Abstract
Aim:
2-(3′,5′-Dimethoxybenzylidene) cyclopentanone (DMBC) is a novel synthetic compound with antinociceptive activities. The aim of this study was to investigate the roles of the autophagic-lysosomal pathway in the antinociceptive effect of DMBC in a mouse acetic acid-writhing model.
Methods:
Mouse acetic acid-writhing test and hotplate test were used to assess the antinociceptive effects of DMBC, 3-MA (autophagy inhibitor) and Clik148 (cathepsin L inhibitor). The drugs were administered peripherally (ip) or centrally (icv).
Results:
Peripheral administration of 3-MA (7.5–30 mg/kg) or Clik148 (10–80 mg/kg) produced potent antinociceptive effect in acetic acid-writhing test. Central administration of 3-MA or Clik148 (12.5–50 nmol/L) produced comparable antinociceptive effect in acetic acid-writhing test. Peripheral administration of DMBC (25–50 mg/kg) produced potent antinociceptive effects in both acetic acid-writhing and hotplate tests. Furthermore, the antinociceptive effect produced by peripheral administration of DMBC (50 mg/kg) in acetic acid-writhing test was antagonized by low doses of 3-MA (3.75 mg/kg) or Clik148 (20 mg/kg) peripherally administered, but was not affected by 3-MA or Clik148 (25 nmol/L) centrally administered.
Conclusion:
Activation of central autophagy and cathepsin L is involved in nociception in mice, whereas peripheral autophagy and cathepsin L contributes, at least in part, to the antinociceptive effect of DMBC in mice.
Keywords: autophagy; cathepsin L; antinociception; 3-MA; Clik148; 2-(3′,5′-dimethoxybenzylidene) cyclopentanone; acetic acid writhing; hotplate
Introduction
Cathepsins are acidic endopeptidases that belong to the papain superfamily of proteases1. Under physiologic conditions, their activity is restricted to the lysosomal compartment, where they participate in protein turnover by degrading unneeded proteins into amino acids2. The cysteine protease cathepsin L is one of the main cathepsins. Cathepsin L has recently been shown to significantly participate in proneuropeptide processing, and it is a protease that is responsible for the production of many neuropeptides, including β-endorphin, enkephalin and dynorphin opioid neuropeptides3,4,5,6,7. The peptides of the endogenous opioid family are best known for their effects on pain modulation8, which suggests that cathepsin L, as a neuropeptide-processing enzyme, participates in the production and regulation of pain.
Autophagy is a highly regulated process that involves the bulk degradation of cytoplasmic macromolecules and organelles in mammalian cells via the lysosomal system. Basal autophagy is a highly regulated process, and any imbalance favoring increases or inhibitions may be detrimental for cell survival. Cellular autophagic activity is usually low under basal conditions but can be markedly up-regulated by numerous stimuli, including nutrient starvation and stressors such as hypoxia, ischemia, energy depletion, endoplasmic reticulum stress, and hormonal stimulation by pharmacological agents such as rapamycin and bacterial, viral, and parasitic infections9. Conversely, autophagy suppression is associated with a number of diseases, including some forms of cancer, neurodegenerative disorders, infectious diseases, and inflammatory bowel disorders10. Recently, Berliocchi et al provided the first report of an impairment of spinal autophagy in a model of neuropathic pain11. Another literature also reported that the activation of autophagy is critical for the improvement of locomotor performance via suppressing the formation of peripheral myelin protein 22 (PMP22) aggregates in a misexpression and cytosolic retention of PMP22 within Schwann cells (SCs)-associated demyelinating neuropathies12. However, the role of the autophagic-lysosomal pathway in the regulation of pain processing is largely unknown. In the present study, we found that 3-methyladenine (3-MA), an inhibitor of autophagy, and Clik148, a specific selective inhibitor of cathepsin L, produced strong analgesic effects when administered systemically and centrally in an acetic acid-induced writhing mouse pain model. In the acetic acid-induced writhing and hotplate mouse pain models, 2-(3′,5′-dimethoxybenzylidene) cyclopentanone (DMBC), a novel synthetic compound (Figure 1), exerted an antinociceptive effect. Further experiments investigated whether 3-MA or Clik148, when administered systemically, could antagonize the analgesic effect of DMBC. Neither 3-MA nor Clik148, when administered centrally, had any obvious effects on DMBC-mediated analgesia, suggesting that inhibition of the peripheral autophagic and cathepsin L mechanisms contributes, at least in part, to the analgesic effects of DMBC. Therefore, our data indicate that both the central and the peripheral autophagic and cathepsin L mechanisms might be involved in the regulation of pain.
