Abstract
B cells are generally considered to be positive regulators of the immune response because of their capability to produce antibodies, including autoantibodies. The production of antibodies facilitates optimal CD4+ T-cell activation because B cells serve as antigen-presenting cells and exert other modulatory functions in immune responses. However, certain B cells can also negatively regulate the immune response by producing regulatory cytokines and directly interacting with pathogenic T cells via cell-to-cell contact. These types of B cells are defined as regulatory B (Breg) cells. The regulatory function of Breg cells has been demonstrated in mouse models of inflammation, cancer, transplantation, and particularly in autoimmunity. In this review, we focus on the recent advances that lead to the understanding of the development and function of Breg cells and the implications of B cells in human autoimmune diseases.
Keywords: autoimmune disease, interleukin-10, regulatory B cells
Introduction
B-cell development in the bone marrow is a dynamic and complex process involving a delicate balance between cell proliferation and apoptotic selection. This balance results in the generation of functional B cells that are responsible for eliciting humoral immunity.1,2,3 The concept that suppressor B cells could regulate the immune response originated in 1974, when the ability of B cells to suppress delayed-type hypersensitivity responses in guinea pigs was described.4,5 However, the term ‘regulatory B cells', which defines B-cell subsets with regulatory properties, was first introduced by Mizoguchi and Bhan nearly 30 years later.6 Similar to regulatory T (Treg) cells, the regulatory function of B cells is exerted via the production of regulatory cytokines, such as IL-10 and TGF-β, and the ability to express inhibitory molecules that suppress pathogenic T cells and autoreactive B cells in a cell-to-cell contact-dependent manner.7 Until recently, the exact origin and molecular identity of regulatory B (Breg) cells remained elusive. Accumulating evidence suggests that the Breg cell population is heterogeneous, meaning that this population can be derived from all B cells under the correct stimulatory context and time.8 It has been postulated that Breg cells can exert their suppressive functions with different mechanisms in various mouse models of disease, including inflammation, cancer and autoimmunity.9 Moreover, dynamic changes in Breg cells have been associated with the progression of human autoimmune diseases.10,11 Here, we review the recent literature studying both the phenotypic and functional characterization of Breg cells and the implications B cells have on the pathogenesis of autoimmune diseases.
Identification of Breg cells
Despite the observations made in the 1970s that B cells with suppressive functions possibly existed, the potential role of B cells with regulatory functions in inflammatory and autoimmune diseases has only been recently appreciated. Janeway and colleagues first observed that B10.PL mice lacking B cells suffered an unusually severe and chronic form of experimental autoimmune encephalomyelitis (EAE), indicating that B cells have regulatory properties in a mouse model of EAE.12 Subsequently, it was found that B cells affected this autoimmune disease by regulating IL-10.13 Mizoguchi and Bhan were the first to introduce the term ‘regulatory B cells' to describe these B-cell subsets with regulatory properties.6 While studying the putative pathogenic role of B cells in the development of colitis, the authors unexpectedly observed that T cell receptor alpha (TCRα)−/− mice that were crossed with B cell-deficient mice spontaneously developed an earlier onset of colitis that was more severe compared to TCRα−/− mice.14 Moreover, Mizoguchi et al. further demonstrated that a certain B-cell subset from gut-associated lymphoid tissues in a chronic inflammatory environment secreted IL-10, upregulated expression of CD1d and suppressed the progression of intestinal inflammation by downregulating inflammatory cascades associated with IL-1 and signal transducer and activator of transcription 3 (STAT3) activation.15
Early studies have revealed that B-1 cells in the peritoneal cavity are a major source of B cell-derived IL-10.16 Upon IL-12 stimulation, B1a cells, but not B-1b cells, have the ability to produce IL-10.17 Recently, the production of Th2 cytokines by conventional B2 cells has been extensively investigated. The marginal-zone (MZ) B cells have been shown to regulate immunity by producing IL-10 in response to CpG stimulation in a mouse model of lupus.18 Moreover, splenic transitional 2-MZ precursor (T2-MZP) B cells that express high amounts of CD21, CD23, CD24, IgM and CD1d from both naive and arthritic mice are capable of producing IL-10. Remarkably, the regulatory function of T2-MZP cells depends on IL-10 production because T2-MZP cells from IL-10−/− DBA mice show no protection against the development of arthritis.19 Tedder and colleagues have identified a subset of IL-10-producing B10 cells that contain the unique phenotype CD1dhiCD5+. These cells share certain phenotypic markers with B-1a, MZ B and T2-MZP cells.20 B10 cells normally represent 1%–2% of splenocytes in wild-type mice and approximately 10% in hCD19 transgenic mice. Notably, IL-10 production has been found to be restricted to this B10 cell subset. Interestingly, IL-10 production was decreased in CD19−/− mice but increased in hCD19 transgenic mice. Rafei et al. have reported a Breg cell subset induced by a granulocyte-macrophage colony-stimulating factor–IL-15 fusion protein known as GIFT15.21 These GIFT15-induced Breg cells possess a phenotype akin to B10 and T2-MZP Breg cells. A study by Ding et al. revealed that T-cell Ig domain and mucin domain protein 1 (TIM-1) is expressed by a large majority of IL-10-producing Breg B cells, which consist of a heterogeneous population including transitional B, MZ B, FO B and CD1dhiCD5+ B10 cells. TIM-1+ B cells express IL-4 and IL-10, promote Th2 response and directly transfer allograft tolerance.22 Recently, Qian et al. have reported that regulatory dendritic cells can program splenic T1, T2, MZ and B1 cells to differentiate into a distinct regulatory B-cell subset with the phenotype CD19hiFcγIIbhi. This B-cell subset exerts potent regulatory functions, such as the secretion of IL-10 both in vitro and in vivo.23
Aside from mouse Breg cells, the existence of human Breg cells has recently been revealed. A number of studies have reported that certain B cells can produce IL-10.24,25 Remarkable progress in identifying the phenotype of human Breg cells has been achieved by the group led by Mauri. In an elegant study, Mauri and colleagues demonstrated that CD19+CD24hiCD38hi B cells, which are cells that have a phenotype that has been previously associated with immature B cells, comprise the highest fraction of IL-10-producing B cells upon CD40 stimulation in human peripheral blood from healthy individuals.10 Separately, Tedder and colleagues also characterized human Breg cells with the phenotype CD24hiCD27+, which is a phenotype related to memory B cells.11
Although great progress has been made in the characterization of Breg cells, the cell surface markers and/or specific transcription factor(s) that are unique to Breg cells have not been defined in mice and humans. Most of the currently identified Breg cells exert their suppressive function at least partially through the production of regulatory cytokines, such as IL-10 and TGF-β. This production of regulatory cytokines has been demonstrated by both in vitro functional assays and in vivo mouse studies.
