Abstract
Graft-versus-host disease (GVHD) is the most common complication after hematopoietic stem cell transplantation. To clarify the role of Toll-like receptor 4 (TLR4), which is a major receptor for bacterial lipopolysaccharides (LPS), in the development of acute GVHD, we used a TLR4-knockout (TLR4−/−) mouse GVHD model and analyzed the underlying immunological mechanisms. When TLR4−/− mice were used as bone marrow and splenocyte cell graft donors or recipients, GVHD symptom occurrence and mortality were delayed compared to wild-type (TLR4+/+) mice. In addition, histopathological analyses revealed that in TLR4−/−→BALB/c chimeras, liver and small intestine tissue damage was reduced with minimal lymphocytic infiltration. In contrast to TLR4+/+, TLR4−/− mice dendritic cells did not express CD80, CD86, CD40, MHC-II or IL-12 during LPS induction and remained in an immature state. Furthermore, the ability of TLR4−/− mice spleen dendritic cells to promote allogeneic T-cell proliferation and, in particular, T-helper cell 1 (Th1) development was obviously attenuated compared with TLR4+/+ mice dendritic cells, and the levels of interferon-γ (IFN-γ) and IL-10, Th2-cell specific cytokines, were significantly higher in the serum of TLR4−/−→BALB/c than in TLR4+/+→BALB/c chimeric mice. Overall, our data revealed that TLR4 may play a role in the pathogenesis of GVHD and that targeted TLR4 gene therapy might provide a new treatment approach to reduce the risk of GVHD.
Keywords: graft-versus-host disease, hematopoietic stem cell transplantation, knockout mouse, Toll-like receptor 4
Introduction
Hematopoietic stem cell transplantation (HSCT) has become one of the main strategies for the treatment of malignant hematological diseases. Unfortunately, the benefit of this treatment is limited by a number of serious side effects, including graft-versus-host disease (GVHD). The complex and intricate pathophysiology of acute GVHD is a consequence of interactions between the donor and host innate and adaptive immune responses.1 Activation of donor T cells by antigen-presenting cells (APCs) is the most important among the three stages described in acute GVHD, as the activated T cells migrate to target organs and cause damage in the recipient target tissue.2 It has been reported that both host-derived and donor-derived APCs are present in secondary recipient lymphoid tissues after allogeneic bone marrow transplantation (BMT). T-cell receptors of the donor T cells can recognize alloantigens on the host APCs (direct presentation) as well as donor APCs (indirect presentation).2,3,4 Recent studies suggest that sufficient CD86, CD80 and CD40 costimulatory molecule expression of the APCs determine whether T cells are fully activated, and an immune response is triggered and inactivation leads to apoptosis or disability in T-cell early responses. It has been reported that dendritic cells (DCs) are a type of APC that have the strongest antigen-presenting ability and can uniquely activate naive T cells, leading to acute graft rejection.1,5 DCs in an immature state demonstrate significantly less antigen-presenting ability, and reduced DCs in a mixed allogeneic T-cell lymphocyte reaction lead to weak T-cell proliferation with a reduced occurrence of GVHD. In addition to human leukocyte antigen mismatching between donors and recipients being the main reason of GVHD, bacterial infections are also an important inducing factor.6 After radio- and chemotherapy cause damage of the intestinal mucosa in recipients, bacteria can pass into the blood via the damaged tissue. The endotoxin receptor Toll-like receptor 4 (TLR4) can recognize endotoxin lipopolysaccharides (LPS) as a pathogen-associated molecular pattern in Gram-negative bacterial cell walls. TLR4 then induces the nuclear factor-kappaB signal pathway,7 leading to the expression of the costimulatory molecules necessary for the activation and differentiation of T lymphocytes,8 including the release of inflammatory factors, such as TNF-α, IL-1, IL-6, NO and other cytokines, finally activating the acquired immune system with concomitant triggering of GVHD.9 Data obtained from mouse GVHD models have shown that disruption of cellular LPS activation effects can significantly reduce GVHD severity.10 As TLR4 has been shown to be a key player in innate immunity and immune tolerance,11,12 TLR4 gene defects have been suggested to alter the function of the receptor, thereby leading to reduced proinflammatory cytokine responses and increased susceptibility to Gram-negative infections.13 Several researchers who focused on TLR4 gene alterations and effects on immune response have found that the distribution of TLR4 gene polymorphisms in Caucasians and Asians is different. Raby and his collaborator reported that TLR4 polymorphisms within the North American cohort/Native American groups in the United States varies with a gene mutation rate of 0.8%–10%.14 Other studies have shown the incidence of being a TLR4 Asp299Gly and Thr399Ile carrier (including homozygotes and heterozygotes) worldwide lies in the range of 2.5%–20.7%.15,16,17,18,19 The TLR4 Asp299Gly polymorphism, in particular, is the highest in African Americans, while in a western Iran population, the highest incidence for a mutation was found for the TLR4 Thr399Ile polymorphism, which is in, contrast, very rare in Asian populations, such as those in Japan and South Korea.20,21 Carriers of the Thr399Ile TLR4 gene mutation have a significantly increased risk of developing GVHD, 42% versus 15% of non-affected patients, whereas transplant-related mortality, overall survival rate and the incidence of infectious complications are not influenced by the mutated gene.22 Histopathological changes in the small intestine are more severe in TLR4-mutant host GVHD recipients than those in TLR4-intact host GVHD recipients at 14 days after GVHD development,23 but the studies reporting these results did not clearly demonstrate the role of TLR4 in the HSCT-related pathogenesis of GVHD. One retrospective study indicated that TLR4 mutations reduced the risk of acute GVHD in humans,24 but other studies have noted that there was no significant influence of TLR4 on the incidence of GVHD.25,26
To elucidate the role of TLR4 in acute GVHD, we used TLR4-knockout mice (TLR4−/−) in myeloablative bone marrow and splenocyte cell donor or recipient models and observed the morphology and GVHD extent of the recipient mice after HSCT. We further analyzed the CD80, CD86, CD40 and MHC-II expression levels of (TLR4−/−) mouse spleen-derived DCs after LPS induction and the interferon-γ (IFN-γ), IL-2, IL-4, IL-10 and other cytokine expression levels in a (TLR4−/−) DC and allogeneic T-cell mixed lymphocyte reaction (MLR). Based on our results, we suggest that TLR4 inhibition might protect against GVHD in recipients after allogeneic HSCT.