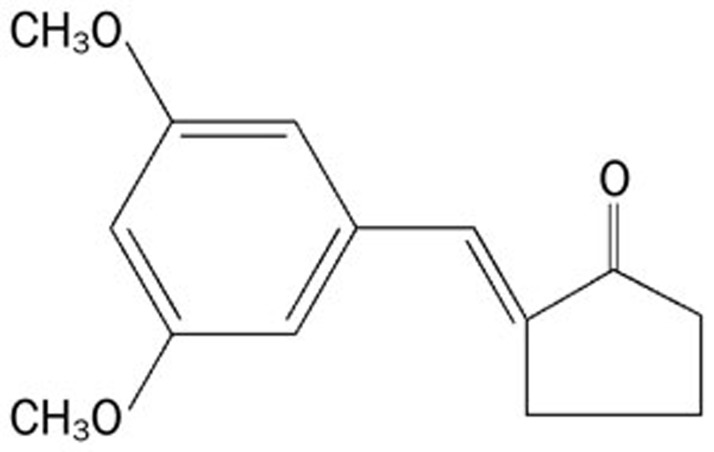
Figure 1.
Chemical structure of 2-(3′,5′-dimethoxybenzylidene) cyclopentanone (DMBC).
Materials and methods
Animals
Male and female ICR mice weighing 18–22 g were purchased from the Center for Medical Experimental Animals, Soochow University, China (Grade 2, Certification No 98018). NIH guidelines for the care and use of laboratory animals were followed during all animal procedures.
Drug treatments
2-(3′,5′-Dimethoxybenzylidene) cyclopentanone (DMBC) and Clik148 were supplied by the College of Pharmaceutical Science (Suzhou, Jiangsu, China) or the Institute for Health Sciences, Tokushima Bumi University (Tokushima, Japan). 3-Methyladenine (3-MA) was purchased from Sigma (St Louis, MO, USA).
To test the effects of DMBC on pain response in the hotplate test, mice were randomly divided into 4 groups (n=10 in each group) that received DMSO as a control or DMBC at dose of 25, 35.4, and 50 mg/kg. Pain thresholds were measured at 30, 60, 90, and 120 min after ip drug administration. To examine the effects of DMBC, 3-MA, and Clik148 on pain response in the acetic acid-writhing test, mice were randomly divided into 4 or 5 groups (n=10 in each group) that received saline or DMSO (controls); DMBC at 25, 35.4, and 50 mg/kg; 3-MA at 3.75, 7.5, 15, and 30 mg/kg or Clik148 at 10, 20, 40 and 80 mg/kg. The effects of 3-MA (ip, icv) or Clik148 (ip, icv) on DMBC-induced analgesia were studied in the acetic acid-writhing test. For these studies, mice were randomly divided into 4 or 6 groups (n=10 in each group) that received DMSO as a control (model group); 3-MA at 3.75 and 7.5 mg/kg (ip) or 3-MA at 25 nmol (icv) (3-MA group); DMBC at 50 mg/kg (DMBC group); and DMBC plus 3-MA (DMBC+3-MA group). For 3-MA icv, 3-MA or normal saline (NS) was administered 30 min before DMBC. Pain thresholds were determined 60 min after DMBC administration. The effect of Clik148 (ip and icv) on DMBC-induced analgesia was evaluated in the acetic acid-writhing test. Mice were randomly divided into 4 or 6 groups (n=10 in each group) that received DMSO as a control (model group); Clik148 at 10 and 20 mg/kg (ip) or Clik148 at 25 nmol (icv) (Clik148 group); DMBC at 50 mg/kg (ip, DMBC group); or DMBC plus Clik148 (DMBC+Clik148 group). For Clik148 icv experiments, Clik148 or DMSO was administered 30 min before DMBC. Pain thresholds were determined 60 min after DMBC administration.
Analgesic assessment
Hotplate test in mice: Female mice were placed on a hot plate with the temperature maintained at 55±1 °C. The latency with which the mice licked their hind paws was recorded as the pain threshold. The baseline pain threshold was obtained by averaging the values for 2 measurements made before drug administration. Mice with 5–20 s pain thresholds were used in the experiments. After drug administration, the pain threshold was determined using the same method, and a cutoff time of 60 s was used to minimize potential harmful effects.
Acetic acid-writhing test in mice: Ten minutes after the administration of 0.1 mL/10 g (ip) 1% acetic acid solution to the mice, the number of writhings between 10 and 20 min after acetic acid injection was counted13.