Breg cells in autoimmune diseases
The regulatory functions of Breg cells have been extensively characterized in various animal models of inflammation, cancer and autoimmune diseases. B cells are generally considered to play a pathogenic role in the development of autoimmune diseases because B cells produce autoantibodies that cause target tissue damage.26 However, autoantibodies can also exert a protective effect via the clearance of apoptotic cells and reduction of autoantigen load.27 Moreover, B cells also act as antigen-presenting cells, which are cells that contribute to the activation and amplification of naive, activated and autoreactive T-cell responses.28,29,30 It has been reported that antigens presented by resting B cells can induce the differentiation of tolerogenic CD4+ T cells.31,32 Furthermore, B cells, similar to T cells, can be defined as B effector 1 and 2 cells. B effector 1 cells produce Th1-associated pro-inflammatory cytokines, including tumor-necrosis factor (TNF)-α, IFN-γ and IL-12, whereas B effector 2 cells produce Th2-associated cytokines, including IL-4 and IL-13.33 Notably, certain regulatory B cells that produce IL-10 or TGF-β have recently been shown to possess inhibitory functions in autoimmune diseases.6 Thus, current studies on the functional implications of Breg cells in the pathogenesis of autoimmune diseases can facilitate the development of combined therapies for autoimmune diseases. In the following sections, the role of Breg cells in mouse models of various autoimmune diseases, including rheumatoid arthritis, autoimmune diabetes, autoimmune encephalomyelitis and lupus, will be discussed.
Breg cells in experimental arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory disease that is characterized by inflammation in the synovium. This inflammation is associated with the infiltration of activated T cells, B cells and macrophages, as well as the progressive destruction of cartilage and bone structures, which eventually leads to joint destruction and deformity.34 RA is a common systemic autoimmune disease that has a prevalence of approximately 0.5%–1% in the adult population.35 An animal model of human RA exists whereby collagen-induced arthritis (CIA) is induced in a susceptible strain of DBA/1J mice that are immunized with heterologous type II collagen emulsified in complete Freund's adjuvant.36 CIA is characterized by severe swelling of the paws, extensive synovial hyperplasia, cartilage damage, bone erosion and joint ankylosis.36,37,38,39 Collagen immunization induces chronic inflammatory arthritis, which is the result of infiltration of CD4+ T cells into the synovial membrane and the production of collagen-specific IgG autoantibodies by B cells.40 Many types of immune cells, including natural killer cells, have been shown to possess a regulatory function in the development of autoimmune arthritis.41 In CIA mice, B-cell depletion using the CD20 monoclonal antibody (mAb) significantly ameliorates disease severity.42 Interestingly, B-cell depletion before collagen immunization delays disease onset and autoantibody production and markedly diminishes the severity of arthritis as indicated by both clinical symptoms and histological changes in joint tissue. However, B-cell depletion after collagen immunization does not show any significant effect on arthritis progression or disease severity. These observations suggest that B cells may play a more prominent regulatory role during the initiation of disease. In CIA mice, IL-10-producing B-cell subsets with varying phenotypes and origins have also been identified during arthritis development. Mauri et al. have performed a comprehensive study that examines whether adoptive transfer of activated B cells from arthritic mice has an inhibitory effect on CIA.43 Mauri and colleagues found that the in vitro activation of splenic arthritogenic B cells with collagen and a CD40 mAb resulted in IL-10 production. B cells that were injected intraperitoneally into recipient DBA/1-T-cell Ag receptor β-transgenic mice that were concurrently immunized with collagen significantly reduced the incidence and severity of arthritis and markedly inhibited Th1 cell differentiation. Moreover, in vitro-activated arthritogenic B cells were also effective in ameliorating the disease. Further studies have demonstrated that IL-10 is essential for the regulatory function of this subset of B cells because B cells isolated from IL-10 knockout mice failed to mediate protective functions. Consistently, B cells isolated from arthritogenic splenocytes treated with anti-IL-10/anti-IL-10R antibodies in vitro were unable to protect recipient mice from developing arthritis. When sorted MZ B, FO B or T2-MZP arthritic B cells were transferred into DBA/1J mice on the day of the second immunization, only T2-MZP B cells significantly delayed the development of arthritis, and approximately 40% of the mice that received T2-MZP cells developed arthritis. Thus, T2-MZP B cells and cells within this phenotypically defined subset can inhibit CIA progression.19 Administration of apoptotic thymocytes to mice up to 1 month before the clinical onset of CIA has also been shown to have protective effects on joint inflammation and bone destruction.44 Activated splenic B cells respond directly to apoptotic cell treatment by increasing the secretion of IL-10, which is important for inducing T cell-derived IL-10. Moreover, the passive transfer of B cells from apoptotic cell-treated mice provides significant protection against developing arthritis. These findings suggest that certain subsets of IL-10-producing B cells generated in vivo can suppress autoimmune pathogenesis. This notion is supported by our recent findings that in vitro induced B10 cells can suppress the development of arthritis and decrease joint pathology in CIA mice.45,46 Adoptive transfer of in vitro expanded B10 cells in mice on the day of the second collagen immunization resulted in a marked delay in the onset of arthritis and a reduced severity of both clinical symptoms and joint damage. This delay of onset was accompanied by a substantial reduction in the number of pathogenic IL-17-producing CD4+ T cells. Thus, Breg cells expanded in vitro can be a potential treatment for autoimmune arthritis.