Materials and methods
Mice
BALB/c (H-2kd) and C57BL/6 (H-2kb) mice, for use as wild-type controls, were obtained from Joint Ventures Sipper BK Experimental Animal Co. (Shanghai, China). TLR4-knockout mice (TLR4−/−) with the same genetic background as C57BL/6 mice (TLR4+/+) were obtained from our breeding colony and originally provided by Shizuo Akira (Osaka University, Japan).27
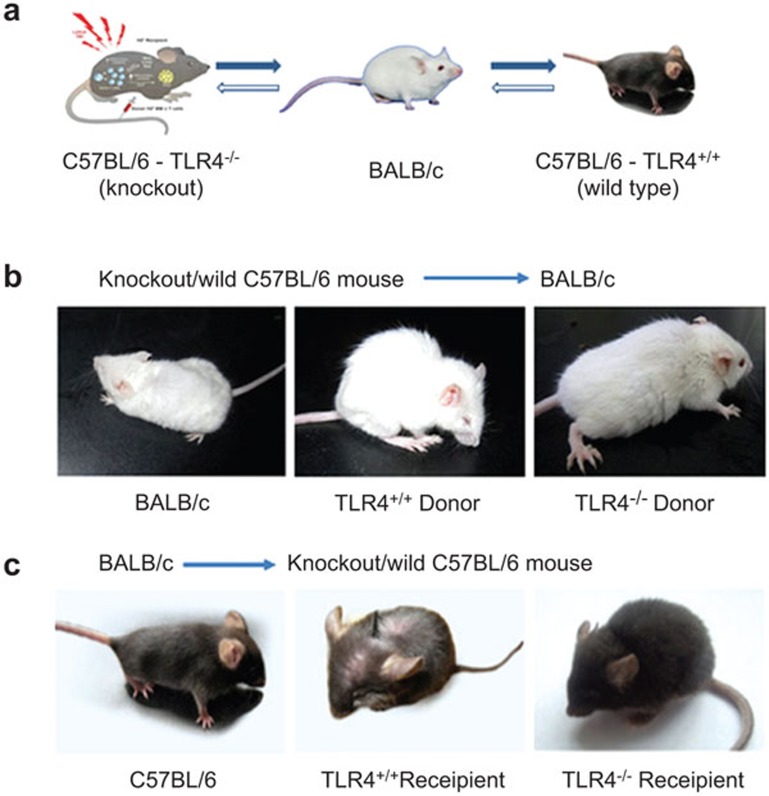
All mice were maintained under pathogen-free conditions and used at 8–12 weeks of age (20–25 g body weight). Animal experiments were performed as shown in Figure 1a. (i) TLR4−/− group: 15 TLR4−/− mice were used as recipients, in which a mixture of BALB/c bone marrow- and spleen-derived cells were injected into the caudal veins. In turn, 15 TLR4−/− mice were used as donors for bone marrow- and spleen-derived cells, which were injected into the caudal vein of BALB/c mice. (ii) TLR4+/+ group: 15 TLR4+/+ mice were used as recipients, in which a mixture of BALB/c bone marrow- and spleen-derived cells were injected into the caudal veins. In turn, 15 TLR4+/+ mice were used as donors for bone marrow- and spleen-derived cells, which were injected into the caudal vein of BALB/c mice. (iii) Control group: six BALB/c or C57BL/six wild-type mice were used as recipients, in which C57BL/6 or BALB/c bone marrow- and spleen-derived cells were injected into the caudal vein. The weights of the recipient TLR4−/− mice and donor TLR4−/− mice did not significantly differ.
Figure 1.
Morphology of recipients after transplantation with bone marrow cells from TLR4+/+ or TLR4−/−mice. Recipient mice (15 mice/group) transplanted with 1×107 bone marrow cells and 2×107 splenocytes from donor mice that were lethally irradiated. (a) Illustration of the transplantations. (b) GVHD phenotype of recipient TLR4+/+→BALB/c (middle panel) and TLR4−/−→BALB/c mice (right panel) after BMT. (c) GVHD-related phenotypes of recipient BALB/c→TLR4+/+ and BALB/c→TLR4−/− mice. GVHD, graft-versus-host disease; TLR4, Toll-like receptor 4.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the approval of the Scientific Investigation Board of the Second Military Medical University, Shanghai, China.
Reagents
RPMI 1640 medium and fetal bovine serum were obtained from PAA Laboratories (Linz, Austria). Recombinant mouse granulocyte-monocyte colony stimulating factor, IL-4 and enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN, USA). Fluorescein-conjugated murine antibodies were purchased from BD Pharmingen (San Diego, CA, USA) and eBioscience (San Diego, CA, USA). Microbead-conjugated murine antibodies against CD3, CD4, CD19 and CD11c were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). 7-Aminoactinomycin D (7-AAD), saponin, indomethacin, brefeldin A, bovine serum albumin, 1-methyltryptophan and carboxyfluorescein diacetate succinimidyl ester (CFSE) were purchased from Sigma (St Louis, MO, USA). Lipopolysaccharide (ultrapure Escherichia coli 0111:B4) was obtained from InvivoGen (San Diego, CA, USA).
Induction of GVHD
Myeloablation of TLR4+/+ and TLR4−/− mice was performed by whole-body irradiation, with a total dose of 1050 cGy (60Co source) given twice with a 3-h interval to minimize gastrointestinal toxicity. This was followed by an injection of BALB/c donor-derived bone marrow and splenocyte cells into the caudal tail veins. Reverse experiments were performed by using irradiated BALB/c mice (900 cGy (60Co source), two doses with a 3-h interval) as recipients and TLR4+/+ or TLR4−/− mice as donors. Bone marrow and splenocyte cell suspensions were prepared as previously described.25 All mice received an oral suspension of gentamicin (320 mg/l) with their drinking water from 7 days before transplantation until 4 weeks after BMT.
Assessment of GVHD
GVHD severity was assessed using a previously described clinical scoring system.28 Each BMT recipient mouse was scored twice weekly for 60 days after transplantation. Weight loss, posture (hunching), activity, fur texture and skin integrity were measured using a scale from 0 to 2, with 0 for absent or normal, 1 for mild abnormal and 2 for severely abnormal. The GVHD clinical index was determined as the sum of the individual criteria scores divided by 10.
Histology
Tissue samples from the small intestines and livers of recipient mice were collected after allogeneic transplantation, and the tissue sections were examined for evidence of GVHD by established protocols.29,30 Cell apoptosis of the small intestine tissue was examined with the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method, and the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Preparation of DCs from mouse bone marrow
Mouse bone marrow-derived DCs were generated by established protocols.31,32,33,34,35 On day 5, the proliferating DC clusters were collected and purified by anti-CD11c magnetic microbeads as immature DCs. Purified immature DCs were stimulated with LPS (1 µg/ml) for 24 h.
Flow cytometric analyses
Detection of cell surface marker, intracellular cytokines and quantification of proliferating T cells was performed using flow cytometry.31,32,33,34,35
In all experiments, the cells were analyzed using FACSCalibur and LSR II flow cytometers (BD Biosciences, San Jose, CA, USA).
Western blot analysis
TLR4+/+- and TLR4−/−-derived DCs were stimulated with 1 µg/ml LPS for the indicated times, harvested, lysed and then analyzed by western blots as described previously.31
MLR cultures
Responder cells were prepared from BALB/c mouse splenocytes, and DC stimulator cells were obtained either from TLR4+/+ or TLR4−/− mice and used at the fifth day after extraction. Cytokine production in the culture supernatants or in serum was detected using ELISA kits. For intracellular cytokine staining, splenocytes were harvested 7 days after transplantation. The protocols were performed as mentioned above.31,32,33,34 Responder cells were prepared from BALB/c mouse splenocytes by negative selection with mouse CD19 microbeads (Miltenyi Biotec) to remove proliferating B cells and to accumulate T cells. Stimulator cells were obtained by positive selection with mouse MHC class II microbeads (Miltenyi Biotec) from spleen cells of C57BL/6 or TLR4-knockout mice not irradiated or lethally irradiated 24 h before the procedure. In experiments using non-irradiated mice, stimulator cells were irradiated (3000 cGy) before coculture. In experiments, which were performed for the evaluation of the TLR4/APC capability to respond to maturation stimuli, LPS (1 µg/ml) and/or TNF-α (10 ng/ml) were added to the stimulator cell medium 24 h before coculture. In all experiments, the proliferation of responder cells was evaluated in the presence of stimulator cells obtained from three different mice per group. Proliferation of T cells was evaluated with the CellTrace CFSE Cell Proliferation Kit (Invitrogen), according to the manufacturer's instructions.
Statistical analysis
The two-tailed unpaired Student's t-test was applied for group comparisons of discrete variables. The log-rank test was used to analyze survival curves. A value of P<0.05 was considered a statistically significant difference.