Intra-cerebral ventricle (icv) injection
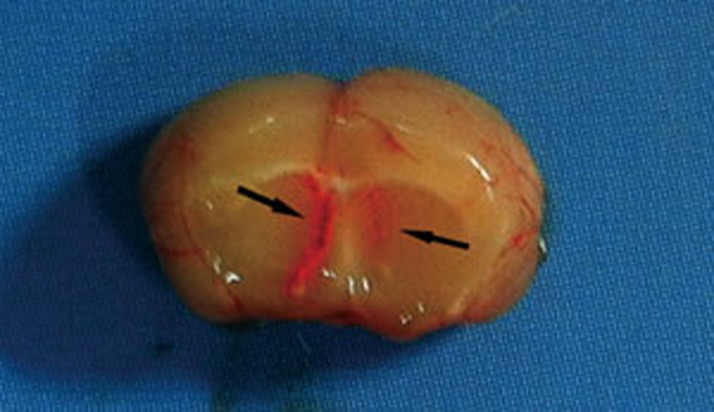
Icv injection of 3-MA or Clik148 was performed as previously described14. Briefly, mice were anesthetized with methoxyflurane and a small incision was made on the skull exposing the bregma. The icv injection was made in a volume of 5 μL through a puncture point 2 mm lateral to bregma using a 10 μL Hamilton syringe with a truncated 27 gauge needle that penetrated the brain 3 mm from the top of the skull. To confirm that the drugs were delivered into the cerebral ventricle, some mice were injected with 5 μL of diluted red ink, and their brains were examined macroscopically after sectioning (Figure 2). This evaluation of the injection technique revealed that 100% of the examined injections were correctly placed. Mice were randomly divided into 2 groups (n=10 in each group) that received saline or DMSO (control) and 3-MA or Clik148. The effects of 3-MA or Clik148 on pain thresholds were measured with acetic acid-writhing test 60 min after 3-MA or Clik148 or saline or DMSO administration.
Figure 2.
Cerebroventricular injection sites. To confirm that the drugs were delivered into the cerebral ventricle, some mice were injected with 5 μL of diluted red ink. The arrow indicates the presence of red in the ventricle; injections were correctly placed in all examined brains.
Statistical analysis
The data are expressed as mean±SD. Statistical significance was determined by one-way analysis of variance (ANOVA).
Results
Analgesic actions of systemically administered 3-MA and Clik148
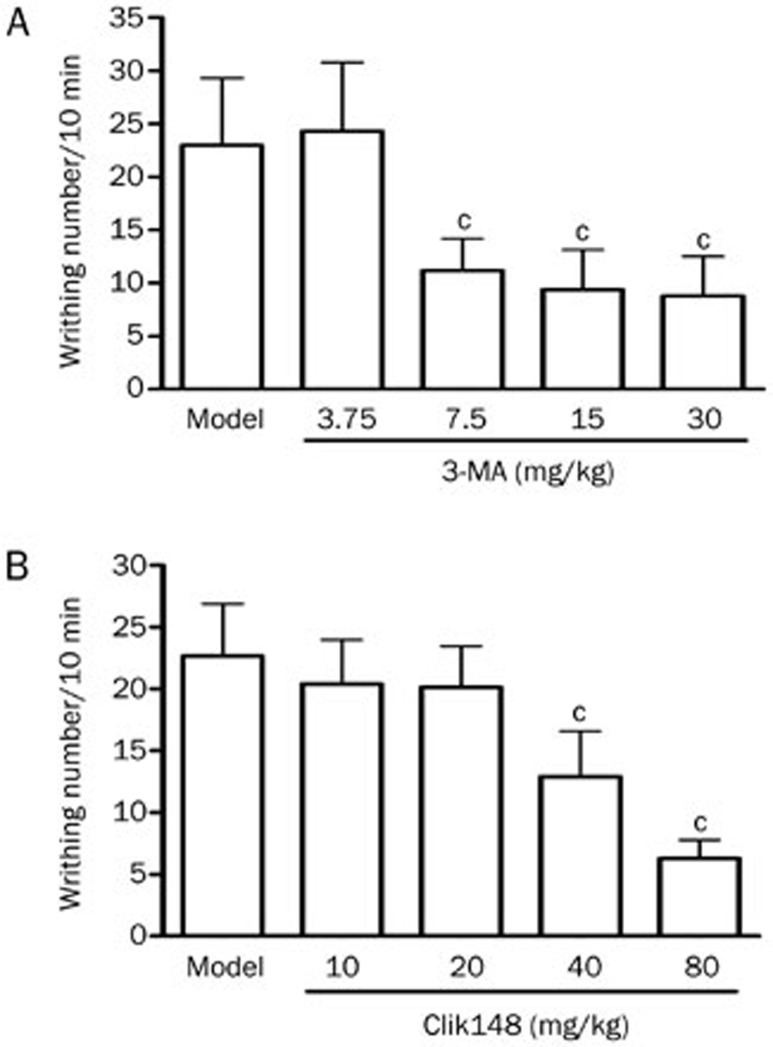
3-MA at 3.75, 7.5, 15, and 30 mg/kg (ip) (Figure 3A) and Clik148 at 10, 20, 40, and 80 mg/kg (ip) (Figure 3B) induced dose-dependent inhibitions of the writhing response in the acetic acid-writhing test, suggesting that 3-MA and Clik148 have analgesic effects and that autophagy or cathepsin L may be involved in the regulation of pain.
Figure 3.
