Abstract
Aim:
To investigate the embryotoxicity of dihydroartemisinin (DHA), the main active metabolite of artemisinin, in zebrafish, and explore the corresponding mechanisms.
Methods:
The embryos of wild type and TG (flk1:GFP) transgenic zebrafish were exposed to DHA. Developmental phenotypes of the embryos were observed. Development of blood vessels was directly observed in living embryos of TG (flk1:GFP) transgenic zebrafish under fluorescence microscope. The expression of angiogenesis marker genes vegfa, flk1, and flt1 in the embryos was detected using real-time PCR and RNA in situ hybridization assays.
Results:
Exposure to DHA (1–10 mg/L) dose-dependently caused abnormal zebrafish embryonic phenotypes in the early developmental stage. Furthermore, exposure to DHA (10 mg/L) resulted in more pronounced embryonic angiogenesis in TG (flk1:GFP) zebrafish line. Exposure to DHA (10 mg/L) significantly increased the mRNA expression of vegfa, flk1, and flt1 in the embryos. Knockdown of the flk1 protein partially blocked the effects of DHA on embryogenesis.
Conclusion:
DHA causes abnormal embryonic phenotypes and promotes angiogenesis in zebrafish early embryonic development, demonstrating the potential embryotoxicity of DHA.
Keywords: artemisinin, dihydroartemisinin, zebrafish, flk1, VEGF, angiogenesis, embryotoxicity
Introduction
Artemisinin, a sesquiterpene lactone derived from the sweet wormwood plant Artemisia annua, is a new antimalarial compounds discovered by Chinese scientists. Artemisinin and its bioactive derivatives are used to effectively treat different forms of malarial parasites1,2. As the main active metabolite of artemisinin, dihydroartemisinin (DHA) has more effective anti-malarial effects than artemisinin. In addition, artemisinin and its derivatives exhibit potent anti-cancer effects in a variety of human cancer cell model systems3,4,5,6 in which the apoptotic response, cell cycle arrest and nuclear receptor response are involved. The anti-cancer activity of artesunate, a semi-synthetic derivative of the sesquiterpene artemisinin, has been verified in mice bearing human xenograft tumors and patients with uveal melanoma and non-small cell lung cancer7,8,9.
However, treatment with artemisinin analogs has been reported to be associated with different types of toxicity, such as neurotoxicity, embryotoxicity and genotoxicity, in cellular and animal models10,11. Among the side effects arising from the use of artemisinin-related compounds, the danger of embryotoxicity appears to be more serious. The typical embryotoxic effects of artemisinin that have been observed in mice, rats, rabbits and monkeys include embryonic death, developmental retardation, skeletal defects and cardiovascular malfunction12,13,14,15,16. In view of these side effects, artemisinin and its derivatives are not recommended for use in the first trimester of pregnancy by the World Health Organization (WHO). Thus, there is an urgent need for artemisinin safety control for patients, particularly pregnant women.
One mechanism of embryotoxicity appears to be related to vasculogenesis and angiogenesis. Artemisinin was shown to inhibit angiogenesis and decrease hypoxia-induced factor 1α (HIF1α) and vascular endothelial growth factor (VEGF) expression in mouse embryonic stem cell-derived embryoid bodies12. DHA caused a reduction of primitive red blood cells in Xenopus larvae and rats13,17. Moreover, artemether, another artemisinin derivative, could induce cardiovascular malformation and embryonic erythroblast defects in rats, rabbits and monkeys14,15,16. However, the detailed mechanisms of artemisinin action require further study. In addition, although several animal models have been used to study the embryotoxicity of artemisinin, fewer studies have reported using zebrafish, particularly for the effect of artemisinin on vasculogenesis and angiogenesis, although zebrafish is a useful model system for the study of vasculogenesis and angiogenesis in embryonic development18.
Thus, we used zebrafish as a model to demonstrate that DHA, the main artemisinin derivative, causes zebrafish embryotoxicity, including pericardial edema and a larger yolk sac, and induces vasculogenesis/angiogenesis and the overexpression of vegfa. Our results not only confirm toxicity phenotypes such as malformation, embryonic death and heart defects in zebrafish, but they also innovatively demonstrate the pro-angiogenic effects of DHA on vessel formation.
Materials and methods
Zebrafish maintenance
Zebrafish maintenance, breeding and staging were performed by standard methods19. The TG (flk1:GFP) transgenic zebrafish line was a kind gift from Dr Ting-xi LIU, Institute of Health Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. The zebrafish facility and zebrafish study were approved by the institutional review board (IRB) of the Institute of Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Protocol Approval Number: 2011-AN-1).