Breg cells in autoimmune diabetes
Type 1 diabetes (T1D) is characterized by the destruction of insulin-producing pancreatic β cells. This destruction is primarily mediated by CD4+ and CD8+ T cells.47 In non-obese diabetic (NOD) mice, which is a spontaneous model of human T1D, the onset of diabetes is initiated at the age of 13–15 weeks, and approximately 80% of female mice and 20% of male mice develop diabetes by the age of 30 weeks.48 There is increasing evidence that B cells play a pathogenic role in the initiation of T1D. B cells are among the earliest cells to infiltrate the pancreatic islets in NOD mice, which is where they organize with T cells into lymphoid structures within germinal centers that promotes the selection of autoreactive B cells.49,50 These ectopic lymphoid structures, which consist of a central zone of T cells surrounded by B cells, begin to generate at the early stage of peri-insulitis.51 Because B cell-deficient NOD mice fail to develop diabetes, targeting B cells may be a potential approach to treating β-cell mediated autoimmune diabetes.52 In 5-week-old female NOD mice treated with CD20 mAbs for a short amount of time, approximately 95% of B cells were depleted, which subsequently led to reduced insulitis. Moreover, diabetes was prevented in more than 60% of the littermates. However, treating 15-week-old NOD female mice with a CD20 mAb substantially delays the onset of diabetes, but does not prevent the disease.53 Recent studies conducted by Grey and colleagues have shown that B-cell depletion delays and reduces diabetes by increasing the number of CD25+Foxp3+CD4+ Treg T cells, thereby enforcing long-term tolerance.54 Moreover, studies by Smith and Tedder have suggested that Breg cells, such as B10 cells, may represent a significant component of the reconstituted B-cell pool after B-cell depletion.55 These findings suggest an involvement of Breg cells in the development of diabetes.
There is compelling evidence that activated B cells can maintain immune tolerance because the transfer of activated B cells protects NOD mice from diabetes.56,57 Repeated intravenous transfer of BCR-activated B cells into 5- to 6-week-old NOD mice delays the onset and reduces the incidence of diabetes, while treatment starting at 9 weeks of age only delays diabetes onset. The therapeutic effect from transfusing activated B cells from NOD mice correlates with the polarization of CD4+ T cells toward a Th2 phenotype in recepient NOD mice.58 Notably, B cell-derived IL-10 is required for protection against T1D because the transfusion of activated NOD-IL-10−/− B cells neither confers protection against diabetes nor reduces the severity of insulitis. Tian et al. have reported that LPS-activated B cells expressed Fas ligand and secreted TGF-β.57 Transfer of the activated B cells into prediabetic NOD mice inhibited spontaneous Th1 immune responses and delayed the onset of diabetes. Cotransfer of activated B cells and diabetogenic splenic T cells into NOD/scid mice prevented the development of diabetes; however, cotransfer of control cells with diabetogenic T cells showed no protective effect. These results suggest that activated B cells may downregulate pathogenic Th1 immunity by triggering the apoptosis of Th1 cells and/or inducing the secretion of the regulatory cytokine TGF-β. Together, these findings indicate that Breg cells play an indispensable role in the initiation of T1D, but have little effect on disease progression in NOD mice.
Breg cells in EAE
Multiple sclerosis (MS) is a prototypic T cell-mediated autoimmune disease that results in the demyelination of cells in the central nervous system (CNS). This demyelination is mediated by CD4+ T cells specific for myelin oligodendrocyte glycoprotein and other autoantigens in the central nervous system.59 EAE is a mouse model of human MS. Accumulating evidence has suggested that B cells also play a pathogenic role in EAE.12,60,61 However, it has been reported that B cell-deficient mice develop a more severe and non-remitting form of EAE.12,60 Moreover, CD20+ B cell depletion after EAE development dramatically suppresses the disease symptoms.62 Although B-cell depletion ameliorates ongoing EAE, B-cell depletion occurring before EAE induction exacerbates the disease, suggesting that the Breg cells that negatively regulate inflammatory reactions are possibly depleted.63 Furthermore, the regulatory functions of B cells during EAE have been linked to the production of IL-10 because adoptive transfer of wild-type B cells, rather than IL-10−/− B cells from μMT mice, decreases the severity of EAE. B cells from recovered mice produce IL-10, which is important for attenuating pro-inflammatory Th1 responses. Importantly, in the absence of IL-10-producing B cells, mice are not able to recover from EAE.60,64 In a recent study, fusokine GIFT15-induced Breg cells are shown to secrete IL-10 and express MHC I, MHC II, and surface IgM and IgD. Moreover, mice with EAE undergo complete remission after the intravenous transfer of GIFT15-Breg cells. These cells function by suppressing neuro-inflammation.21 Therefore, IL-10-producing B cells have been identified as important regulators in controlling EAE. Recently, Tedder and colleagues characterized the overlapping and differential roles of Breg and Treg cells in shaping the course of EAE immunopathogenesis.65 Adoptively transferred B10 cells can directly influence EAE pathogenesis by producing IL-10. Interestingly, the number of B10 cells expands quickly in the spleen but not in the CNS, which is consistent with the regulatory function of B10 cells involved in disease initiation. Furthermore, transfer of antigen-sensitized B10 cells into wild-type mice dramatically reduces EAE initiation, but B10 cells could not inhibit ongoing EAE progression. However, the number of Treg cells expanded markedly within the CNS during disease progression. This expansion negatively regulated the late phase of EAE. Thus, these findings suggest that Breg cells play a predominant role in the control of disease initiation, whereas Treg cells exert their regulatory function during the late-phase of disease.