Dose-dependent analgesic effects of ip-administered 3-MA and Clik148 in mice. (A) Analgesic effects of 3-MA in the mouse acetic acid writhing test. Nociception was measured as the number of writhings induced by 1% acetic acid 10–20 min after ip administration. 3-MA at 3.75, 7.5, 15, and 30 mg/kg or saline was administered ip 60 min before the test. (B) Analgesic effects of Clik148 in the mouse acetic acid writhing test. Clik148 at 10, 20, 40, and 80 mg/kg or 2% DMSO was administered ip 60 min before the test. Values represent the mean±SD (n=10 in all groups). Statistical analysis was carried out with ANOVA followed by Dunnett's t-test. cP<0.01 vs saline or 2% DMSO control group.
Analgesic actions of 3-MA or Clik148 administered centrally
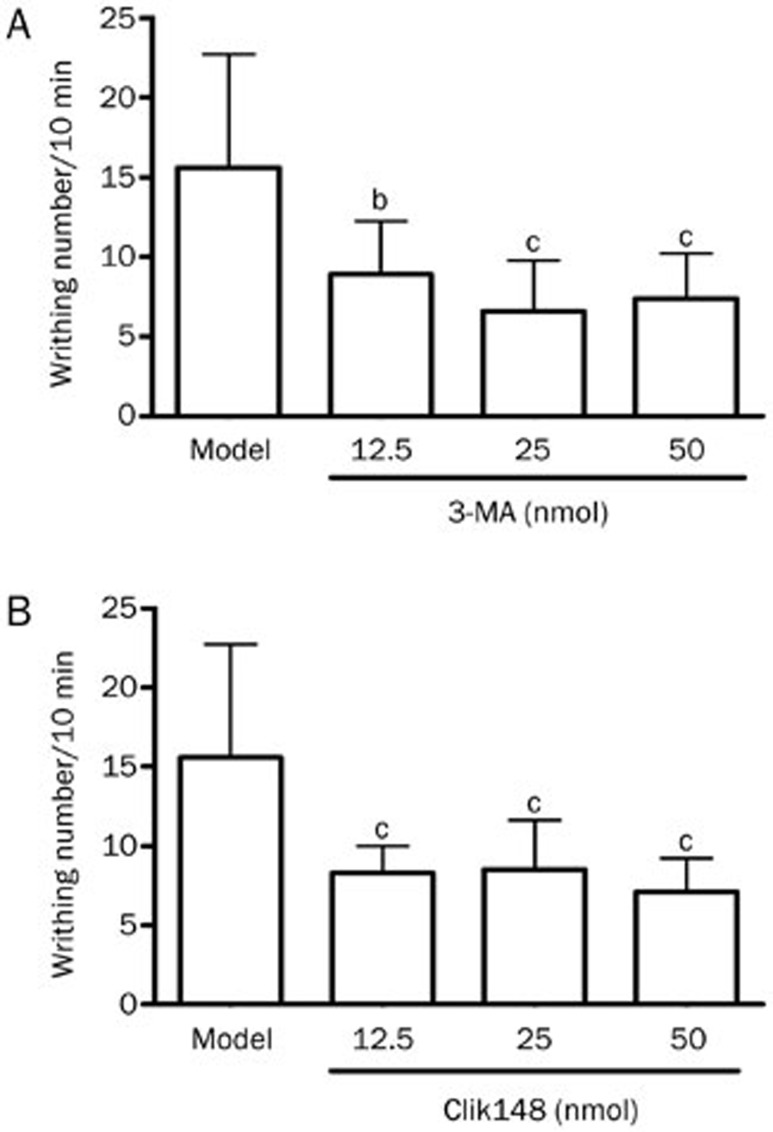
In mice, icv administration of 3-MA (12.5, 25, and 50 nmol) (Figure 4A) and Clik148 (12.5, 25, and 50 nmol) (Figure 4B) significantly reduced the writhing responses induced by acetic acid, indicating that 3-MA and Clik148 have central antinociceptive effects.
Figure 4.
Analgesic actions of centrally administered 3-MA and Clik148. (A) Analgesic effect of icv injection of 3-MA in the mouse acetic acid writhing test. Pain thresholds were measured as the number of writhings induced by 1% acetic acid between 10 and 20 min after administration. 3-MA at 12.5, 25, and 50 nmol or saline was administered icv 60 min before the test. (B) Analgesic effects of Clik148 in the mouse acetic acid writhing test. Clik148 at 12.5, 25, and 50 nmol or 2.57% DMSO were administered icv, and nociception was determined 60 min after drug administration. Values represent the mean±SD (n=10 in all groups). Statistical analysis was carried out with ANOVA followed by Dunnett's t-test. bP<0.05, cP<0.01 vs saline or 2.57% DMSO control group.