Collection and treatment of zebrafish embryos
Embryos were collected and staged following standard methods19. The stock solutions for the different concentration of each compound were prepared in DMSO. The treatment solutions were diluted 1000 times from the stock solutions with system water. All stock and treatment solutions were freshly made before each use. For exposure, 10 hpf (hours post fertilization) embryos were placed in 6-cm Petri dishes containing 10 mL of treatment solution. Untreated embryos and embryos treated with 0.1% DMSO were used as control groups. Exposures were performed at 28.5 °C for 38 h–6 d. The control/treatment solutions were changed daily. Three replicates of 60 embryos were used for each concentration group. The developmental phenotypes of zebrafish embryos were directly observed in the dish using an Olympus SZX9 system (Tokyo, Japan) every 12 h. Such phenotypes, including mortality, spontaneous movement, hatching success, heartbeat, pericardial edema and curved body axis, were selected and examined to determine the embryotoxicity effects of the compounds. Artemisinin and its derivative DHA were kind gifts from Zhejiang Yiwu Golden Fine Chemical Co Ltd.
Real-time PCR
Total RNA was extracted from 20 zebrafish embryos using TRIzol reagent (Invitrogen, Shanghai, China). RNA was reverse transcribed using the PrimeScript RT reagent kit (TaKaRa, Shiga, Japan). Real-time quantitative PCR for all genes was performed with the fluorescent dye SYBR Green. Amplification reactions were performed in a total volume of 20 μL with Real-time PCR Master Mix (TOYOBO, Osaka, Japan) and 10 pmol of each primer. PCR was performed for 40 cycles consisting of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 30 s using the CFX96™ real-time system (Bio-Rad, Hercules, CA, USA). The gene-specific primers are shown in Table 1. The results were normalized to β-actin, which served as a loading control.
Table 1. Primers for real-time PCR.
| Genes | Sense (5′–3′) | Antisense (5′–3′) |
|---|---|---|
| β-Actin | ATGCCCCTCGTGCTGTTTTC | GCCTCATCTCCCACATAGGA |
| vegf | TCTCCTCCATCTGTCTGC | CAGGAGCATTTACAGGTG |
| flt1 | AGGCTTTTGCAGGACAGAAA | TGTGATGGTGAGGTTCTGGA |
| flk1 | GATGGAGATACACACCTTCAG | TGCGTACCGATGACACATTTC |
RNA in situ hybridization
For whole-mount RNA in situ hybridization (WISH), 48 hpf embryos were collected and fixed in 4% paraformaldehyde overnight at 4 °C. cDNA sequences of specific genes were PCR-amplified and cloned into the pGEM-T Easy vector (Promega, Madison, WI, USA) as templates to generate an antisense riboprobe for in situ hybridization (Roche, Shanghai, China). WISH was performed using the standard methods20, and the primers are shown in Table 2.
Table 2. Primers for real-time PCR.
| Genes | Sense (5′–3′) | Antisense (5′–3′) |
|---|---|---|
| vegf | TGCCCACATACCCAAAGAAG | GCAAGGCTCACAGTGGTTTTC |
| flt1 | GCCGTTGATGGTCATTGTTG | TCTCCGTTCCGCACATAGTCT |
| flk1 | GAGATGGAGGAGGAACTGGTG | GATAGCCGCTGGTCTGATTG |
Knockdown of flk1 and flt1 expression with antisense morpholino oligonucleotides
Antisense morpholino oligonucleotides (MO) for flk1 and flt1 were obtained from Gene Tools, LLC (Philomath, OR, USA). The sequences used are as follows: 5′-CCG AAT GAT ACT CCG TAT GTC AC-3′ for flk1, 5′-CAG CAG TTC ACT CAC ATC TCC GTT C-3′ for flt121, and 5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′ for the control. For microinjection, a 1-nl volume was injected into 1- to 2-cell stage embryos.
Statistical analysis
Experimental data were expressed as the mean±SD unless indicated, and the significance of differences was analyzed using Student's t-test, as appropriate.
Results
DHA induces morphological abnormalities during the early embryonic developmental stages of zebrafish
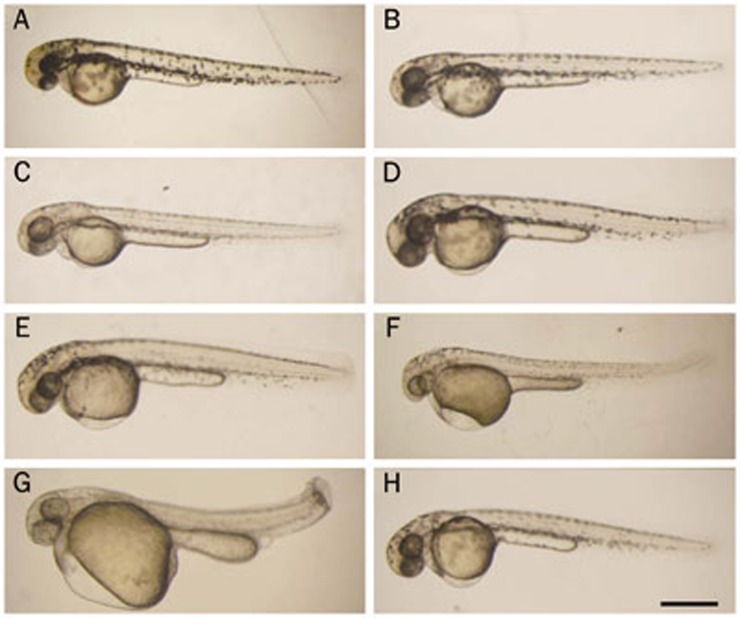
To investigate the overall effects of DHA and artemisinin on zebrafish early embryonic development, embryos were exposed to different concentrations of drugs beginning at 10 hpf. Morphologically, the treated embryos began to exhibit obvious abnormalities at the 48 hpf stage from 1 mg/L drug treatment, including pericardial edema, a larger yolk sac, shorter yolk sac extension, a curving trunk, a shorter trunk axis and less melanin pigmentation, compared with untreated and DMSO-treated embryos (Figure 1A–1H). Moreover, the drug caused the dilation of pericardial edema and other abnormal phenotypes in a dose-dependent manner (Figure 1C–1G and data not shown). In addition, we found that DHA more severely induced morphological abnormalities in embryos than artemisinin (Figure 1F and 1H). Therefore, DHA was selected for subsequent experiments.
