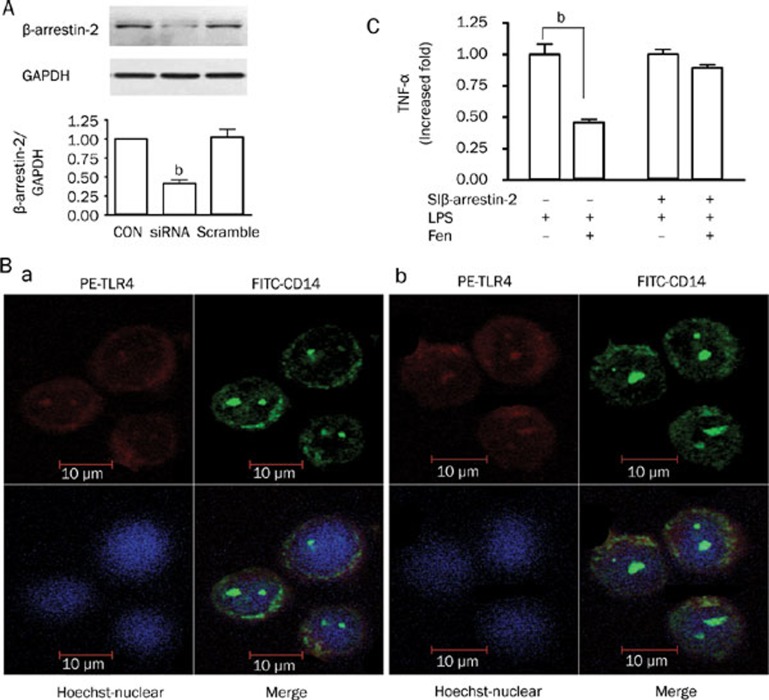
Silencing β-arrestin-2 abolished the anti-inflammatory effects and redistribution of LPS-induced TLR4/CD14 complex stimulated by β2-AR
The siRNA used almost abrogated β-arrestin-2 expression in THP-1 cells (Figure 4A). To determine whether the β-arrestin-2 siRNA could affect anti-inflammatory effects and redistribution of LPS-stimulated TLR4/CD14 complex on stimulation with β2-AR, afer transfection with siRNA designed against β-arrestin-2 or control siRNA, THP-1 cells were stimulated with LPS in the presence or absence of fenoterol as described before. As shown in (Figure 4B, 4C), anti-inflammatory effects and redistribution of CD14 and TLR4/CD14 complex mediated by β2-AR stimulation were abolished by siRNA-mediated knockdown of β-arrestin-2, while not abolished by control siRNA (data not shown).
Figure 4.
Effects of β-arrestin-2′ downregulation on the anti-inflammation and redistribution of LPS-stimulated TLR4/CD14 complex stimulated by β2-AR. (A) Effect of β-arrestin-2 small interfering RNA (siRNA) for 72 h on the expression of the β-arrestin-2 protein. Data are presented as mean±SEM. bP<0.05 vs control or scramble. GAPDH was used as an internal loading control. (B) After transfection with siRNA designed against β-arrestin-2 for 72 h, confocal analysis of LPS-stimulated TLR4/CD14 complex in THP-1 cells (a); Confocal analysis of redistribution of LPS-stimulated TLR4/CD14 complex in THP-1 cells under stimulation with β2-AR (b). (C) After transfection with siRNA designed aga inst β-arrestin-2 for 72 h, THP-1 cells were stimulated with LPS for 24 h in the presence or absence of fenoterol, anti-inflammatory effects stimulated by β2-AR was abolished when β-arrestin-2 was knocked down. bP<0.05 vs LPS 0.1 μg/mL.
Discussion
LPS-induced inflammatory response was abolished in mice deficient in MyD88−/−, an important downstream signaling molecule of TLRs, suggesting that TLRs play a central role in the pathogenic microorganism-mediated inflammatory response13, 17. On the other hand, we noticed that β2-AR signaling exerted anti-inflammatory effect6, 7. Therefore, further study is needed to elucidate the relationship between β2-AR-mediated anti-inflammatory effects and TLR signaling pathway.