Effects of DMBC on pain responses
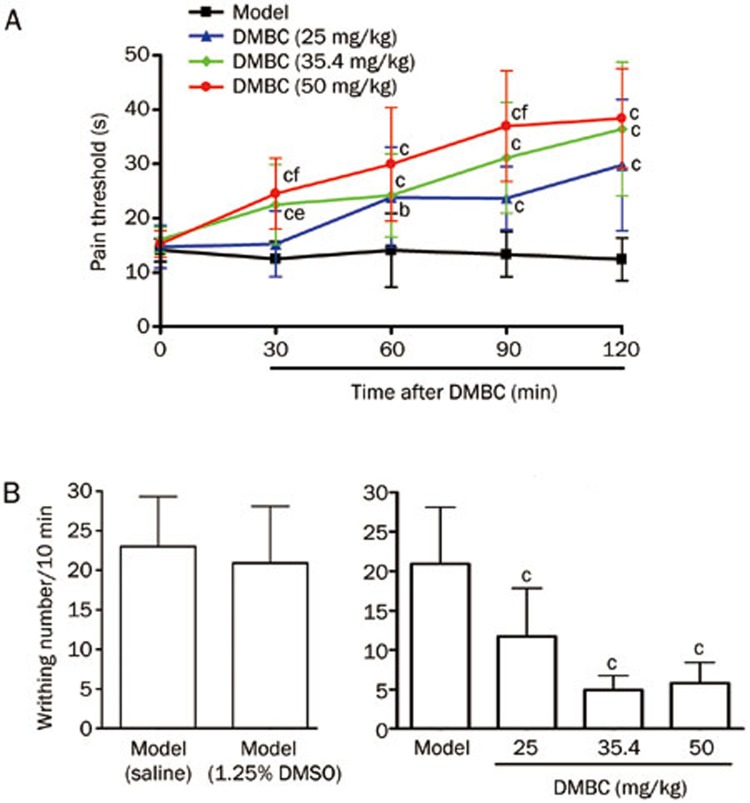
Intraperitoneal administration of 1.25% DMSO did not produce antinociceptive effects (Figure 5B). However, ip administration of DMBC (25, 35.4, and 50 mg/kg) produced significant dose-dependent antinociceptive effects during the mouse hotplate test; the analgesic effects appeared at 30 min, peaked at 90 min and lasted for 120 min (Figure 5A). Similarly, ip administration of DMBC (25, 35.4, and 50 mg/kg) produced a marked, dose-dependent antinociceptive effect in the mouse writhing test (Figure 5B).
Figure 5.
Analgesic effects of 2-(3′,5′-dimethoxybenzylidene) cyclopentanone (DMBC) on pain response. (A) Analgesic effects of DMBC in the mouse hotplate test. Pain thresholds were determined as the latency to licking the hind paws. The basal threshold (0 min) was obtained by averaging two measures made before drug administration. DMBC at 25, 35.4, and 50 mg/kg or 1.25% DMSO was administered ip, and pain thresholds were determined at 30, 60, 90, and 120 min post injection. (B) Analgesic effects of DMBC in the mouse acetic acid writhing test. Nociception was measured as the number of writhings induced by 1% acetic acid between 10 and 20 min after administration. DMBC at 25, 35.4, and 50 mg/kg or 1.25% DMSO was administered ip 60 min before the test. Values represent the mean±SD (n=10 in all groups). Statistical analysis was carried out with ANOVA followed by Dunnett's t-test. bP<0.05, cP<0.01 vs 1.25% DMSO control group. eP<0.05, fP<0.01 vs the 25 mg/kg group.
Effects of 3-MA on the analgesic actions of DMBC
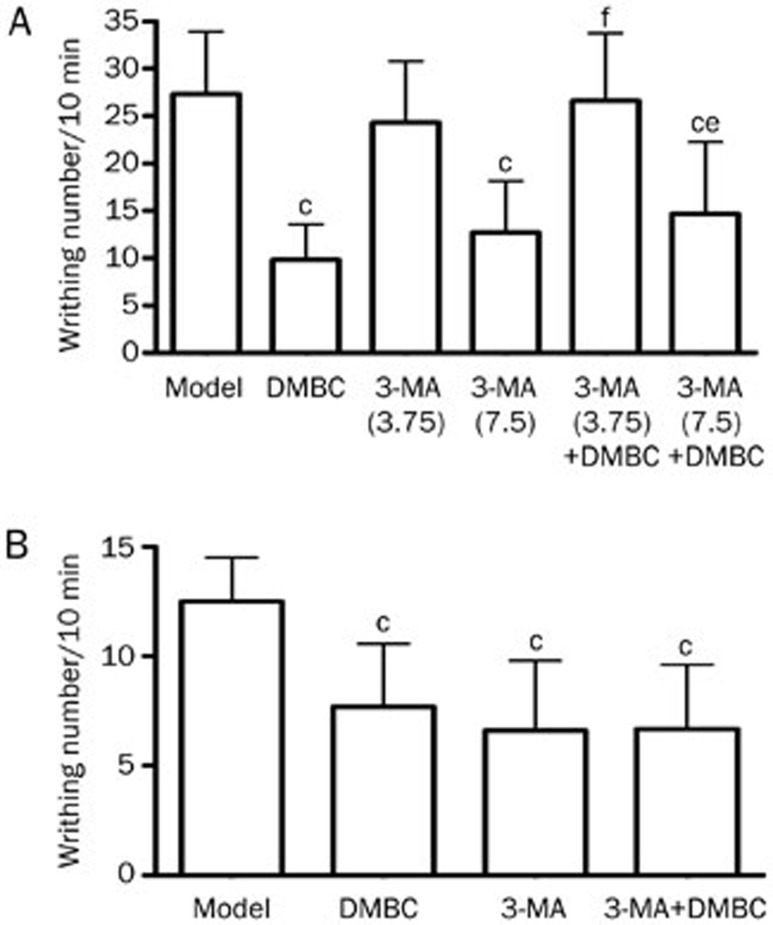
We next observed the effects of systemically administered 3-MA on the analgesic actions of DMBC. In the mouse acetic acid writhing test, we used low doses of 3-MA; the dose of 3.75 mg/kg showed no analgesic effects. However, 7.5 mg/kg showed some analgesic effects. We found that 50 mg/kg DMBC alone significantly inhibited writhing responses, 3.75 mg/kg (ip) 3-MA abolished the DMBC-induced analgesia, and 7.5 mg/kg (ip) 3-MA attenuated the DMBC-induced analgesia (Figure 6A); these results suggest that low-dose 3-MA can antagonize the antinociceptive actions