Figure 1.
Morphological changes induced by dihydroartemisinin (DHA) and artemisinin during zebrafish early embryonic development. Embryos treated with different concentrations of compounds were imaged at 48 hpf. (A) No treatment; (B) 0.1% DMSO; (C) 1.0 mg/L DHA; (D) 2.5 mg/L DHA; (E) 5.0 mg/L DHA; (F) 10 mg/L DHA; (G) 15 mg/L DHA; (H) 10 mg/L artemisinin. The scale bar represents 500 μm for all panels.
DHA exposure slows down heartbeats and causes hatching failure
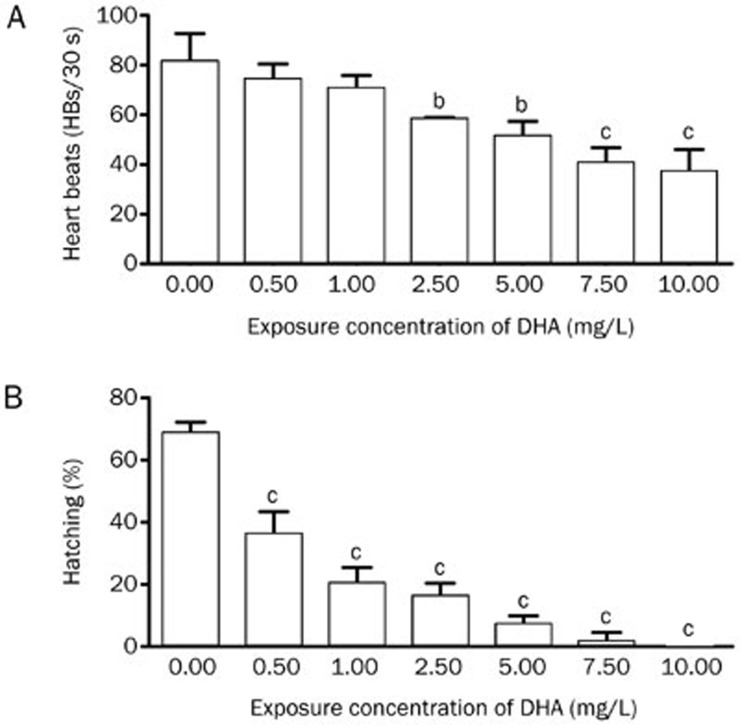
After treatment with DHA for 50 h at different concentrations, all embryos were alive, even in the highest concentration group (20 mg/L). However, the treated embryos began to die at 72 hpf from 5 mg/L treatment (when the heart in the embryo stopped beating, the embryo was considered to be dead). During this process, the heartbeat rates of embryos began to decrease in the DHA-exposed embryos, beginning at 60 hpf, compared with untreated and DMSO-treated embryos. Thus, we examined the heartbeat rates at various DHA concentrations at 60 hpf, and the results showed that DHA exposure decreased heartbeat rates in a dose-dependent manner (Figure 2A).
Figure 2.
The heartbeats and hatching success of embryos exhibit a dose-dependent decrease. (A) Embryos were treated, and the heartbeats were examined at 60 hpf. Three replicates of 60 embryos were used for each concentration group. (B) A total of 60 embryos were used per group. Shown are results representative from three independent experiments. bP<0.05, cP<0.01 compared with the control embryos. DHA, dihydroartemisinin.
Zebrafish embryo hatching in the untreated and DMSO-treated groups normally began at 48 hpf and ended at 72 hpf. However, the hatching ability of DHA-treated embryos was significantly impaired. At 60 hpf, the hatching rate began to decrease with increasing DHA concentrations ranging from 0.5 to 10 mg/L (Figure 2B). High concentrations (≥5 mg/L) of DHA significantly inhibited embryo hatching with only sporadic hatching.