Breg cells in murine lupus
Systemic lupus erythematous (SLE) is a systemic autoimmune disease that is characterized by high autoantibody production, increased immune complex deposition, and multiple organ damage. Both T and B cells contribute to the pathogenesis of human SLE.66,67 NZB/NZW (NZB/W) F1 hybrid mice develop a spontaneous lupus-like disease, which is characterized by immune complex-mediated glomerulonephritis that is associated with IgG autoantibody production against nuclear Ags, including dsDNA, RNA, chromatin and histones.68 The prominent characteristics of NZB/W F1 mice include the expansion of B-1a and MZ B cells, activation of polyclonal B cells, and high serum levels of IgM and IgG.69,70,71,72 MRL/lpr mice also spontaneously develop a similar lupus-like disease.73 Therefore, both NZB/W F1 and MRL/lpr mouse models have been extensively used for studying human SLE. When 12- to 28-week-old NZB/W F1 mice receive treatment with a low dose of the CD20 mAb, spontaneous disease progression is significantly delayed. In contrast, B-cell depletion initiated in 4-week-old mice promotes disease onset, most likely due to the depletion of IL-10-producing Breg cells.74 It has been shown that there is significant expansion of B10 cells in young NZB/W F1 mice.65 Therefore, different B-cell populations can play either protective or pathogenic roles during disease pathogenesis because the timing of B-cell depletion has significant effects on disease progression in NZB/W F1 mice. To examine the roles of B cells in disease pathogenesis in NZB/W F1 mice, CD19−/− NZB/W mice were generated. The production of anti-nuclear Abs was remarkably delayed in CD19−/− NZB/W mice compared with wild-type NZB/W mice. However, CD19−/− NZB/W mice developed nephritis significantly earlier and had a substantially reduced survival rate. These results emphasized that B cells play both pathogenic and protective roles in lupus pathogenesis.75 B10 cells were increased in wild-type NZB/W mice during disease progression, whereas CD19−/− NZB/W mice lacked B10 cells, which is similar to the findings in a previous report.20 Moreover, transfer of splenic B10 cells from wild-type NZB/W mice into CD19−/− NZB/W recipients significantly prolonged the survival rate of NZB/W mice. This increased survival was accompanied by expansion of Treg cells, which suggests that regulatory B10 cells play a protective role in lupus pathogenesis.75 Moreover, Blair et al. have demonstrated that the transfer of in vitro anti-CD40-induced T2 Breg cells significantly improved the severity of renal disease and survival rate in MPL/lpr mice in an IL-10-dependent manner.76 Thus, both B10 cells and T2-MZP B cells can effectively protect mice from developing lupus.
Breg cells in human autoimmune diseases
Extensive studies in mice have demonstrated that Breg cells play important roles in the suppression of autoimmune diseases, but relatively little is known about human Breg cells in healthy individuals and patients. A remarkable study by Mauri and colleagues has identified a specific subset of human Breg cells with a phenotype of CD19+CD24hiCD38hi in the peripheral blood of healthy individuals.10 This phenotype has been previously associated with immature transitional B cells.77,78 However, these B cells were able to produce IL-10 in response to CD40 stimulation. However, CD19+CD24hiCD38hi B cells isolated from the peripheral blood of patients with SLE lacked the suppressive capacity possessed by their counterparts in healthy individuals. Moreover, comparisons between B cells from SLE patients and healthy controls indicate that the defect in IL-10-production in response to CD40 stimulation in B cells from SLE patients is possibly due to altered activation of STAT3.10 Interestingly, SLE patients who received rituximab treatment had an increased ratio of CD19+CD24hiCD38hi B cells to memory B cells, which supports the notion that B cell depletion may result in an increased generation of tolerogenic B cells.77,79
Tedder and colleagues have identified a subset of human B10 cells with a phenotype of CD24hiCD27+; approximately 60% of these B10 cells express CD38.11 CD27 is a well-characterized marker for human memory B cells. Moreover, CD27+ B cells can expand during autoimmune diseases and act as a biomarker for disease activity.80,81 B10 cells in the blood have a higher proliferative capacity than other B cells in response to mitogen stimulation, which indicates that these B cells have not recently emigrated from the bone marrow.11 The frequency of CD24hiCD27+ B10 cells in human blood was even higher in the autoimmune diseases including SLE, RA, autoimmune skin disease and MS. Recently, Bouaziz et al. have demonstrated that human B cells stimulated with anti-Ig and CpG produced IL-10 and enriched both CD27+ memory and CD38hi transitional B cell compartments.82 Although current findings cannot reconcile the different phenotypes of human Breg cells, it is clear that Breg cells exist in human blood and lymphoid organs. These cells have regulatory functions that are partially dependent on IL-10 production.
Similar to findings in animal models, the relative contribution of different immune components to the pathogenesis of human autoimmune disease differs from one disease to another. B cells may play either a crucial role in the initiation of the disease or contribute to autoimmune pathogenesis after disease onset.40 Recently, B-cell depletion strategies have been used to treat patients with autoimmune diseases. In RA patients, B-cell depletion using rituximab significantly diminished ongoing joint inflammation, but the recrudescence of disease activity was often accompanied by B-cell recovery.83,84,85 In human MS, B-cell depletion appears to be more effective after the onset of disease. B-cell depletion after the onset of the symptoms can ameliorate disease progression.86 Clinical studies have shown that B-cell depletion with rituximab improves the clinical manifestations of SLE.87 These clinical studies have suggested that B-cell depletion is an effective therapy for treating autoimmune diseases. However, B-cell depletion may exacerbate disease in some autoimmune conditions. For example, B-cell depletion has been shown to exacerbate ulcerative colitis and trigger psoriasis, which are both Th1-mediated autoimmune conditions.88,89 These intriguing findings may indicate the existence of Breg cells that modulate T cell-mediated inflammatory responses in vivo.
Taken together, B cells, autoantibodies and T cells are all involved in the development of autoimmune diseases and have unique functions in each autoimmune disease. B cells not only produce autoantibodies and act as antigen-presenting cells for CD4+ T-cell activation, but also serve as negative regulators that dampen harmful immune responses. Therefore, the time window for depleting B cells or transferring Breg cells is important because the changes in immunological balance may result in either exacerbation or amelioration of disease progression.