To understand the mechanism of β2-AR-mediated TLR regulation, TLR binding structure and its co-factors first need to be considered. All TLRs are type I transmembrane receptors, characteristic of a highly variable extracellular region, including a leucine-rich repeat domain involved in ligand binding and an intracellular tail containing a highly conserved region, the Toll/Interleukin-1 Receptor (TIR) homology domain, which mediates interaction between TLRs and downstream signaling molecules13. Activation of TLR4 is initiated as follows: the binding of the LPS binding protein (LBP)/LPS complex to membrane CD14 (mCD14), then binding and forming the TLR4/CD14 complex and activating TLR4, which activates signal transduction pathways and induces inflammatory gene expression13. Thus, we speculated that a change in the membrane-bound TLR4/CD14 complex level might affect the activation of TLR4. Furthermore, in the present study, we identified that reduced inflammatory response mediated by β2-AR stimulation was related to the change of membrane-bound TLR4/CD14 complex (Figure 2) but not total protein expression of TLR4 in monocytes (Figure 3A). Interestingly, despite no significant change in total protein expression of TLR4 with β2-AR stimulation, confocal microscopy revealed redistribution of the TLR4/CD14 complex (Figure 3B). A previous study showed that human corneal epithelial cells express TLR2 and TLR4 intracellularly but not at the cell surface and fails to respond to LPS even on artificial translocation of LPS18. Thus, membrane-bound TLRs play a central role in LPS-induced inflammatory response, and β2-AR mediated reduction of membrane-bound TLRs was responsible for the reduced inflammatory response in monocytes.
Whether the β2-AR-mediated anti-inflammatory effect depends on the inhibition of the receptor level or downstream signaling of TLRs is still in debate. There have been some reports that the anti-inflammatory effect of β-receptor activation was associated with a change in content of IκB/NF-κB, extracellular signal-regulated kinase 1/2 (ERK1/2) or p386, 19, whether these changes were the direct effect of β-receptor stimulation or resulted from down-regulation of TLRs is still unknown. A recent study revealed that β2-AR agonist exerts its anti-inflammatory effect through inhibiting the expression of membrane-bound CD14, a co-factor of TLRs, on monocytes 7. The regulation of TLRs might be a potential target of the β2-AR agonist. Our results further demonstrated that the reduced level of membrane-bound TLRs was responsible for the anti-inflammatory effect of β2-AR agonist (Figure 2). As well, the decreased activation of NF-κB signaling was attributed to the down-regulation of membrane-bound TLRs. Whether the signaling of TLRs is a specific pathway for the β2-AR-mediated anti-inflammatory effect still needs to be elucidated.
Upon agonist binding, β-arrestins1/2 is recruited to the plasma membrane and mediates desensitization and internalization of G-protein-coupled receptor (GPCR)20. However, β-arrestins have been considered as novel non-G protein-dependent signaling molecules and play functional roles in the regulation of a variety of signaling pathways and in the mediation of cross-talk between receptors21, 22, 23. For example, β-arrestin-2-dependent stabilization of cytosolic IκBα and inhibition of NF-κB activation following LPS stimulation are essential for rapid and sufficient production of NO in response to microbial attack14. Moreover, there is accumulating evidence that β-arrestin-2, which is expressed abundantly in the spleen, is functionally involved in some important immune responses, such as regulation of lymphocyte chemotaxis and homing24, 25. In the present study we used RNA interference against β-arrestin-2 to test its role in anti-inflammatory effects stimulated by β2-AR. The specificity and efficiency of siRNA against β-arrestin-2 was demonstrated by Western blotting (Figure 4A). The translocation of β-arrestins1/2 to the plasma membrane was reported to interact directly with two structural components of clathrin-coated pits, clathrin and AP-2, promoting the endocytosis of β2-AR into early endosomes via clathrin-coated vesicles9, 10. Moreover, TLR4 was also endocytosed by a dynamin and clathrin dependent mechanism and colocalized with LPS into early/sorting endosomes11. Therefore, we hypothesized that β-arrestins' translocation to the cell surface was associated with redistribution of TLRs on stimulation of β2-AR. Meanwhile, fenoterol increased membrane-bound β-arrestin-2 expression, suggesting that β-arrestin-2 translocated to the cell surface on stimulation of β2-AR in THP-1 cells (Figure 3C). Our study indicated that depletion of β-arrestin-2 abolished redistribution of CD14 and TLR4/CD14 complex mediated by β2-AR activation (Figure 4B), suggesting that β-arrestin-2′ translocation and β-arrestin-2/clathrin-dependent redistribution of TLRs was required for anti-inflammatory effects stimulated by β2-AR. Further study needs to clarify β-arrestin-2/clathrin mediated redistribution of TLRs on stimulation of β2-AR.
Taken together, we provided evidence that β2-AR agonist exerts anti-inflammatory effects by down-regulating membrane-bound TLRs through β-arrestin-2. Down-regulation of β-arrestin-2 significantly attenuates the anti-inflammatory effects mediated by fenoterol, suggesting that β-arrestin-2 is beneficial to protecting organism against invading pathogens. This finding has implications not only with regard to our understanding of molecular mechanism for the β2-AR agonists' anti-inflammatory effects but also for the development of therapeutic agents targeting these pathways, which may be helpful for treatment of acute and chronic inflammatory diseases.