DHA accelerates the vasculogenesis and angiogenesis processes
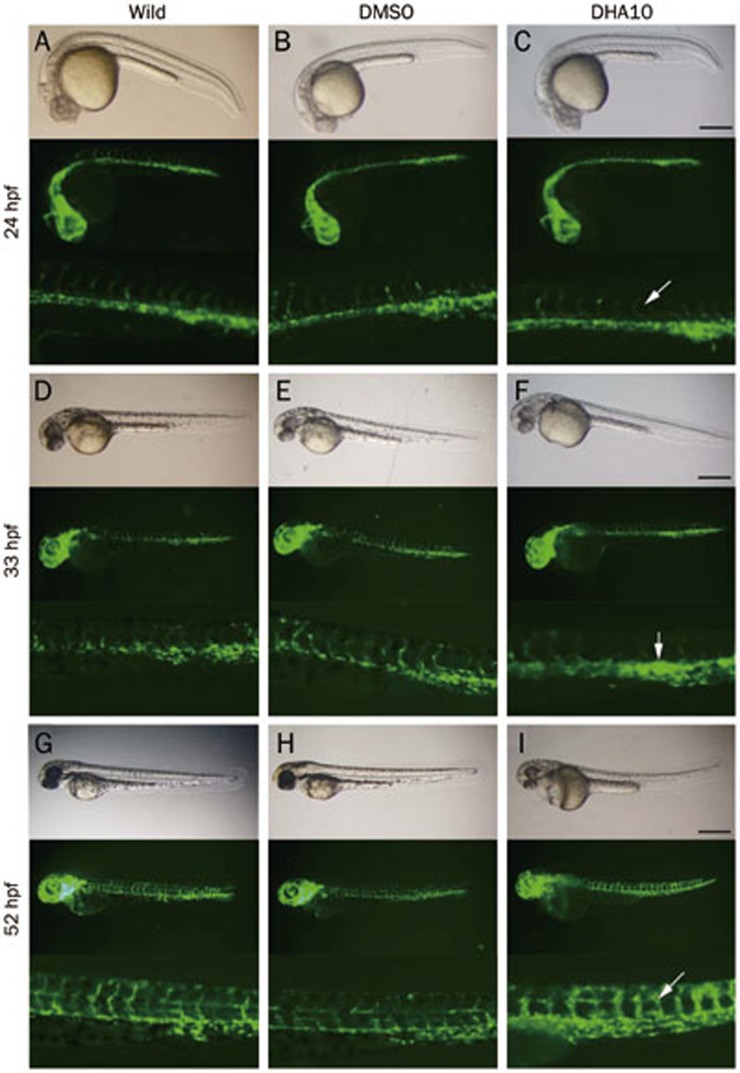
It has been reported that pericardial edema may result from a change in vascular permeability18. To investigate the effect of DHA on vasculature development, the TG (flk1:GFP) zebrafish model was used. In this model, the development of GFP-labeled blood vessels could be directly observed in live embryos under a fluorescence microscope. At 24 hpf, the characteristic “S” pattern of the intersegmental blood vessel (ISV) was present along the dorsal-ventral axis (Figure 3A–3C). Compared with untreated and DMSO-treated embryos, the curvature of the “S” pattern increased with the DHA exposure (Figure 3C), suggesting that DHA could promote the extension of the ISV. At 33 hpf, the dorsal aorta (DA) and pericardial vein (PCV) were more emanative and showed stronger GFP fluorescence intensity under DHA exposure (Figure 3D–3F), demonstrating that DHA could promote trunk intersomitic vessel formation. At 52 hpf, the effect of DHA on ISV became more profound (Figure 3G–3I). In addition, we found that abnormal vasculogenesis together with other abnormal phenotypes, such as pericardial edema and a curving trunk, occurred in a dose-dependent manner (data not shown).
Figure 3.
Embryos from the TG (flk1:GFP) zebrafish line display angiogenesis abnormalities with dihydroartemisinin (DHA) exposure. Three groups of embryos were used to compare changes including the untreated, DMSO-treated and DHA-treated (10 mg/L DHA) groups, and different developmental stages were selected to show changes, including 24 hpf (A, B, C), 33 hpf (D, E, F), and 55 hpf (G, H, I). The white arrows in panels C, F, and I indicate abnormalities in embryos compared with untreated and DMSO-treated embryos. The embryos shown in all panels are lateral views. The scale bar represents 500 μm for the first and second row in each panel and 1500 μm for the third row in each panel.