Micro-environmental signaling in modulating Breg cell generation
No common surface markers or specific transcription factor(s) have been identified yet that define both human and mouse Breg cells. Moreover, Breg cells can be generated with the appropriate stimulation both in vitro and in vivo, which reinforces the notion that factors present in the microenvironment may play a crucial role in the induction of Breg cells.8 Certain TLR agonists have been demonstrated to be potent inducers of B cells with suppressive functions. LPS from Gram-negative bacteria and CpG-containing oligonucleotides that mimic bacterial DNA have been shown to induce IL-10-producing B cells and inhibit disease progression in a mouse model of EAE.90 Mice containing B-cell deletions of Tlr2, Tlr4 or the TLR adaptor myeloid differentiation primary-response gene 88 (MyD88) could not recover from EAE, suggesting that TLRs are directly involved in modulating the regulatory function of B cells.90
TLR-signaling has been shown to initiate IL-10 production in naive B cells. However, B cells also require CD40 and BCR ligation to enable further IL-10-production.60 Accumulating data support a two-step model for the establishment of B-cell-mediated suppression. During the initial stage, TLR stimulation induces only a few IL-10-producing B cells. During the second phase, BCR and CD40 ligation, which are classically involved in B-cell survival and expansion, further amplifies the population of IL-10-producing B cells, which results in sufficient IL-10 production for effective suppression.90 Yanaba et al. have demonstrated that splenic B cells treated with LPS, PMA and ionomycin in vitro for 5 h results in optimal IL-10 production.91 Moreover, LPS or LPS plus CD40 stimulation for 48 h induces additional splenic CD1dhiCD5+ B10 cells to express IL-10 following PMA plus ionomycin stimulation. Human B cells express TLR9, which is a receptor for CpG, but not TLR4. Bouaziz et al. have shown that TLR9 is a potent inducer of IL-10 production. Moreover, the optimal stimulus for human B cells found in the blood is the combination of CpG and anti-Ig, which can act synergistically to induce human B cells to produce IL-10.82 Interestingly, CpG and anti-Ig stimuli can effectively induce memory B cells (CD27+), CD5+ B cells and immature transitional B cells (CD38hiCD24hi) to produce IL-10.10,11,92 These findings suggest that TLR signaling plays an important role in the induction of Breg cells.
CD40 engagement has been found to be required for the suppression of both EAE and CIA, suggesting that an interaction between B cells and CD40L-expressing CD4+ T helper cells is necessary for B cell-mediated suppression.43,60,76 Mauri et al. have demonstrated that stimulation of splenocytes from CIA mice during remission with anti-CD40 mAb induces the differentiation of IL-10-producing B cells. Furthermore, B cells from normal mice that have recovered from EAE produced IL-10 upon the ligation of CD40.43 In contrast, mice with chimeric bone marrow that contained B cells lacking CD40 expression failed to recover from EAE. However, the transfer of B cells from mice that had recovered from EAE into these chimeric mice compensated for their inability to recover from EAE.60 In humans, B cells from the blood that were treated with CD40L effectively induced CD4hiCD25+Foxp3+ Treg cells, which suppress CD4+CD25− T cells.93 Blair et al. have reported that CD40L stimulation induces CD19+CD24hiCD38hi B-cell expansion and suppresses Th1 cell differentiation. However, CD19+CD24hiCD38hi B cells from the peripheral blood of SLE patients were insensitive to stimulation by CD40L and produced a reduced amount of IL-10. Consequently, these B cells were unable to suppress CD4+CD25− T cells from healthy donors.10 These results suggest that anti-CD40 ligation is critically involved in Breg cell activation.
B-cell activating factor (BAFF), which is a member of the TNF family, acts as a key regulator of B-cell maturation and survival. Analyses of BAFF-deficient mice reveal a fundamental role of BAFF in promoting the maturation of T1 B cells to T2 B cells.94 In addition to the crucial role BAFF plays in the maintenance of the peripheral B cell pool, BAFF has been found to be essential for MZ B-cell development.95 Moreover, new evidence from BAFF-transgenic mice indicates that BAFF induces CD4+Foxp3+ T cells to suppress T-cell responses in an indirect, B cell-dependent manner, which suggests a regulatory role of BAFF in vivo.96 Recently, we have shown that low dosages of BAFF can induce B cells of the phenotype CD1dhiCD5+ to induce IL-10, which is similar to B10 cells. BAFF stimulation can selectively induce the expansion of IL-10-producing B cells after 3-days in culture. Moreover, BAFF treatment in vivo increased the number of IL-10-producing B cells in the marginal zone regions.46 These findings reveal a previously unappreciated function of BAFF, which is to induce B cells with regulatory function.
In addition, other signals have been reported to be important in the generation of Breg cells. For example, apoptotic cells (ACs) have been shown to act as endogenous signals that trigger IL-10 production, leading to the amelioration of CIA.97 ACs are able to induce splenic B cells to secrete IL-10, which further enhances antigen-specific T cells to secrete IL-10 and exert immunosuppressive functions. Moreover, ACs can preferentially induce MZ B cells, rather than FO B cells, to secrete IL-10, which is most likely related to the fact that MZ B cells reside on the border between the red and white pulp in the spleen.98 Interestingly, studies by Rafei et al. have shown that GIFT15 can induce Breg cells to have a phenotype that is a hybrid between CD1dhiCD5+ B10 and T2-MZP Breg cells and plasma cells expressing CD138.21 Recently, Qian et al. have reported that regulatory dendritic cells (DCs) can induce splenic B cells to differentiate into IL-10-producing B cells that have the phenotype CD19hiFcγIIbhi through IFN-β and CD40L.23 These results suggest that Breg cells can be generated in the appropriate temporal and spatial microenvironment. Future studies identifying more signals involved in the differentiation of B cells into Breg cells are anticipated.