of DMBC. Furthermore, we investigated the effects of centrally administered 3-MA on the analgesic actions of DMBC. 3-MA (25 nmol, icv) had no obvious effects on DMBC-induced analgesia (Figure 6B). These results indicate that DMBC might have peripheral antinociceptive actions and that peripheral autophagic mechanisms contribute, at least partially, to DMBC-induced analgesia.
Figure 6.
Effects of 3-MA on the analgesic actions of DMBC in the mouse acetic acid writhing test. (A) Effects of peripheral administration of 3-MA on the analgesic actions of DMBC. 3-MA (3.75 and 7.5 mg/kg) and/or DMBC (50 mg/kg) was administered ip 1 h prior to the acetic acid test. Nociception was measured as the number of writhings induced by 1% acetic acid between 10 and 20 min after administration. (B) Effects of central administration of 3-MA on the analgesic actions of DMBC. 3-MA (25 nmol, icv) was administered 30 min before DMBC ip (50 mg/kg), and the acetic acid test was performed 1 h after DMBC administration. Values represent the mean±SD (n=10 in all groups). Statistical analysis was carried out with ANOVA followed by Dunnett's t-test. cP<0.01 vs control group. eP<0.05, fP<0.01 vs DMBC group.
Effects of Clik148 on the analgesic actions of DMBC
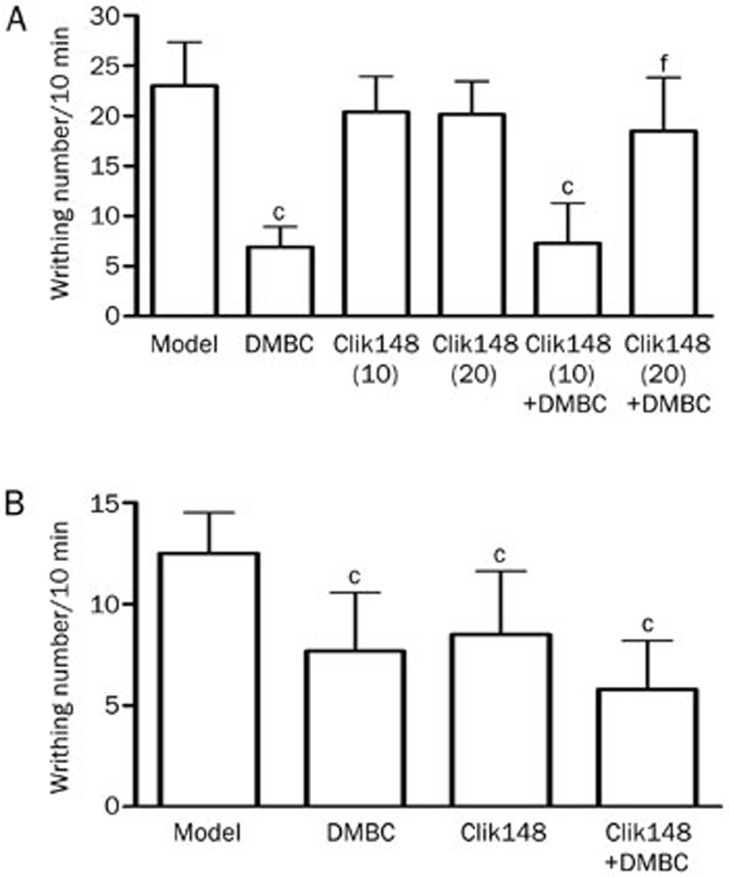
We next observed the effects of systemically administered Clik148 on the analgesic actions of DMBC. In the mouse acetic acid writhing test, we used low doses of Clik148 (10 and 20 mg/kg) that had little or no analgesic effects. We found that 50 mg/kg DMBC alone significantly inhibited writhing responses, and Clik148 at 10 mg/kg (ip) had no obvious effects on DMBC-induced analgesia. Clik148 at 20 mg/kg (ip) abolished DMSO-induced analgesia (Figure 7A), suggesting that Clik148 can antagonize the antinociceptive actions of DMBC. Furthermore, we investigated the effects of centrally administered Clik148 on the analgesic actions of DMBC. Clik148 (25 nmol, icv) had no obvious effects on DMBC-induced analgesia (Figure 7B). These results indicate that DMBC might have peripheral antinociceptive properties and that peripheral cathepsin L mechanisms contribute, at least in part, to the analgesic effects of DMBC.