DHA exerts the embryotoxicity effect through the VEGF signaling pathway
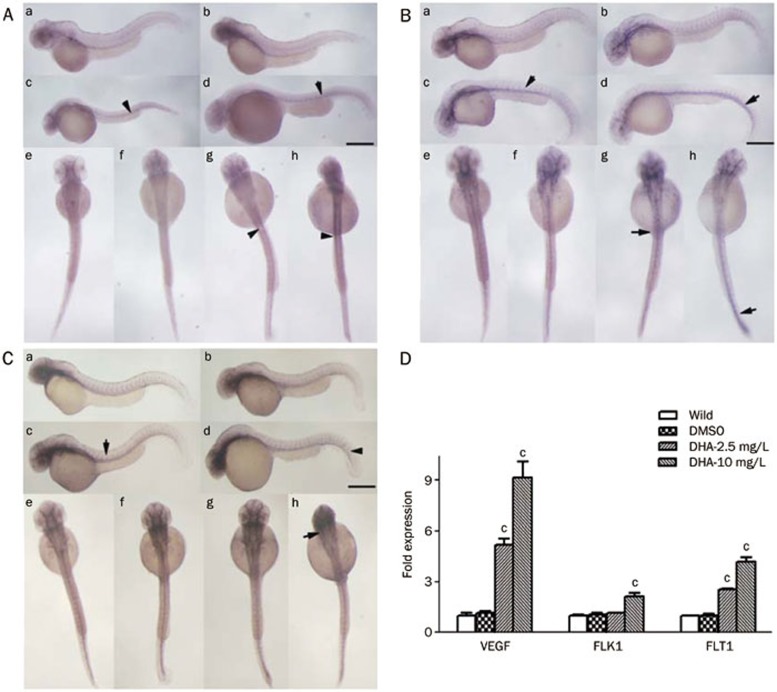
It is well known that the VEGF signaling pathway plays important roles in vasculogenesis and angiogenesis22,23. We determined the expression of VEGF family genes in zebrafish embryos treated with DHA at 48 hpf. By performing whole-mount in situ hybridization, we found that the mRNA levels of vegfa, a key factor among VEGF family genes in zebrafish, and its two receptor genes, flk1 and flt1, were upregulated compared with the control groups in a dose-dependent manner (Figure 4A–4C). The expression of vegfa was strongly enhanced in the adjacent somite areas along the vasculature (Figure 4Ac, 4Ad, 4Ag, 4Ah), while the expression of flt1 and flk1 was highly increased in the entire vasculature of the embryos compared with the control embryos (Figure 4Bc, 4Bd, 4Bg, 4Bh, 4Cc, 4Cd, 4Cg, 4Ch). The real-time PCR results consistently showed that the mRNA levels of vegfa, flk1, and flt1 were significantly upregulated in DHA-treated embryos at 48 hpf (Figure 4D). Therefore, we speculated that the VEGF pathway might play an important role in the abnormal embryonic angiogenesis induced by DHA.
Figure 4.
Dihydroartemisinin (DHA) enhances the expression of angiogenesis marker genes in zebrafish embryonic development. (A–C) The WISH results for vegfa (A), flk1 (B), and flt1 (C) expression induced by DHA during early embryogenesis at 48 hpf. The embryos in panels A (a, e), B (a, e), and C (a, e) were untreated, the embryos in panels A (b, f), B (b, f), and C (b, f) were DMSO-treated, the embryos in panels A (c, g), B (c, g), and C (c, g) were treated with 2.5 mg/L DHA, and the embryos in panels A (d, h), B (d, h), and C (d, h) were treated with 10 mg/L DHA. Panels A (a–d), B (a–d), C (a–d) are lateral views and the others are dorsal views. The scale bar represents 650 μm for panels A (a–d), B (a–d), C (a–d), and 150 μm for panels A (e–h), B (e–h), C (e–h). (D) The mRNA expression level of vegfa, flk1, and flt1 at 48 hpf was examined by real-time PCR. Data shown are the mean±SEM for at least three independent experiments. cP<0.01 compared with the control embryos.
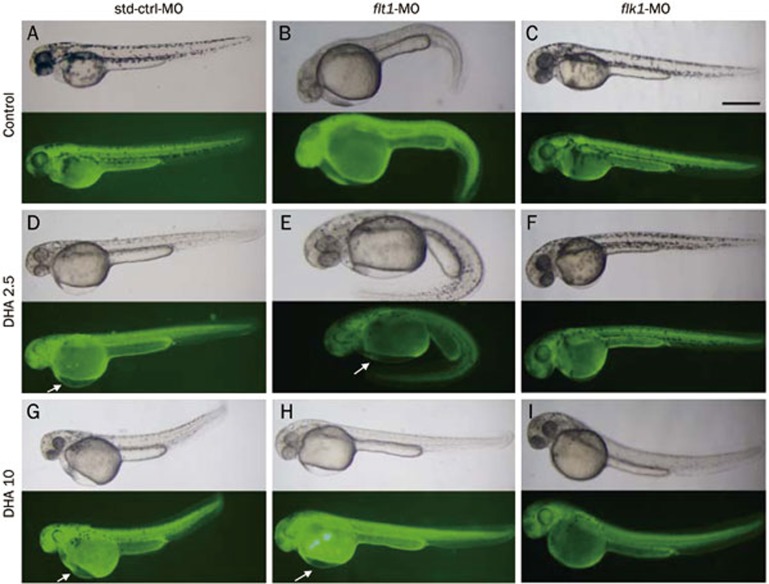
The knockdown of flk1 blocks DHA-induced pericardial edema during zebrafish embryogenesis
To further confirm the role of flt1 and flk1 in the abnormal vascular development and pericardial edema of the DHA-treated embryos, we knocked down the expression of flt1 and flk1 separately by injecting flk1-MO or flt1-MO into embryos at the 1- to 2-cell stage. Untreated embryos and embryos injected with std-ctrl-MO were used as controls. The MOs were tagged with green fluorescein at the 3′-end, and a fluorescence signal indicated the successful injection and distribution of the respective MO in the injected-embryos. At 48 hpf, we found that ectopic phenotypes eg, dilated pericardial edema, were not present in flk1-MO-injected embryos compared with the controls (Figure 5A, 5D, 5G, 5C, 5F, and 5I). However, abnormal edema still occurred in flt1-MO-injected embryos (Figure 5A, 5D, 5G, 5B, 5E, and 5H). These results indicate that flk1 might play an important role in DHA-induced pericardial edema.