Results
Reduced acute GVHD severity after TLR4−/− mouse donor-derived bone marrow and splenocyte cell transplantation into irradiated BALB/c mice
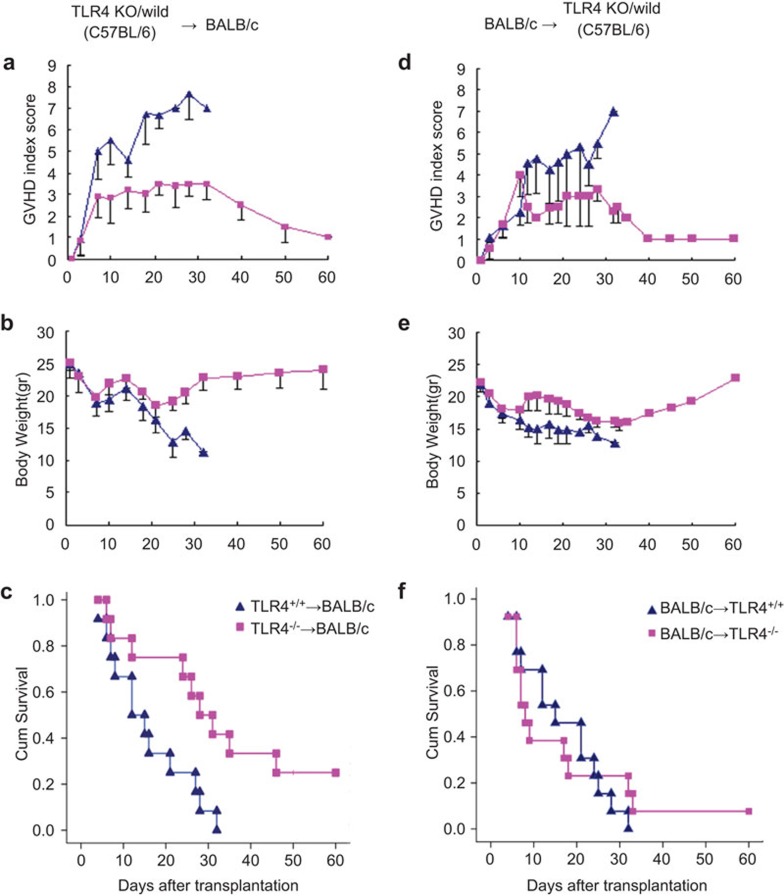
BALB/c recipient mice (15/group) were myeloablative-irradiated and received 1×107 bone marrow and 2×107 splenocyte cells obtained from the TLR4−/− or TLR4+/+ donor mice. Three mice from each recipient group were selected randomly as control mice and received a phosphate-buffered saline injection. All recipient mice were monitored for survival rate, weight changes and clinical signs of GVHD. The control mice all died of bone marrow failure within 5–10 days post-transplantation with a white blood cell count less than 0.5×109/l. We sampled eye blood from both groups of the recipient BALB/c (H-2kd) mice. The blood was marked with specific antibodies on day 14 and subjected to fluorescence-activated cell sorting (FACS) that indicated that the blood of all recipient mice contained more than 90% donor cells (H-2kb). We also examined the chimeric spleen-derived donor and recipient DCs 14 days after BMT. The cells were stained with anti-H2kb, anti-H2kd as well as anti-CD11c antibodies and analyzed by FACS. A CD11c population analysis performed on living cells revealed that 85% of DCs were derived from the donors (data not shown). We compared the severity and mortality of GVHD by a GVHD clinical scoring system. On median day 7.2 post-transplantation, TLR4+/+→BALB/c mice began to present typical signs of GVHD, including significant weight loss, severe hunching and fur texture ruffling (Figure 1b, middle panel). In contrast, TLR4−/−→BALB/c mice did not present any characteristics of GVHD before 12 days and they had obvious symptoms of GVHD by a median of 12.3 days after transplantation (Figure 1b, right panel). Their GVHD clinical score was also significantly lower than that in the TLR4+/+→BALB/c mice (P=0.004) (Figure 2a), with significantly less weight loss (P<0.001) (Figure 2b). In addition, TLR4−/−→BALB/c mice presented a significantly higher survival rate, with two of twelve mice still alive at the end of the experiment (day 60) (Figure 2c, P=0.01).
Figure 2.
Reduced acute GVHD severity of TLR4−/− mouse donor-derived BMT or TLR4−/− mice as BMT recipients receiving cells from BALB/c mice. (▴) indicates TLR4+/+ recipient, (▪) indicates TLR4−/− recipient BALB/c mice. TLR4−/−→BALB/c allogeneic BMT experiment. (a) Mean GVHD index score: (▴) vs. (▪), P=0.004. (b) Mean body weight: (▴) vs. (▪), P<0.001. (c) Percentage of survival: donor TLR4+/+ (▴) vs. donor TLR4−/− (▪), P=0.01. Results of the subsequent reverse-direction allogeneic BMT experiments with BALB/c→TLR4+/+ mice. (d) Mean GVHD index score: (▴) vs. (▪), P=0.002. Compared to BALB/c→TLR4−/− mice, BALB/c→TLR4+/+ mice developed obvious symptoms of GVHD 14 days after BMT. (e) Mean body weight: (▴) vs. (▪), P=0.002. (f) The survival curves of TLR4+/+ recipients (▴) compared with TLR4−/− recipients (▪) indicates no significant difference. Data shown are derived from at least two independent experiments. BMT, bone marrow transplantation; GVHD, graft-versus-host disease; TLR4, Toll-like receptor 4.
The effect of reduced acute GVHD severity also occurred with irradiated TLR4−/− mice as recipients of BALB/c mouse-derived bone marrow and splenocyte cell transplantations
Reverse to what was performed in the allogeneic BMT experiment, TLR4−/− and TLR4+/+ mice (15 mice/group/strain) were myeloablative-irradiated and treated with 1×107 bone marrow cells and 2×107 splenocytes obtained from BALB/c donors. The results were similar. Three mice of each recipient group were selected randomly as control mice with a phosphate-buffered saline injection only. All control mice died of bone marrow failure within 1 week post-transplantation. At day 14, all surviving recipient mice contained 100% donor cells (H-2kd) as measured by FACS. At median day 8 post-transplantation, BALB/c→TLR4+/+ mice began to present typical characteristics of GVHD (Figure 1c, middle panel), but at the same time, BALB/c→TLR4−/− mice did not present any GVHD symptoms (Figure 1c, right panel) and had obvious signs of GVHD at median day 16.1, and they appeared to a lesser extent (P=0.002) (Figure 2d). BALB/c→TLR4+/+ mice, when compared with BALB/c→TLR4−/− mice (Figure 2d), developed obvious symptoms of GVHD 14 days after BMT, and their weight loss was lower as well (P=0.002) (Figure 2e). TLR4−/− recipient mice displayed a slightly higher survival rate, with one of twelve mice still alive at the end of the experiment (day 60), but all TLR4+/+ recipient mice died within 32 days. However, the difference in survival rates between the two groups was not significant (Figure 2b).