Mechanisms underlying Breg cell function
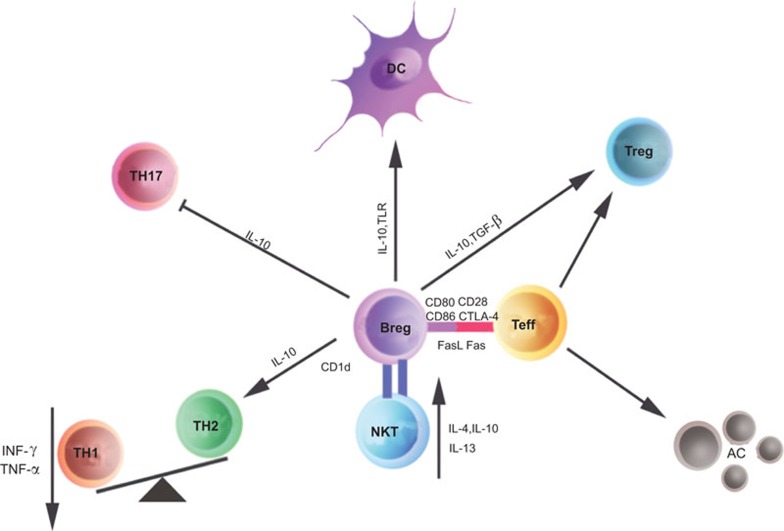
There are several direct and indirect mechanisms by which Breg cells exert their regulatory functions during the immune response (Figure 1). In mice, there are currently two well-characterized subsets of IL-10-producing B cells. The first is a B10 cell subset with the phenotype CD19hiCD1dhiCD5+, and the second is a T2-MZP cell subset with the phenotype CD19+CD23+CD21+CD1dhi.20,99 Although the frequency of naturally existing IL-10-producing regulatory B cells is extremely low, Breg cells can be expanded in vitro. This expansion allows for the enrichment of Breg cells and permits a more comprehensive study of the mechanisms by which Breg cells mediate immune suppression. These two Breg subsets produce IL-10 and suppress both the proliferation of T cells and cytokine production (IFN-γ and TNF-α) by Th1 cells.20,46,99 Moreover, transfer of a relatively low number of in vitro expanded Breg cells maintains long-term protection against several autoimmune diseases in animal models, which suggests that Breg cells can either further proliferate in vivo or initiate an efficient immunosuppressive cascade with other immune suppressive cells.100 Breg cells can not only suppress Th1-mediated immune responses but also convert effector T cells into regulatory Tr1 cells.76,97,101 Gray et al. have clearly shown that ACs induce B and T cells to produce IL-10. Moreover, B cell-derived IL-10 has been shown to be essential in the induction of T cells to secrete IL-10 in vitro.97 Furthermore, Mauri et al. have observed that there is a longer contact time between CD4+CD25− T cells and IL-10-producing B cells than IL-10-deficient B cells. This longer contact time enables IL-10+ B cells to convert effector T cells into Tr1 cells, which is mediated by IL-10 that is produced by B cells.101 B cells can also promote DCs to not only secrete IL-4 but also downregulate IL-12, which affects the Th1/Th2 balance.102 In addition to IL-10-producing Breg cells, TGF-β1-producing Breg cells have been identified in response to LPS stimulation in vitro.103,104 These B cells can trigger pathogenic Th1 cells to undergo apoptosis through Fas–FasL interactions and/or the inhibition of antigen-presenting cell activity via the secretion of TGF-β1.103
Figure 1.
Mechanisms of action for Breg cells in immune responses. The possible mechanisms by which Breg cells modulate immune responses may include the following: Breg cells restore the Th1/Th2 balance by producing IL-10; Breg cells inhibit Th1 and Th17 cell differentiation, but promote Treg cell expansion. These effects are mediated not only through the release of soluble factors such as IL-10 and TGF-β, but also via cell-to-cell contact involving CD80, CD86 and FasL, etc. Interactions between Breg and Teffs) can result in the induction of ACs as well as the induction of both Foxp3+ Treg cells and IL-10-producing Tr1 cells. Breg cells can dampen the activation of DCs and macrophages. Moreover, Breg cells express CD1d, which may activate iNKT cells with regulatory functions. AC, apoptotic cell; Breg, regulatory B; DC, dendritic cell; iNKT, invariant natural killer T; Teff, effector T cell; TNF, tumor-necrosis factor; Treg, regulatory T.
In addition to regulating the Th1/Th2 balance, Breg cells have been shown to affect the balance between Foxp3+ and IL-17–producing T cells.101,105 Worm-induced Breg cells have been shown to suppress allergic airway inflammation by promoting pulmonary infiltration of CD4+CD25+Foxp3+ Treg cells, which is a IL-10-dependent but TGF-β-independent mechanism.105,106 Carter et al. have elegantly demonstrated that endogenous IL-10-producing B cell-deficient mice develop an exacerbated case of arthritis and exhibit an increased frequency of Th1/Th17 pro-inflammatory cells, but a decreased frequency of Treg cells.101,107 Consistent with these findings, we have also demonstrated that B10 cells induced in vitro can suppress Th17 cell differentiation by decreasing the phosphorylation levels of Stat3, which subsequently reduces the levels of RORγt, and partially inhibits the Th17 cell population in an IL-10-dependent manner.45 Based on these current findings, it is likely that Breg cells play an important role in T-cell plasticity.
Apart from cytokine-mediated suppression, B cells can also exert their regulatory effects by cellular interactions. Both B10 and T2-MZP Breg cells share the phenotype CD1dhi, which is a MZ B cell marker. CD1d-expressing MZ B cells have been shown to activate invariant natural killer T (iNKT) cells in the presence of DCs and aid in the establishment of peripheral tolerance by the induction of Tr1 cells, which is a process that is dependent on the activation of iNKT cells via CD1d.108,109 Moreover, CD1dhi MZ B cells are capable of presenting glycolipids through CD1d. These glycolipids are recognized by NKT cells, which are cells that have been shown to play important roles in autoimmune development. Interestingly, EAE is exacerbated in CD1d−/− mice, which lack NKT cells.110 Recently, human transitional B cells (CD19+CD24hiCD38hi) have been shown to play an essential role in iNKT cell expansion and activation in healthy individuals, but not in SLE patients because transitional B cells from SLE patients have defects in CD1d recycling.111 Thus, CD1d-expressing Breg cells can exert their regulatory functions by activating NKT cells.
The mechanisms for regulating the immune response are mediated by either the release of suppressive soluble cytokines, including IL-10 and TGF-β, by regulatory cells or promotion of activation-induced cell death (or apoptosis), which is mediated by death-inducing ligands, including FasL, TNF-related apoptosis-inducing ligand, and programmed death ligands 1 and 2 (PD-L1 and PD-L2), etc.112,113,114 B cells can express FasL and other death-inducing ligands under many circumstances. Both FasL and IL-10 are highly expressed in the CD5+ B-cell population, which indicates that CD5+ B cells may exert regulatory effects by their killing ability.7 Interestingly, a recent study by Ray et al. has suggested that B cells can induce the proliferation of Treg cells in the CNS during the development of EAE via the expression of glucocorticoid-induced TNF receptor ligand rather than IL-10.115 In addition, costimulatory molecules are also involved in Breg-mediated suppression. The synergistic effects of IL-10, CD80 and CD86 interactions have been demonstrated in both mouse and humans.10,116 Thus, signaling through CD80 and CD86 is an additional effector mechanism for immune suppression.