Figure 5.
Antisense knockdown of flk1 in zebrafish abolishes dihydroartemisinin (DHA)-induced pericardial edema. (A–C) Embryos were treated with DMSO. (D–F) Embryos were treated with 2.5 mg/L DHA. (G–I) Embryos were treated with 10 mg/L DHA. NC-MO injected embryos are shown in panels A, D, and G. flt1-MO injected embryos are shown in panels B, E, and H. flk1-MO injected embryos are shown in panels C, F, and I. The scale bar represents 500 μm for all panels.
Discussion
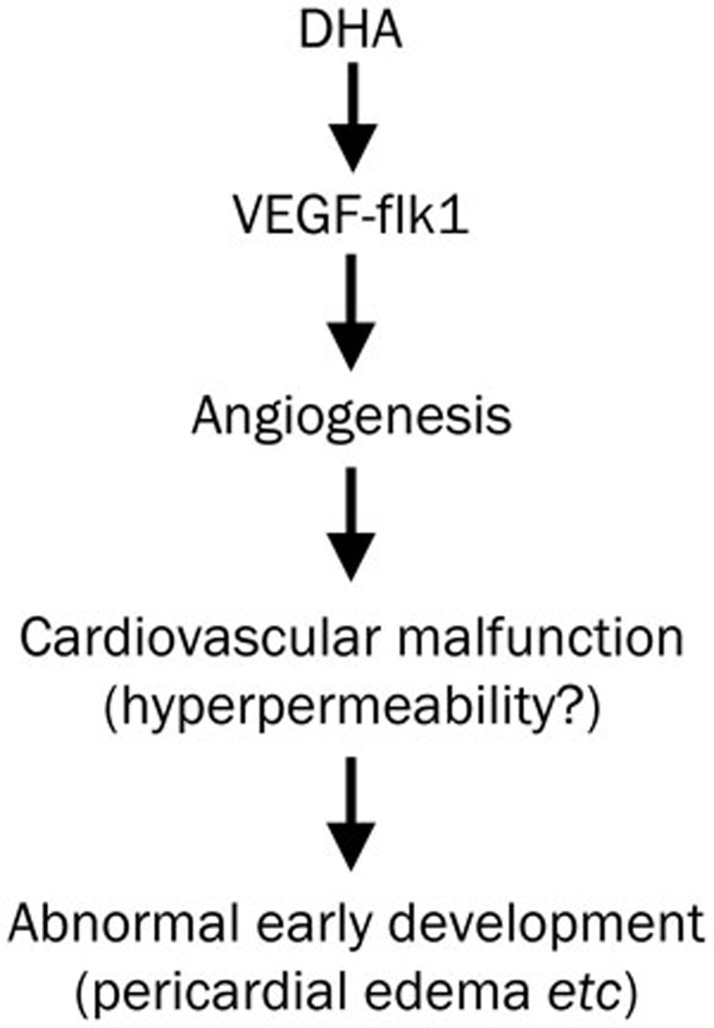
In this study, we investigated the embryotoxicity of DHA exposure during zebrafish early embryonic development and found that DHA exposure could cause abnormal phenotypes including pericardial edema, a larger yolk sac, heart rate inhibition and hatching failure. Using the TG (flk1:GFP) zebrafish line, we detected a pro-angiogenic property of DHA, and flk1 might play a key role in this process. In summary, we propose a potential mechanism in which DHA may cause embryotoxicity (Figure 6). DHA induces VEGF-flk1 overexpression, promotes angiogenesis and results in abnormal phenotypes in early development, including pericardial edema, which might be related to cardiovascular malfunctions such as hyperpermeability.
Figure 6.
The proposed mechanism by which dihydroartemisinin (DHA) exposure causes abnormal early embryonic development in zebrafish.