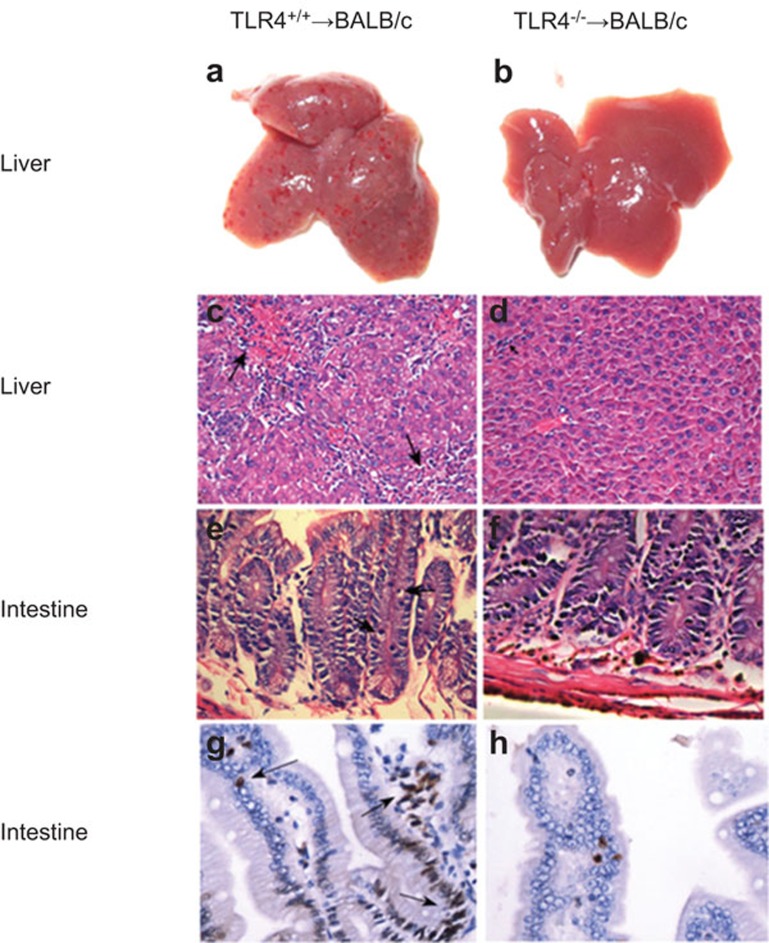
TLR4−/− bone marrow and splenocyte cell transplantation into irradiated BALB/c recipient mice caused fewer pathohistological changes of the liver and intestine than transplantation of TLR4+/+ donor cells
Histological analyses were performed on livers (Figure 3a–d) and small intestines (Figure 3e–h) from BALB/c mice at day 21 post-transplantation of 1×107 bone marrow cells and 2×107 splenocytes from TLR4+/+ or TLR4−/− donor mice. The tissue damage in TLR4−/− mice cell recipients was minimal (Figure 3b, d and f), while the surface of TLR4+/+ cell-transplanted mice livers presented with more obvious and diffuse bleeding blots (Figure 3a) that were heavily infiltrated with lymphocytes in the portal area and displayed endothelialitis of a hepatic vein as well as a disordered histological architecture (Figure 3c). In contrast, no obvious bleeding spots on the surface of TLR4−/− cell-transplanted mice livers (Figure 3b) were visible, and minimal lymphocyte infiltration in the portal area took place with rare inflammatory cell invasion into the bile ducts (Figure 3d). In addition, the small intestines of the TLR4+/+ cell-transplanted mice exhibited more severe villus blunting, crypt destruction changes and greater crypt atrophy than TLR4−/− cell-transplanted mice. Figure 3e and f depicts apoptotic crypt cells of the small intestine, stained with hematoxylin and eosin. Figure 3g and h depicts TUNEL staining.
Figure 3.
Histological analysis of TLR4+/+ and TLR4−/− transplanted mice. The liver (a–d) and small intestine (e–h) tissues from BALB/c mice analyzed 21 days after transplantation with 1×107 bone marrow cells and 2×107 splenocytes from TLR4+/+ or TLR4−/− donor mice. Tissue damage following TLR4−/− mice cell BMT was minimal (b, d and f). The liver surface of TLR4+/+ mice cell BMT presented more obvious bleeding (a), and the histology of the mice livers revealed endothelialitis of a hepatic vein (upper arrow), hepatocytes undergoing necrosis and heavy lymphocytic infiltrations (lower arrow) (c) compared with TLR4−/− mice (b, d). The small intestines of TLR4+/+ mice exhibited more severe villus blunting, crypt destruction changes and crypt atrophy (e). Apoptotic crypt cells of the small intestines are shown in e (arrows, stained with H&E) and in g (arrows, stained with TUNEL) compared with TLR4−/− mice (f, h). Representative samples from each mouse group were processed and stained with H&E or TUNEL. Original magnification: c and d, ×200; e–h, ×400. BMT, bone marrow transplantation; H&E, hematoxylin and eosin; TLR4, Toll-like receptor 4; TUNEL, terminal deoxynucleoitidyl transferase-mediated dUTP nick end-labeling.
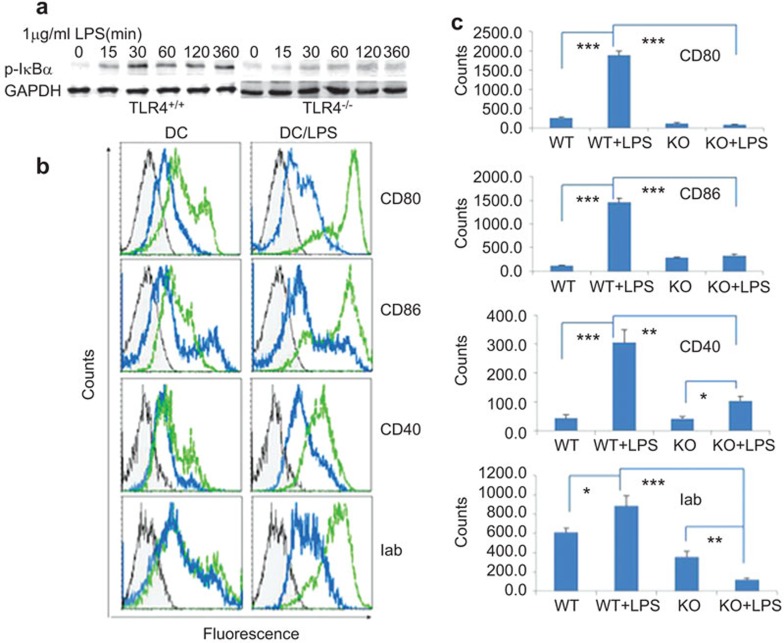
TLR4−/− mice-derived DCs remained immature after LPS stimulation
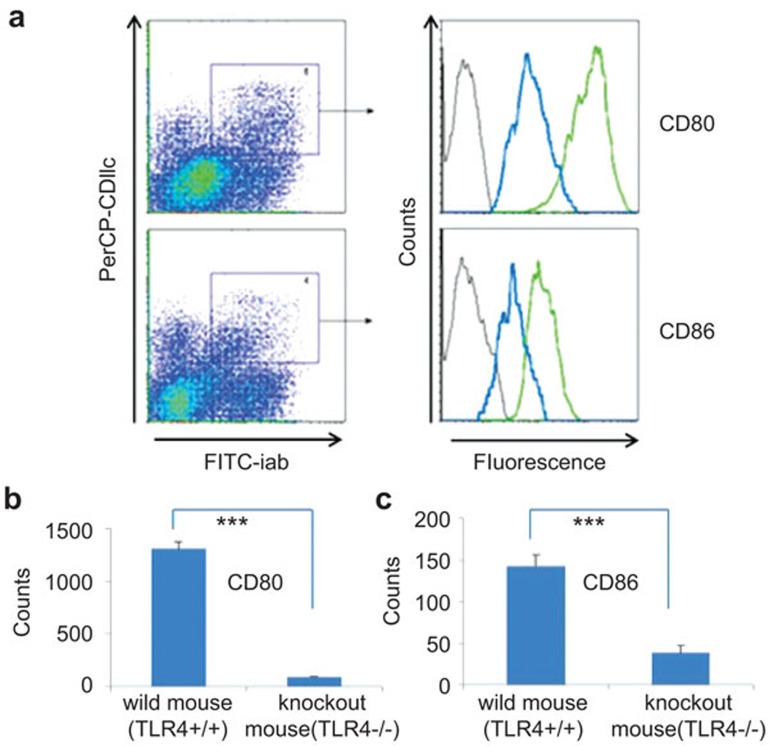
To test the assumption that the TLR4−/− gene mutation might inhibit DC maturation, we stimulated TLR4−/− DCs with LPS and analyzed the Phospho-I κBα levels by western blotting. As visible in Figure 4a, in contrast to TLR4+/+ DCs, there was only a marginal enhancement of Phospho-I κBα protein levels in TLR4−/− DCs after LPS stimulation. To further elucidate the role of TLR4−/−-derived DCs on T-cell activation, we analyzed the costimulatory molecule expressions of 5-day-old TLR4+/+ or TLR4−/− mice bone marrow cells, with or without 24 h LPS (1 µg/ml) stimulation before cell harvest. TLR4−/−-derived DCs did not obviously express CD80, CD86, CD40 and MHC-II with and without LPS stimulation, while the expression of costimulatory markers in TLR4+/+ DCs was obviously upregulated upon LPS stimulation (CD80: 1887.6±99 in TLR4+/+ mice vs. 85.9±11.2 in TLR4−/− mice, P<0.001; CD86: 1461.5±85.3 in TLR4+/+ mice vs. 327.1±26.5 in TLR4−/− mice, P<0.001; CD40: 305.4±45 in TLR4+/+ mice vs. 104.9±16.1 in TLR4−/− mice, P<0.01; MHC-II: 884.7±104.4 in TLR4+/+ mice vs. 123.3±13.3 in TLR4−/− mice, P<0.01) (Figure 4b and c).