Figure 7.
Effects of Clik148 on the analgesic actions of DMBC in the mouse acetic acid writhing test. (A) Effects of peripheral administration of Clik148 on the analgesic actions of DMBC. Clik148 (10 and 20 mg/kg) or DMBC (50 mg/kg) was administered ip 1 h prior to the acetic acid test. Nociception was measured as the number of writhings induced by 1% acetic acid between 10 and 20 min after administration. (B) Effects of central administration of Clik148 on the analgesic actions of DMBC. Clik148 (25 nmol, icv) was administered 30 min before DMBC (50 mg/kg, ip), and the acetic acid test was performed 1 h after DMBC administration. Values represent the mean±SD (n=10 in all groups). Statistical analysis was carried out with ANOVA followed by Dunnett's t-test. cP<0.01 vs control group. eP<0.05, fP<0.01 vs DMBC group.
Discussion
In the current study, acetic acid writhing and hotplate tests were used to demonstrate the following: (1) 3-methyladenine (3-MA), an inhibitor of autophagy, and Clik148, a specific selective inhibitor of cathepsin L, produced strong analgesic effects when administered systemically and centrally, suggesting that the central autophagic and cathepsin L mechanisms are at least partially involved in the regulation of pain; and (2) 2-(3′,5′-dimethoxybenzylidene) cyclopentanone (DMBC), a novel synthetic compound, had analgesic effects. Furthermore, 3-MA administered systemically at lower dosage and Clik148 administered systemically at higher dosage antagonized the antinociceptive effect of DMBC. In contrast, when centrally administered, neither 3-MA nor Clik148 had any obvious effect on DMBC-mediated analgesia, suggesting that DMBC might have peripheral antinociceptive actions and that inhibition of the peripheral autophagic and cathepsin L mechanisms contributes, at least in part, to the DMBC-induced analgesia. Therefore, our data indicate that both the central and the peripheral autophagic and cathepsin L mechanisms might be involved in the regulation of pain. Interestingly, in the current study, we found that 3-MA at 3.75 mg/kg (ip; which produced no analgesic effects on its own) abolished DMSO-induced analgesia, Clik148 at 10 mg/kg (which showed no analgesic effect on its own) had no obvious effects on DMBC-induced analgesia, and Clik148 at 20 mg/kg (ip) abolished DMSO-induced analgesia. The difference in the minimum doses of 3-MA and Clik148 that antagonized DMSO-induced analgesia may be the result of different antagonistic mechanisms of action.
Recent studies have demonstrated an impairment of spinal autophagy in a model of neuropathic pain11 and that the activation of autophagy is critical for the improvement of locomotor performance via suppressing the formation of peripheral myelin protein 22 (PMP22) aggregates in a misexpression and cytosolic retention of PMP22 within Schwann cells (SCs)-associated demyelinating neuropathies12. In addition, Cathepsin L has recently been shown to significantly participate in proneuropeptide processing; it is a protease that is responsible for the production of many neuropeptides6,7, including β-endorphin, enkephalin and dynorphin opioid neuropeptides3,4,5. The peptides of the endogenous opioid family are best known for their effects on pain modulation8, which suggests that cathepsin L, as a neuropeptide processing enzyme, participates in the production and regulation of pain. However, our present data differ from the above observations and show that the inhibition of central autophagic mechanisms or cathepsin L mechanisms produces analgesic effects. This discrepancy might be the result of the different pain models used. In addition, we found that inhibition of the peripheral autophagic and cathepsin L contributes, at least in part, to the analgesic effects of DMBC, suggesting that peripheral autophagic and cathepsin L mechanisms are also involved in pain regulation. However, at the doses required to produce analgesia in this study, we do not know whether DMBC crossed the blood brain barrier into the CNS following systemic administration. Therefore, we cannot rule out the possibility that DMBC exerted its antinociceptive effects via the central inhibition of autophagic and cathepsin L mechanisms. DMBC is a novel synthetic compound synthesized by our institute. The anti-inflammatory compounds α- and β-unsaturated ketone and polyoxygenated phenyl may contribute to the analgesics effects of DMBC (Figure 1).