Previous animal studies on rats, rabbits and monkeys have shown that artemisinin and its derivatives cause angiogenesis defects, embryonic death, developmental retardation and cardiovascular malfunction12,13,14,15,16. We consistently found that DHA induces anomalies in zebrafish, including embryonic death, developmental retardation and cardiovascular malfunction. The difference is that we found that DHA promoted angiogenesis in zebrafish early development. This inconsistency may be attributed not only to different species but also to different experimental settings. In these studies, artemisinin was delivered po in the gestational period for approximately 10 d (rats and rabbits) or more than 30 d (monkeys). In the current study, we directly added the drug into the system water and cultured the zebrafish embryos continuously for up to approximately 6 d, which represents a distinct culture system and drug delivery route. In addition, in vitro studies show that the activity of DHA varies with different cell types, suggesting that the physiological environment might also confer this variability. Because the culture condition and drug delivery route are distinct between zebrafish and other lab animals, we cannot compare the concentrations of DHA among them. However, in patients, the plasma level of artemisinin derivatives varies from 0.51 to 200 μg/mL, depending on the drug delivery route, and these levels are close to the concentrations that we used10.
Previous studies have shown that the overexpression of VEGF can induce pericardial edema18,24, and our real-time PCR and in situ hybridization results also supported the notion that VEGF and its receptors play important roles in this process. In zebrafish, flk1 has an essential role in vasculogenesis, angiogenesis and hematopoiesis, which can mediate vascular dilation, endothelial cell proliferation and epidermal hyperplasia by binding VEGF25. In our studies, the knockdown of flk1 with antisense MOs blocked the pericardial edema phenotype, which confirmed that DHA exerted its effects through the VEGF-flk1 pathway.
In addition to inducing pericardial edema, VEGF plays important roles in angiogenesis. Studies with in vitro cell models, such as endothelial cells, and ex vivo models, such as the microvessel-like formation assay, indicate that DHA, along with other derivatives, can downregulate VEGF expression and inhibit angiogenesis24,26,27,28. Moreover, the cytotoxicity of DHA might play a role in its anti-angiogenic function in ex vivo models inhibiting the proliferation of vessels in the medium by killing single cells. However, we found that DHA increased vegfa expression and promoted angiogenesis. This inconsistency may be attributed to a difference between the in vitro and in vivo contexts. Considering that our studies were performed in live animals, the pro-angiogenic effect observed in zebrafish embryos may have more biological relevance.
This study demonstrated the potential toxicity of DHA on the early embryonic development of zebrafish and its corresponding mechanisms. Interestingly, we found that DHA exerted a distinct effect on angiogenesis in zebrafish in which the VEGF-flk1 pathway might be involved. Further studies to uncover the mechanism of the embryotoxicity of DHA are warranted to guide the clinical use of artemisinin and its derivatives with less toxicity and more significantly anti-malarial and anti-tumor effects.
Author contribution
Hui WANG and Rui-ai CHU designed the research and revised the manuscript; Qian BA, Juan DUAN, and Jia-qiang TIAN performed the research and wrote the manuscript; Zi-liang WANG analyzed the data; Tao CHEN, Xiao-guang LI, Pei-zhan CHEN, Song-jie WU, Li XIANG, and Jing-quan LI performed the experiments.
Acknowledgments
We thank Dr Ting-xi LIU (Institute of Health Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for the kind gift of the TG (flk1: GFP) transgenic zebrafish line. This study was supported by grants from the Ministry of Science and Technology of China (2012BAK01B00, 2011BAK10B00, and 2009CB919000), the National Natural Science Foundation (81125020, 91029715, 31070680, 31101261, 81242002, and 31200569), the Science and Technology Commission of Shanghai Municipality (12XD1407000, 12431900500, and 10391902100), Xuhui Central Hospital (CRC2011001 and CRC2011004), Director Foundation (20090101), and the Key Labortory of Food Safety Research of Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
References
- Cui L, Su XZ. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev Anti Infect Ther. 2009;7:999–1013. doi: 10.1586/eri.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–55. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- Chen T, Li M, Zhang R, Wang H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J Cell Mol Med. 2009;13:1358–70. doi: 10.1111/j.1582-4934.2008.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone GL, Sundar SN. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med. 2009;11:e32. doi: 10.1017/S1462399409001239. [DOI] [PubMed] [Google Scholar]
- Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354:28–33. doi: 10.1016/j.ijpharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Ba Q, Zhou N, Duan J, Chen T, Hao M, Yang X, et al. Dihydroartemisinin exerts Its anticancer activity through depleting cellular Iron via transferrin receptor-1. PLoS One. 2012;7:e42703. doi: 10.1371/journal.pone.0042703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Eva R, Pfeffer U, Vene R, Anfosso L, Forlani A, Albini A, et al. Inhibition of angiogenesis in vivo and growth of Kaposi's sarcoma xenograft tumors by the anti-malarial artesunate. Biochem Pharmacol. 2004;68:2359–66. doi: 10.1016/j.bcp.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Berger TG, Dieckmann D, Efferth T, Schultz ES, Funk JO, Baur A, et al. Artesunate in the treatment of metastatic uveal melanoma — first experiences. Oncol Rep. 2005;14:1599–603. [PubMed] [Google Scholar]
- Zhang ZY, Yu SQ, Miao LY, Huang XY, Zhang XP, Zhu YP, et al. Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: a randomized controlled trial Zhong Xi Yi Jie He Xue Bao 20086134–8.Chinese. [DOI] [PubMed] [Google Scholar]
- Efferth T, Kaina B. Toxicity of the antimalarial artemisinin and its dervatives. Crit Rev Toxicol. 2010;40:405–21. doi: 10.3109/10408441003610571. [DOI] [PubMed] [Google Scholar]
- Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32:1655–60. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- Wartenberg M, Wolf S, Budde P, Grunheck F, Acker H, Hescheler J, et al. The antimalaria agent artemisinin exerts antiangiogenic effects in mouse embryonic stem cell-derived embryoid bodies. Lab Invest. 2003;83:1647–55. doi: 10.1097/01.lab.0000098424.38003.ff. [DOI] [PubMed] [Google Scholar]
- Longo M, Zanoncelli S, Torre PD, Riflettuto M, Cocco F, Pesenti M, et al. In vivo and in vitro investigations of the effects of the antimalarial drug dihydroartemisinin (DHA) on rat embryos. Reprod Toxicol. 2006;22:797–810. doi: 10.1016/j.reprotox.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Clark RL. Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester. Reprod Toxicol. 2009;28:285–96. doi: 10.1016/j.reprotox.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Clark RL, Arima A, Makori N, Nakata Y, Bernard F, Gristwood W, et al. Artesunate: developmental toxicity and toxicokinetics in monkeys. Birth Defects Res B Dev Reprod Toxicol. 2008;83:418–34. doi: 10.1002/bdrb.20163. [DOI] [PubMed] [Google Scholar]
- Clark RL, White TE, S AC, Gaunt I, Winstanley P, Ward SA. Developmental toxicity of artesunate and an artesunate combination in the rat and rabbit. Birth Defects Res B Dev Reprod Toxicol. 2004;71:380–94. doi: 10.1002/bdrb.20027. [DOI] [PubMed] [Google Scholar]
- Longo M, Zanoncelli S, Della Torre P, Rosa F, Giusti A, Colombo P, et al. Investigations of the effects of the antimalarial drug dihydroartemisinin (DHA) using the Frog Embryo Teratogenesis Assay-Xenopus (FETAX) Reprod Toxicol. 2008;25:433–41. doi: 10.1016/j.reprotox.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Liang D, Chang JR, Chin AJ, Smith A, Kelly C, Weinberg ES, et al. The role of vascular endothelial growth factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in zebrafish development. Mech Dev. 2001;108:29–43. doi: 10.1016/s0925-4773(01)00468-3. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, et al. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98:643–51. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- Rottbauer W, Just S, Wessels G, Trano N, Most P, Katus HA, et al. VEGF-PLCgamma1 pathway controls cardiac contractility in the embryonic heart. Genes Dev. 2005;19:1624–34. doi: 10.1101/gad.1319405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Keyt B. Vascular endothelial growth factor: basic biology and clinical implications. EXS. 1997;79:209–32. doi: 10.1007/978-3-0348-9006-9_9. [DOI] [PubMed] [Google Scholar]
- Ho QT, Kuo CJ. Vascular endothelial growth factor: biology and therapeutic applications. Int J Biochem Cell Biol. 2007;39:1349–57. doi: 10.1016/j.biocel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang B, Guo Y, Li G, Xie Q, Zhu B, et al. Artemisinin inhibits tumor lymphangiogenesis by suppression of vascular endothelial growth factor C. Pharmacology. 2008;82:148–55. doi: 10.1159/000148261. [DOI] [PubMed] [Google Scholar]
- Goishi K, Klagsbrun M. Vascular endothelial growth factor and its receptors in embryonic zebrafish blood vessel development. Curr Top Dev Biol. 2004;62:127–52. doi: 10.1016/S0070-2153(04)62005-9. [DOI] [PubMed] [Google Scholar]
- He Y, Fan J, Lin H, Yang X, Ye Y, Liang L, et al. The anti-malaria agent artesunate inhibits expression of vascular endothelial growth factor and hypoxia-inducible factor-1alpha in human rheumatoid arthritis fibroblast-like synoviocyte. Rheumatol Int. 2011;31:53–60. doi: 10.1007/s00296-009-1218-7. [DOI] [PubMed] [Google Scholar]
- Lee J, Zhou HJ, Wu XH. Dihydroartemisinin downregulates vascular endothelial growth factor expression and induces apoptosis in chronic myeloid leukemia K562 cells. Cancer Chemother Pharmacol. 2006;57:213–20. doi: 10.1007/s00280-005-0002-y. [DOI] [PubMed] [Google Scholar]
- Zhou HJ, Wang WQ, Wu GD, Lee J, Li A. Artesunate inhibits angiogenesis and downregulates vascular endothelial growth factor expression in chronic myeloid leukemia K562 cells. Vascul Pharmacol. 2007;47:131–8. doi: 10.1016/j.vph.2007.05.002. [DOI] [PubMed] [Google Scholar]