Figure 4.
TLR4−/−-derived DCs remained immature after LPS stimulation. (a) The Phospho-I κBα levels of the NF-κB pathway was not activated in TLR4−/−-derived DC after LPS stimulation. TLR4+/+- or TLR4−/−-derived DCs were incubated with or without LPS (1 µg/ml) for 15, 30, 60, 120 and 360 min and a western blot analysis of the TLR4 signal pathway was performed in DCs with GAPDH as a control. b and c display the expression of costimulatory markers on DCs from TLR4+/+ or TLR4−/− mice. The bone marrow of day-5 DCs from TLR4+/+ or TLR4−/− mice and for 24 h LPS-treated day-5 DCs were purified with microbead-conjugated anti-CD11c mouse antibodies and the purified DCs were stained with FITC-anti-CD80, CD86, CD40 and MHC-II antibodies. The gray represent isotype controls. The green lines represent TLR4+/+ mice and the blue lines represent TLR4−/− mice. DC, dendritic cell; FITC, fluorescein isothiocyanate; KO, knockout; LPS, lipopolysaccharide; NF-κB, nuclear factor-kappaB; TLR4, Toll-like receptor 4; WT, wild-type.
We also detected the expression of CD80 and CD86 in DCs from the spleen cells of TLR4+/+→BALB/c and TLR4−/−→BALB/c mice 21 days after BMT. The expression of CD80 and CD86 costimulatory molecules in DCs from TLR4+/+→BALB/c mice significantly increased compared to TLR4−/−→BALB/c mice (CD80: 1313.3±65.1 in donor TLR4+/+ mice vs. 93.6±4.5 in donor TLR4−/− mice, P<0.001; CD86: 143.4±13.3 in donor TLR4+/+ mice vs. 38.9±8.6 in donor TLR4−/− mice, P<0.001) (Figure 5).
Figure 5.
TLR4−/−-derived DCs remained immature after TLR4+/+→BALB/c or TLR4−/−→BALB/c transplantations. Phenotypic analysis by flow cytometry of in vivo experiments: upregulation of costimulatory markers on splenic DCs from TLR4+/+→BALB/c mice after BMT. Expression of CD80 and CD86 in DCs obtained from spleen cells of TLR4+/+→BALB/c or TLR4−/−→BALB/C mice 21 days after BMT. Gray represents an isotype control. The green line represents TLR4+/+→BALB/c cells and the blue line represents TLR4−/−→BALB/c cells. (a) Cells were stained with FITC-anti-MHC-II antibodies, PE-anti-CD80 or CD86 antibodies, PerCP-anti-CD11c antibodies and analyzed by flow cytometry. The analysis was performed on living cells gated by the CD11chiMHC-IIhi population. Both CD80 and CD86 were expressed in a lower amount by TLR4−/− mice cells (b and c). Two independent experiments, with two mice for each, were performed. BMT, bone marrow transplantation; DC, dendritic cell; TLR4, Toll-like receptor 4.
TLR4−/−-derived DCs partly lost their ability to activate allogeneic T cells
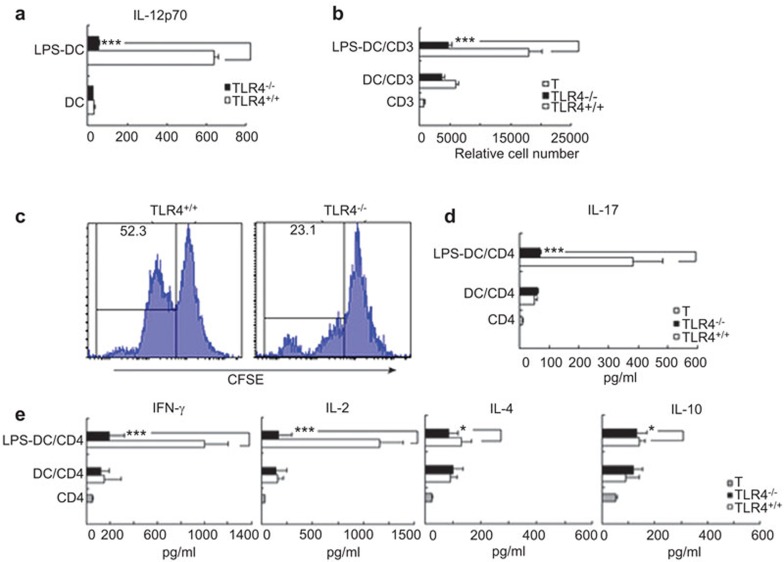
We analyzed the IL-12 levels in the culture medium supernatants of TLR4+/+ and TLR4−/− mouse-derived DCs and found that there was no difference between them in 5-day-old DC culture medium supernatants. However, after LPS (1 µg/ml) treatment for 24 h, the level of IL-12 from TLR4+/+ mouse-derived DC culture supernatants was higher than that of the culture medium supernatants of TLR4−/− mice (Figure 6a).
Figure 6.
Bone marrow DCs from TLR4−/− reduces allostimulatory activities and induces allogeneic T-cell hyporesponsiveness. (a) IL-12 production of the cultured day-5 DCs derived from TLR4+/+ or TLR4−/− mice stimulated with or without LPS (1 µg/ml) for 24 h, as measured by ELISA. Results are expressed as the mean±s.d. of triplicate wells. (b) BALB/c spleen CD3+ T cells were cocultured with allogeneic TLR4+/+- or TLR4−/−-derived DCs and LPS-treated DCs with a stimulator: responder ratio of 1∶10 in a MLR. After 5 days, the relative number of viable T (CD4+7AAD−) cells in each well was counted by flow cytometry. (c) Proliferation of BALB/c CD3+ T cells was labeled with CFSE, cocultured with LPS-treated DCs derived from TLR4+/+ or TLR4−/− mice for 5 days and the amount of proliferation was determined by FACS. (d, e) The levels of cytokines produced (IFN-γ, IL-2, IL-4, IL-10 and IL-17) by the activated CD4+ T cells in a MLR culture media supernatant, as assayed by ELISA. Data shown are mean±s.d. of three independent experiments performed. (*P<0.05, ***P<0.001). 7-AAD, 7-Aminoactinomycin D; CFSE, carboxyfluorescein diacetate succinimidyl ester; DC, dendritic cell; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescent activated cell sorting; IFN-γ, interferon-γ MLR, mixed lymphocyte reaction; LPS, lipopolysaccharide; TLR4, Toll-like receptor 4.