A growing body of evidence indicates that autophagic-lysosomal mechanisms are a double-edged sword in the regulation of cell death and survival in diseases such as heart disease, cancer, a number of neurodegenerative disorders and cerebral ischemic disease that depends on the balance between the amount of intracellular substrate targeted for autophagy and the capacity of the cellular autophagic machinery15,16,17,18. Our present data and that of other groups indicate that autophagic-lysosomal mechanisms might also act as a double-edged sword that mediates pain or induces analgesia depending on the type of pain stimuli and the duration and severity of pain. Further investigations to clarify these issues regarding autophagic-lysosomal mechanisms in pain regulation are warranted.
Author contribution
Yong-ming ZHU, Shi-chang SUN, Qiang ZHOU, and Jia-hong FAN conducted the experiments and collected the data; Wei-wei GU wrote the first draft of the manuscript and analyzed data; Gui-zhen AO provided 2-(3′,5′-dimethoxybenzylidene) cyclopentanone (DMBC) and wrote part of the first draft of the manuscript; Katunuma NOBUHIKO and Kazumi ISHIDOH provided Clik148; Xiu-mei GAO participated in the animal experiments and revised the manuscript; and Hui-ling ZHANG conceived and designed the study, revised the manuscript and provided funding and technical support.
Acknowledgments
This work was supported by grants from the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, China and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
References
- Turk B, Turk V, Turk D. Structural and functional aspects of papain-like cysteine proteinases and their protein inhibitors. Biol Chem. 1997;378:141–50. [PubMed] [Google Scholar]
- Kirschke H, Langner J, Reimann S, Weideranders B, Ansorge S, Bohley P. Lysosomal cystein propteinases. In: protein degradation in health and disease, Ciba Foundation Symposium (Evered D, Whelan J, eds) Amsterdam: Excerpta Medica. 1980. pp. p5–35.
- Yasothornsrikul S, Greenbaum D, Medzihrazky KF, Toneff T, Bundey R, Miller R, et al. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci U S A. 2003;100:9590–5. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SR, Garza C, Mosier C, Toneff T, Wunderlich E, Goldsmith P, et al. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretion. J Biol Chem. 2007;282:9556–63. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- Funkelstein L, Toneff T, Hwang SR, Beuschlein F, Lichetnauer UD, Reinheckel T, et al. Major role of cathepsin L for producing the peptide hormones ACTH, beta-endorphin, and alpha-MSH, illustrated by gene knockout and expression. J Biol Chem. 2008;283:35652–9. doi: 10.1074/jbc.M709010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkelstein L, Toneff T, Hwang SR, Reinheckel T, Peters C, Hook V. Cathepsin L participates in the production of neuropeptide Y in secretory vesicles, demonstrated by protease gene knockout and expression. J Neurochem. 2008;106:384–9. doi: 10.1111/j.1471-4159.2008.05408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinfeld MC, Funkelstein L, Foulon T, Cadel S, Kitagawa K, Toneff T, et al. Cathepsin L plays a major role in cholecystokinin production in mouse brain cortex and in pituitary AtT-20 cells: protease gene knockout and inhibitor studies. Peptide. 2009;30:1882–91. doi: 10.1016/j.peptides.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Owens C, Gutstein H, Taylor L, Currran E, Watson S. Endogenous opioids: overview and current issues. Drug Alcohol Depend. 1998;51:127–40. doi: 10.1016/s0376-8716(98)00071-4. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliocchi L, Russo R, Maiarù M, Levato A, Bagetta G, Corasaniti MT. Autophagy impairment in a mouse model of neuropathic pain. Pain. 2011;7:83. doi: 10.1186/1744-8069-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaraju S, Verrier JD, Madorsky I, Nicks J, Dunn WA, Jr, Notterpek L. Rapamycin activates autophagy and improves myelination in explant cultures from neuropathic mice. J Neurosci. 2010;30:11388–97. doi: 10.1523/JNEUROSCI.1356-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HL, Han R, Chen ZX, Chen BW, Gu ZL, Laurence N, et al. Opiate and acetylcholine-independent analgesic actions of crotoxin isolated from crotalus durissus terrificus venom. Toxicon. 2006;48:175–82. doi: 10.1016/j.toxicon.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Pedigo NW, Dewey WL, Harris LS. Determination and characterization of the antinociceptive activity of intraventricularly administered acetylcholine in mice. J Pharmacol. 1975;193:845–52. [PubMed] [Google Scholar]
- White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–16. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet W, Agostinis P, Vanhoecke B, Dewaele M, De Meyer GR. Autophagy in disease: a double-edged sword with therapeutic potential. Clin Sci (Lond) 2009;116:697–712. doi: 10.1042/CS20080508. [DOI] [PubMed] [Google Scholar]
- Wei K, Wang P, Miao CY. A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury. CNS Neurosci Ther. 2012;18:879–86. doi: 10.1111/cns.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung YT, Wang BJ, Hu MK, Hsu WM, Lee H, Huang WP, et al. Autophagy: a double-edged sword in Alzheimer's disease. J Biosci. 2012;37:157–65. doi: 10.1007/s12038-011-9176-0. [DOI] [PubMed] [Google Scholar]