Author contribution
Bei HE, Ming XU, You-yi ZHANG designed research; Wei WANG performed research; Wei WANG contributed new analytical tools and reagents; Wei WANG, Bei HE, Ming XU wrote the paper.
Acknowledgments
Project was supported by the National Key Basic Research Program (NKBRP) of People's Republic of China (No 2006CB503806) and the National Natural Science Foundation of China (No 30770939 and 30821001).
The authors would like to thank Dr Laura HERATY for language revision, and Dr Pei ZHANG for performing the confocal laser scanning microscope.
References
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Bourbeau J, Christodoulopoulos P, Maltais F. Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: a randomised controlled trial. Thorax. 2007;62:938–43. doi: 10.1136/thx.2006.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears MR, Ottosson A, Radner F, Suissa S. Long-acting beta-agonists: a review of formoterol safety data from asthma clinical trials. Eur Respir J. 2009;33:3–5. doi: 10.1183/09031936.00145006. [DOI] [PubMed] [Google Scholar]
- Delavoie F, Molinari M, Milliot M, Zahm JM, Coraux C, Michel J, et al. Salmeterol restores secretory functions in cystic fibrosis airway submucosal gland serous cells. Am J Respir Cell Mol Biol. 2009;40:388–97. doi: 10.1165/rcmb.2008-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Lee J. Beta2 adrenergic agonist therapy may enhance alveolar epithelial repair in patients with acute lung injury. Thorax. 2008;63:189–90. doi: 10.1136/thx.2007.086256. [DOI] [PubMed] [Google Scholar]
- Farmer P, Pugin J. β-Adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IκB/NF-κB pathway. Am J Physiol Lung Cell Mol Physiol. 2000;279:675–82. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- Kuroki K, Takahashi HK, Iwagaki H, Murakami T, Kuinose M, Hamanaka S, et al. β2-adrenergic receptor stimulation-induced immunosuppressive effects possibly through down-regulation of co-stimulatory molecules, ICAM-1, CD40 and CD14 on monocytes. J Int Med Res. 2004;32:465–83. doi: 10.1177/147323000403200503. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:343–5. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA, et al. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem. 1997;272:31051–7. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, et al. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–7. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husebye H, Halaas Ø, Stenmark H, Tunheim G, Sandanger Ø, Bogen B, et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–92. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remer KA, Brcic M, Sauter KS, Jungi TW. Human monocytoid cells as a model to study Toll–like receptor-mediated activation. J Immunol Methods. 2006;313:1–10. doi: 10.1016/j.jim.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Kizaki T, Izawa T, Sakurai T, Haga S, Taniguchi N, Tajiri H, et al. Beta2-adrenergic receptor regulates Toll-like receptor-4-induced nuclear factor-kappaB activation through beta-arrestin 2. Immunology. 2008;124:348–56. doi: 10.1111/j.1365-2567.2007.02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioca DP, Aoki Y, Kiyosawa K. RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Therapy. 2003;10:125–33. doi: 10.1038/sj.cgt.7700544. [DOI] [PubMed] [Google Scholar]
- Eldstrom JR, La K, Mathers DA. Polycationic lipids translocate lipopolysaccharide into HeLa cells. Biotechniques. 2000;28:510–6. doi: 10.2144/00283st10. [DOI] [PubMed] [Google Scholar]
- Miggin SM, O'Neill LA. New insights into the regulation of TLR signaling. J Leukoc Biol. 2006;80:220–6. doi: 10.1189/jlb.1105672. [DOI] [PubMed] [Google Scholar]
- Ueta M, Nochi T, Jang MH, Park EJ, Igarashi O, Hino A, et al. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J Immunol. 2004;173:3337–47. doi: 10.4049/jimmunol.173.5.3337. [DOI] [PubMed] [Google Scholar]
- Szelenyi J, Selmeczy Z, Brozik A, Medgyesi D, Magocsi M. Dual β-adrenergic modulation in the immune system: Stimulus-dependent effect of isoproterenol on MAPK activation and inflammatory mediator production in macrophages. Neurochem Int. 2006;49:94–103. doi: 10.1016/j.neuint.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Ma L, Pei G. Beta-arrestin signaling and regulation of transcription. J Cell Sci. 2007;120:213–8. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–52. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, et al. Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–73. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. 2006;281:18081–9. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Cheng Z, Ma L, Pei G. β-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–9. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci USA. 2002;99:7478–83. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]