We further studied the difference of antigen presentation and lymphocyte proliferation mediating ability between TLR4+/+ and TLR4−/− mouse-derived DCs with and without LPS stimulation. CD3+ T cells from BALB/c mice spleen were cocultured with TLR4+/+ or TLR4−/− mouse-derived immature untreated and LPS-treated DCs with a responder ratio of 1∶10 in a MLR. After 5 days, the relative number of viable T (CD3+7AAD−) cells in each well were determined by flow cytometry, and the alloreactive BALB/c T-cell number was significantly lower in cultures stimulated by TLR4−/− than in cultures stimulated by TLR4+/+ mouse-derived DCs (P<0.001) (Figure 6b). In addition, the proliferation of BALB/c CD3+ T cells cocultured with stimulator LPS-treated DCs derived from TLR4+/+ or TLR4−/− mice for 5 days was labeled with the proliferation marker CFSE and analyzed by FACS. Proliferating CFSE-marked cells, following TLR4−/− mouse-derived DCs stimulation, were reduced (mean±s.d.: 23.1%±1.1%) compared with those that were TLR+/+ DC stimulated (52.3%±1.2%) (Figure 6c).
T cells cocultured with TLR4−/− mouse-derived DCs secreted fewer cytokines than T cells cocultured with TLR4+/+ mouse-derived DCs
Next, we examined cytokine secretion in media supernatants after 5 days of BALB/c T-cell coculturing with a 24-h treatment of LPS (1 µg/ml)-stimulated TLR4−/− and TLR4+/+ DCs to assess the role of DCs on TLR4−/− on allogeneic T-cell differentiation. For the analysis, 120 µl/well culture supernatants were collected, and the levels of IFN-γ, IL-2, IL-4, IL-10 and IL-17 secreted by the activated T cells in the supernatants of the MLR culture media were assayed by ELISA. The levels of IFN-γ, IL-2, IL-17 in the TLR4−/− group decreased significantly compared to the TLR4+/+ group (P<0.001). Interestingly, IL-10, IL-4 levels representing T-helper cell 2 (Th2) cytokines changed little in both groups (P>0.05) (Figure 6d and e).
Cytokine expression of Ths in mice 7 days after BMT
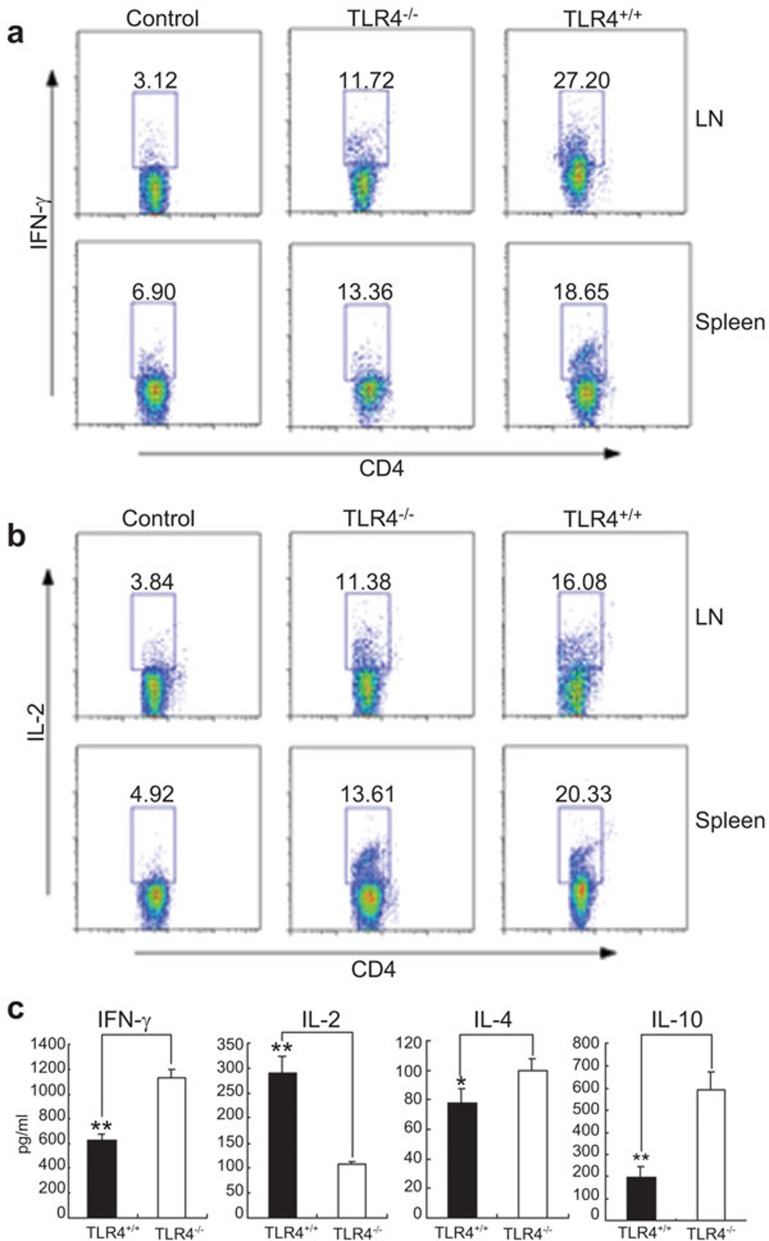
To gain insight into the effect of TLR4−/− DCs on T-cell differentiation, we removed and ground the spleens and lymph nodes of chimeric mice 7 days after transplantation. We then incubated the mixtures in 1640 medium with phorbol myristate acetate, ionomycin and brefeldin A for 6 h. The percentage of CD4+ T cells secreting intracellular cytokines, such as IFN-γ and IL-2, was determined by FACS. The percentages of T cells secreting IFN-γ and IL-2 in the TLR4−/− cell recipient BALB/c mice group were significantly lower than the levels observed in TLR4+/+-cell recipient BALB/c mice (P<0.05) (Figure 7a and b).
Figure 7.
Expression of Th cytokines in mice after BMT. Spleen and lymph nodes of chimeric mice 7 days after transplantation were removed, ground and then incubated in RPMI medium with phorbol myristate acetate, ionomycin and brefeldin A for 6 h. The percentages of CD4+ T cells secreting cytokines such as IFN-γ (a) and IL-2 (b) in TLR4−/− mice and TLR4+/+ mice were determined by FACS. The percentage of T cells secreting IFN-γ and IL-2 in the TLR4−/− mice group compared to TLR4+/+ mice was significantly decreased (P<0.05). (c) TLR4+/+ or TLR4−/− as donor mice: the production of cytokines in sera derived from recipient BALB/c mice was analyzed on day 7 post-transplantation by ELISA. The levels of cytokines (IFN-γ and IL-10) in the TLR4−/− group, as compared to the TLR4+/+ donor mice group, were significantly increased. IL-2 levels in the TLR4−/− mice group were significantly lower than in TLR4+/+ group (P<0.05), while IL-4 levels were not significantly different between both groups (Figure 7c). Data shown are the mean±s.d. from at least three mice (*P<0.05, **P<0.01). BMT, bone marrow transplantation; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescent activated cell sorting; IFN-γ, interferon-γ TLR4, Toll-like receptor 4.
We also tested the levels of IFN-γ, IL-2, IL-10 and IL-4 in the sera of recipient BALB/c mice 7 days after transplantation. To our surprise, the IFN-γ levels in recipient BALB/c mice serum gradually increased after TLR4+/+ BMT and reached its peak 7 days after transplantation, while the IFN-γ levels in BALB/c mice serum that received TLR4−/− mice cells were significantly higher than in TLR4+/+ mice cell recipients (P<0.05). IL-2 levels in the TLR4−/− mice cell recipient BALB/c mice serum were significantly lower than in TLR4+/+ mice cell recipients (P<0.05). IL-10 levels representing Th2-related subsets of cytokines in TLR4−/− mice cell recipient BALB/c mice serum were significantly higher (P<0.05), while IL-4 levels were not significantly different between both groups (Figure 7c).
Discussion
To evaluate the effects of TLR4−/− mouse-derived DCs on GVHD with direct and indirect presentation, we designed a mouse GVHD model with TLR4+/+ and TLR4−/− mice as the recipients and injected bone marrow and splenocyte cells obtained from BALB/c mice as donors. In a subsequent reverse-direction experiment, we used BALB/c mice as recipients and TLR4+/+ and TLR4−/− mice as donors. The transplantation rate of our chimeric mice was in accordance with a previous report, which indicated that approximately 90% of DCs in the recipient mice spleens were donor derived 14 days after transplantation.4 Our results demonstrated that TLR4−/− mice, either as donors or recipients, led to chimera that developed less severe and delayed GVHD (median time: 16.1 days vs. 12.3 days), with reduced weight loss and minor liver and small intestine damage.
We need to point out that there was no significant difference in the survival curves between the TLR4−/− or TLR4+/+ mice as recipient groups with otherwise significant higher BALB/c recipient mortality with TLR4+/+ donor cells after allogeneic BMT. We consider that TLR4−/− mice may have been more sensitive to radiation, as we found that TLR4−/− mice were more prone to radiation sickness under myeloablative irradiation. Upon investigating the reduced GVHD severity after allogeneic BMT related to TLR4 knockout, we first found that even after LPS stimulation, they did not express Phospho-I κBα, in contrast to TLR4+/+-derived DCs, indicating that the nuclear factor-kappaB pathway was no longer functional.
Recent studies suggest that DCs express the highest level of MHC class II molecules and CD86, CD80 and CD40 on their surface, thereby playing a major role in the activation of naive T lymphocytes. In cases where the APC cannot express enough costimulatory molecules, T cells may not be completely activated, leading to anergy or apoptosis.1,5 Our FACS data demonstrate that day-5 DCs from TLR4+/+ mice, after being stimulated by exogenous LPS (1 µg/ml) for 24 h, expressed high levels of CD80, CD86, CD40 and MHC-II molecules and displayed mature DC phenotypes and increased IL-12 secretion levels, while TLR4−/−-derived day-5 DCs still maintained immature phenotypes without costimulatory CD80, CD86, CD40, MHC-II molecule upregulation and their IL-12 secretion did not differ with or without LPS stimulation.
In an MLR experiment, we found that TLR4−/−-derived DCs had an attenuating effect on allogeneic T-cell activation compared to the TLR4+/+ group. In addition, an in vivo experiment demonstrated that the expression of the costimulatory molecules CD80 and CD86 in DCs, obtained from the spleen cells of TLR4−/−→BALB/c mice 21 days after BMT, was significantly decreased compared to TLR4+/+→BALB/c. IL-12, mainly produced by dendritic cells in response to LPS, can induce IFN-γ production and trigger CD4+ T cells to differentiate into Th1 phenotypes,36 while the proliferation of T cells is inhibited by insufficient IL-12 expression. The immature phenotype-displaying TLR4−/−-derived DCs significantly lost their antigen-presenting ability after LPS treatment, and reduced MLR stimulation of allogeneic T cells by these DCs led to weaker T-cell proliferation and induction.
It has been suggested that the Th1/Th2 polarization of the T helper cells plays an important role in the development of GVHD. The Th1 cell switch to Th2 can change the local graft immune response and inhibit cell-mediated rejections.37,38 As the cytokines produced by the Th1 subset are associated with the severity and mortality of GVHD,39,40 we examined the effect of TLR4−/− DCs on allogeneic CD4+ T-cell differentiation in a MLR cocultivation experiment, and we analyzed the expression of Th-related cytokines in the supernatant of the MLR culture media. We concluded that the levels of cytokines produced by the Th1 subset, IFN-γ and IL-2, significantly decreased in TLR4−/− mice DC and allogeneic CD4+ T-cell cocultures (P<0.01), while the levels of cytokines produced by the Th2 subset, IL-10 and IL-4, also significantly decreased at the same time (P<0.05).
These results underline that DCs from TLR4−/− mice cannot fully activate the proliferation of Th1 subset, and there is no Th1 switch to Th2. To further investigate the relative levels of cytokines produced by the Th1/Th2 subsets after BMT in our mouse GVHD model, we used the ELISA method to quantify serum cytokines in chimeric mice 7 days post-transplantation and found that the level of the Th1 subset representing IL-2 was significantly lower than that in TLR4−/− donor mice. Surprisingly, the level of IFN-γ was significantly higher than that of TLR4+/+→BALB/c mice. We suggest that other cells, such as natural killer cells and APCs, in addition to T cells, also probably secreted higher amounts of IFN-γ. However, our results are consistent with other findings that IFN-γ can reduce the occurrence of GVHD by negatively regulating alloreactive T cells via inhibiting cell differentiation.41,42
With TLR4−/− mice as donors, in the serum of recipient BALB/c mice on day 7 post-transplantation, the production of IL-10 as a characteristic sign of the Th2 response did significantly increase. Studies found that Th2 cytokines can be associated with downregulation of cell-mediated immune responses by antagonizing the effect of Th1 cytokines, thereby reducing GVHD.40,43 The expression of IL-17 in the supernatant of MLR with TLR4+/+ mice cells was significantly higher than that of TLR4−/−mouse cell MLRs, which suggests that IL-17 may be an additional GVHD progression-promoting factor. IL-17 expression is related to a third subset of T cells and plays a critical role in the extracellular pathogen response, which may lead to inflammation and is also related to severe autoimmune diseases.15
In summary, our results indicated that TLR4 gene deficiency keeps DCs in an immature state where they do not express high levels of CD80, CD86, CD40 or MHC-II, and their IL-12 secretion is blocked. This, in turn, leads to reduced CD4+ T-cell maturation into Th1 phenotypes. With reduced T-cell proliferation and maturation, the severity of GVHD is lower in TLR4−/−-related BMT.
Conclusions
The LPS/TLR4 signal transduction pathway plays an important role in the immune response, which is mediated and primed by allogeneic T lymphocytes. Based upon this, we concluded that T cells from TLR4−/−-knockout mice cannot be effectively activated and do not proliferate under the stimulation of APCs. Therefore, TLR4−/− can lead to immune tolerance of allogeneic organ transplantation and thus significantly reduce the risk of developing GVHD.
Acknowledgments
We are grateful to Miao Chen, Qiangguo Gao and Yiqi Liu (Second Military Medical University, Shanghai, China) for technical support and offer special thanks to Professor Qing Yi (M.D. Anderson Cancer Center; Houston, TX, USA) for helpful guidance in the experiments. We thank Shizuo Akira (Osaka University, Osaka, Japan) for originally providing key mouse strains. This work was supported by grants of the National Natural Science Foundation of China (no. 30772502 and 30973455), Zhejiang Major Medical and the Health Science and Technology & Ministry of Health of the Chinese Government (no. WKJ2009-2-022). This work was also supported by the Major Research Plan of the Chinese National Natural Science Foundation (no. 91029740), Zhejiang Province Science and Technology Department Foundation (no. 2009C03012-2) and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents.
The authors declare that there are no conflicts of interest.
References
- Paczesny S, Hanauer D, Sun Y, Reddy P. New perspectives on the biology of acute GVHD. Bone Marrow Transplant. 2010;45:1–11. doi: 10.1038/bmt.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffermann-Gretzinger S, Lossos IS, Vayntrub TA, Leong W, Grumet FC, Blume KG, et al. Rapid establishment of dendritic cell chimerism in allogeneic hematopoietic cell transplant recipients. Blood. 2002;99:1442–1448. doi: 10.1182/blood.v99.4.1442. [DOI] [PubMed] [Google Scholar]
- Gonzalez B, Guerra C, Morris D. Dendritic cells in infectious disease, hypersensitivity, and autoimmunity. Int J Interferon Cytokine Mediator Res. 2010;2:137–147. [Google Scholar]
- Mullighan CG, Bardy PG. New directions in the genomics of allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:127–144. doi: 10.1016/j.bbmt.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Cooke KR, Olkiewicz K, Erickson N, Ferrara JL.The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res 2002. Sect. 441–448. [DOI] [PubMed]
- Netea MG, van Deuren M, Kullberg BJ, Cavaillon JM, van der Meer JW. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors. Trends Immunol. 2002;23:135–139. doi: 10.1016/s1471-4906(01)02169-x. [DOI] [PubMed] [Google Scholar]
- Beutler B, Poltorak A. The sole gateway to endotoxin response: how LPS was identified as Tlr4, and its role in innate immunity. Drug Metab Dispos. 2001;29:474–478. [PubMed] [Google Scholar]
- Ferwerda B, McCall MB, Verheijen K, Kullberg BJ, van der Ven A, van der Meer JW, et al. Functional consequences of Toll-like receptor 4 polymorphisms. Mol Med. 2008;14:346–352. doi: 10.2119/2007-00135.Ferwerda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raby BA, Klimecki WT, Laprise C, Renaud Y, Faith J, Lemire M, et al. Polymorphisms in Toll-like receptor 4 are not associated with asthma or atopy-related phenotypes. Am J Respir Crit Care Med. 2002;166:1449–1456. doi: 10.1164/rccm.200207-634OC. [DOI] [PubMed] [Google Scholar]
- Carvalho A, Marques A, Maciel P, Rodrigues F. Study of disease-relevant polymorphisms in the TLR4 and TLR9 genes: a novel method applied to the analysis of the Portuguese population. Mol Cell Probes. 2007;21:316–320. doi: 10.1016/j.mcp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Berdeli A, Emingil G, Han Saygan B, Gurkan A, Atilla G, Kose T, et al. TLR2 Arg753Gly, TLR4 Asp299Gly and Thr399Ile gene polymorphisms are not associated with chronic periodontitis in a Turkish population. J Clin Periodontol. 2007;34:551–557. doi: 10.1111/j.1600-051X.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Nebel A, Flachsbart F, Schafer A, Nothnagel M, Nikolaus S, Mokhtari NE, et al. Role of the toll-like receptor 4 polymorphism Asp299Gly in longevity and myocardial infarction in German men. Mech Ageing Dev. 2007;128:409–411. doi: 10.1016/j.mad.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Awomoyi AA, Rallabhandi P, Pollin TI, Lorenz E, Sztein MB, Boukhvalova MS, et al. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol. 2007;179:3171–3177. doi: 10.4049/jimmunol.179.5.3171. [DOI] [PubMed] [Google Scholar]
- Rezazadeh M, Hajilooi M, Rafiei A, Haidari M, Nikoopour E, Kerammat F, et al. TLR4 polymorphism in Iranian patients with brucellosis. J Infect. 2006;53:206–210. doi: 10.1016/j.jinf.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Okayama N, Fujimura K, Suehiro Y, Hamanaka Y, Fujiwara M, Matsubara T, et al. Simple genotype analysis of the Asp299Gly polymorphism of the Toll-like receptor-4 gene that is associated with lipopolysaccharide hyporesponsiveness. J Clin Lab Anal. 2002;16:56–58. doi: 10.1002/jcla.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Hwang YJ, Kim SY, Yang SM, Lee KY, Park Ie B. Rarity of TLR4 Asp299Gly and Thr399Ile polymorphisms in the Korean population. Yonsei Med J. 2008;49:58–62. doi: 10.3349/ymj.2008.49.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmaagacli AH, Koldehoff M, Hindahl H, Steckel NK, Trenschel R, Peceny R, et al. Mutations in innate immune system NOD2/CARD 15 and TLR-4 (Thr399Ile) genes influence the risk for severe acute graft-versus-host disease in patients who underwent an allogeneic transplantation. Transplantation. 2006;81:247–254. doi: 10.1097/01.tp.0000188671.94646.16. [DOI] [PubMed] [Google Scholar]
- Imado T, Iwasaki T, Kitano S, Satake A, Kuroiwa T, Tsunemi S, et al. The protective role of host Toll-like receptor-4 in acute graft-versus-host disease. Transplantation. 2010;90:1063–1070. doi: 10.1097/TP.0b013e3181f86947. [DOI] [PubMed] [Google Scholar]
- Lorenz E, Schwartz DA, Martin PJ, Gooley T, Lin MT, Chien JW, et al. Association of TLR4 mutations and the risk for acute GVHD after HLA-matched-sibling hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:384–387. doi: 10.1053/bbmt.2001.v7.pm11529488. [DOI] [PubMed] [Google Scholar]
- Calcaterra C, Sfondrini L, Rossini A, Sommariva M, Rumio C, Menard S, et al. Critical role of TLR9 in acute graft-versus-host disease. J Immunol. 2008;181:6132–6139. doi: 10.4049/jimmunol.181.9.6132. [DOI] [PubMed] [Google Scholar]
- Yaa MN, Paolo P, Peter S, Eric PG, Jaya S, Papanicolaou GA. Toll-like receptor 4 polymorphisms and risk of Gram-negative bacteremia after allogeneic stem cell transplantation. A prospective pilot study. Biol Blood Marrow Transplant. 2009;15:1130–1133. doi: 10.1016/j.bbmt.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr, Crawford JM, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- Asai O, Longo DL, Tian ZG, Hornung RL, Taub DD, Ruscetti FW, et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998;101:1835–1842. doi: 10.1172/JCI1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson JS, Jennings CD, Lowery DM, Carlson SL, Pflugh DL, Caywood BE, et al. Rejection of an MHC class II negative tumor following induction of murine syngeneic graft-versus-host disease. Bone Marrow Transplant. 1999;23:363–372. doi: 10.1038/sj.bmt.1701557. [DOI] [PubMed] [Google Scholar]
- Xu S, Liu X, Bao Y, Zhu X, Han C, Zhang P, et al. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps–SHP-2 pathway. Nat Immunol. 2012;13:551–559. doi: 10.1038/ni.2283. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P, et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat Immunol. 2011;12:416–424. doi: 10.1038/ni.2015. [DOI] [PubMed] [Google Scholar]
- Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol Sci. 2010;11:789–806. doi: 10.3390/ijms11030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrup F, Vestweber D, Borges E, Lhning M, Bruer R, Herz U, et al. P-and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105:1289–1298. doi: 10.1172/JCI7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Tawara I, Toubai T, Reddy P. Pathophysiology of acute graft-versus-host disease: recent advances. Transl Res. 2007;150:197–214. doi: 10.1016/j.trsl.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawara I, Maeda Y, Sun Y, Lowler KP, Liu C, Toubai T, et al. Combined Th2 cytokine deficiency in donor T cells aggravates experimental acute graft versus host disease. Exp Hematol. 2008;36:988–996. doi: 10.1016/j.exphem.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brok HP, Heidt PJ, van der Meide PH, Zurcher C, Vossen JM. Interferon-gamma prevents graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Immunol. 1993;151:6451–6459. [PubMed] [Google Scholar]
- Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102:2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy WJ, Welniak LA, Taub DD, Wiltrout RH, Taylor PA, Vallera DA, et al. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102:1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]