In summary, Breg cells can exert their suppressive effects by secreting anti-inflammatory cytokines, such as IL-10 and TGF-β, and engaging in cell-to-cell contact via activating cell death markers or costimulatory molecules. Moreover, Breg cells can not only regulate the balance of T helper cells, but also induce tolerogenic DC or invariant NKT cells to further influence T helper cell plasticity.
Concluding remarks: Breg cells from bench to bedside
The pathogenic roles of B cells in autoimmune diseases have been extensively characterized. These roles have been further confirmed by the efficacy of B-cell depletion in treating autoimmune diseases in both humans and mice. Although B cell-targeted therapies are very promising, long-term B-cell depletion may lead to the development of immunopathology. CD20 is expressed on B cells ranging from the pre-B to mature stages, but not on plasma cells, which suggests that long-lived plasma cells that produce autoantibodies may not be affected by anti-CD20 mAb treatment. Moreover, current approaches to target B cells cannot distinguish between pathogenic B and Breg cells. Recently, the anti-BAFF mAb has been shown to be effective in treating autoimmune diseases such as SLE. Although blocking BAFF can inhibit the survival and maturation of B cells that contribute to autoimmune pathogenesis, this approach may also reduce the number of IL-10-producing Breg cells. Because low dosages of BAFF induce the generation of Breg cells, the timing and choice of suitable antibodies for B cell depletion is critical depending on the pathogenic features of each autoimmune disease. Increasing evidence indicates that B-cell depletion results in long-term remission, which is most likely due to the expansion of Treg and Breg cells. In particular, recent studies from various mouse models have suggested that B-cell depletion leads to an increased Breg cell subset in the reconstituted B-cell population. Thus, it would be of interest to determine the effects of the adoptive transfer of Breg cells either alone or in combination with B-cell depletion for the treatment of autoimmune diseases.
The adoptive transfer of Breg cells is a potential therapeutic strategy. IL-10-producing Breg cells can continually secrete IL-10, whereas the direct administration of IL-10 has a restricted therapeutic effect due to its short half-life. There is also compelling evidence that transferred Breg cells can migrate to local inflammatory sites and reside in joint tissue for more than 3 weeks in CIA mice. Hence, Breg cells may exert regulatory functions in local sites depending on their homing capacity and the survival signals present in the local environment. However, there are also some critical questions that need to be addressed before the clinical application of Breg cells can be considered. Similar to the therapeutic application of Treg cells, one of the major challenges is the functional stability of transferred Breg cells in vivo. Thus, further characterization of the functional features of Breg cells in vivo will provide a more complete understanding of the roles Breg cells play in autoimmune pathogenesis. The knowledge gained is essential to facilitate the development of Breg cells as a potential cellular therapy for human autoimmune diseases.
Acknowledgments
The authors dedicate this review manuscript to Dr Dennis G Osmond at McGill University for his mentorship. Dr Lu is a Croucher Senior Research Fellow supported by Hong Kong Croucher Foundation. This work was supported by grants from the National Basic Research Program of China (Grant No. 2010 CB 529100) and Research Grants Council of Hong Kong. The authors apologize to those researchers whose work could not be cited due to space limitations.
References
- Osmond DG. B cell development in the bone marrow. Semin Immunol. 1990;2:173–180. [PubMed] [Google Scholar]
- Cooper MD. Exploring lymphocyte differentiation pathways. Immunol Rev. 2002;185:175–185. doi: 10.1034/j.1600-065x.2002.18515.x. [DOI] [PubMed] [Google Scholar]
- Welner RS, Pelayo R, Kincade PW. Evolving views on the genealogy of B cells. Nat Rev Immunol. 2008;8:95–106. doi: 10.1038/nri2234. [DOI] [PubMed] [Google Scholar]
- Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974;251:550–551. doi: 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- Neta R, Salvin SB. Specific suppression of delayed hypersensitivity: the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J Immunol. 1974;113:1716–1725. [PubMed] [Google Scholar]
- Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- Lundy SK. Killer B lymphocytes: the evidence and the potential. Inflamm Res. 2009;58:345–357. doi: 10.1007/s00011-009-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D, Gray M. What are regulatory B cells. Eur J Immunol. 2010;40:2677–2679. doi: 10.1002/eji.201040961. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Matsushita T, Tedder TF.B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer Ann NY Acad Sci 2010118338–57.20146707 [Google Scholar]
- Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- Spencer NF, Daynes RA. IL-12 directly stimulates expression of IL-10 by CD5+ B cells and IL-6 by both CD5+ and CD5− B cells: possible involvement in age-associated cytokine dysregulation. Int Immunol. 1997;9:745–754. doi: 10.1093/intimm/9.5.745. [DOI] [PubMed] [Google Scholar]
- Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Rafei M, Hsieh J, Zehntner S, Li M, Forner K, Birman E, et al. A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat Med. 2009;15:1038–1045. doi: 10.1038/nm.2003. [DOI] [PubMed] [Google Scholar]
- Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Qian C, Chen Y, Bai Y, Bao Y, Lu L, et al. Regulatory dendritic cells program B cells to differentiate into CD19hiFcgammaIIbhi regulatory B cells through IFN-beta and CD40L. Blood. 2012;120:581–591. doi: 10.1182/blood-2011-08-377242. [DOI] [PubMed] [Google Scholar]
- Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation. J Immunol. 2004;172:3422–3427. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Cambridge G, Abrahams VM. Do self-perpetuating B lymphocytes drive human autoimmune disease. Immunology. 1999;97:188–196. doi: 10.1046/j.1365-2567.1999.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Mizoguchi E, Sugimoto K, Kibe R, Benno Y, Mizoguchi A, et al. Regulatory role of B-1 B cells in chronic colitis. Int Immunol. 2008;20:729–737. doi: 10.1093/intimm/dxn031. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pinto D, Moreno J. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154−CD40-dependent manner. Eur J Immunol. 2005;35:1097–1105. doi: 10.1002/eji.200425732. [DOI] [PubMed] [Google Scholar]
- Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Osmond DG. Apoptosis and its modulation during B lymphopoiesis in mouse bone marrow. Immunol Rev. 2000;175:158–174. [PubMed] [Google Scholar]
- Zhang M, Srivastava G, Lu L. The pre-B cell receptor and its function during B cell development. Cell Mol Immunol. 2004;1:89–94. [PubMed] [Google Scholar]
- Zhang M, Ko KH, Lam QL, Lo CK, Srivastava G, Zheng B, et al. Expression and function of TNF family member B cell-activating factor in the development of autoimmune arthritis. Int Immunol. 2005;17:1081–1092. doi: 10.1093/intimm/dxh287. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Lo CK, Lam QL, Sun L, Wang S, Ko KH, Xu H, et al. Natural killer cell degeneration exacerbates experimental arthritis in mice via enhanced interleukin-17 production. Arthritis Rheum. 2008;58:2700–2711. doi: 10.1002/art.23760. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, St Clair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci USA. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Deng J, Liu Y, Ko KH, Wang X, Jiao Z, et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol. 2012;180:2375–2385. doi: 10.1016/j.ajpath.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184:3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- Silveira PA, Grey ST. B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes. Trends Endocrinol Metab. 2006;17:128–135. doi: 10.1016/j.tem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Danska JS. Independent genetic regulation of T-cell and antigen-presenting cell participation in autoimmune islet inflammation. Diabetes. 1998;47:331–338. doi: 10.2337/diabetes.47.3.331. [DOI] [PubMed] [Google Scholar]
- Kendall PL, Yu G, Woodward EJ, Thomas JW. Tertiary lymphoid structures in the pancreas promote selection of B lymphocytes in autoimmune diabetes. J Immunol. 2007;178:5643–5651. doi: 10.4049/jimmunol.178.9.5643. [DOI] [PubMed] [Google Scholar]
- Henry RA, Kendall PL. CXCL13 blockade disrupts B lymphocyte organization in tertiary lymphoid structures without altering B cell receptor bias or preventing diabetes in nonobese diabetic mice. J Immunol. 2010;185:1460–1465. doi: 10.4049/jimmunol.0903710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, et al. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- Marino E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4+CD25+ T-cells control autoimmunity in the absence of B-cells. Diabetes. 2009;58:1568–1577. doi: 10.2337/db08-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SH, Tedder TF. Targeting B-cells mitigates autoimmune diabetes in NOD mice: what is plan B. Diabetes. 2009;58:1479–1481. doi: 10.2337/db09-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Delovitch TL. Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. J Immunol. 2007;179:7225–7232. doi: 10.4049/jimmunol.179.11.7225. [DOI] [PubMed] [Google Scholar]
- Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- Hussain S, Delovitch TL. Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. J Immunol. 2007;179:7225–7232. doi: 10.4049/jimmunol.179.11.7225. [DOI] [PubMed] [Google Scholar]
- Williams KC, Ulvestad E, Hickey WF. Immunology of multiple sclerosis. Clin Neurosci. 1994;2:229–245. [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Cross AH, Trotter JL, Lyons J. B cells and antibodies in CNS demyelinating disease. J Neuroimmunol. 2001;112:1–14. doi: 10.1016/s0165-5728(00)00409-4. [DOI] [PubMed] [Google Scholar]
- Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Mann MK, Basu S, Dittel BN. A case for regulatory B cells in controlling the severity of autoimmune-mediated inflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neuroimmunol. 2011;230:1–9. doi: 10.1016/j.jneuroim.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2:764–766. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- Grammer AC, Slota R, Fischer R, Gur H, Girschick H, Yarboro C, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154–CD40 interactions. J Clin Invest. 2003;112:1506–1520. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountz J. Animal models of systemic lupus erythematosus and Sjogren's syndrome. Curr Opin Rheumatol. 1990;2:740–748. doi: 10.1097/00002281-199002050-00010. [DOI] [PubMed] [Google Scholar]
- Wither JE, Roy V, Brennan LA. Activated B cells express increased levels of costimulatory molecules in young autoimmune NZB and (NZB×NZW)F1 mice. Clin Immunol. 2000;94:51–63. doi: 10.1006/clim.1999.4806. [DOI] [PubMed] [Google Scholar]
- Schuster H, Martin T, Marcellin L, Garaud JC, Pasquali JL, Korganow AS. Expansion of marginal zone B cells is not sufficient for the development of renal disease in NZB×NZW F1 mice. Lupus. 2002;11:277–286. doi: 10.1191/0961203302lu191oa. [DOI] [PubMed] [Google Scholar]
- Atencio S, Amano H, Izui S, Kotzin BL. Separation of the New Zealand Black genetic contribution to lupus from New Zealand Black determined expansions of marginal zone B and B1a cells. J Immunol. 2004;172:4159–4166. doi: 10.4049/jimmunol.172.7.4159. [DOI] [PubMed] [Google Scholar]
- Vyse TJ, Halterman RK, Rozzo SJ, Izui S, Kotzin BL. Control of separate pathogenic autoantibody responses marks MHC gene contributions to murine lupus. Proc Natl Acad Sci USA. 1999;96:8098–8103. doi: 10.1073/pnas.96.14.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999;169:107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H, et al. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010;184:4789–4800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–4809. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, et al. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182:5982–5993. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani A, Lougaris V, Soresina A, Meini A, Zunino F, Losi CG, et al. A novel immunodeficiency characterized by the exclusive presence of transitional B cells unresponsive to CpG. Immunology. 2007;121:183–188. doi: 10.1111/j.1365-2567.2006.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56:3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–206. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, et al. IL-10 produced by activated human B cells regulates CD4+ T-cell activation in vitro. . Eur J Immunol. 2010;40:2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford) . 2001;40:205–211. doi: 10.1093/rheumatology/40.2.205. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis. 2007;13:1365–1368. doi: 10.1002/ibd.20215. [DOI] [PubMed] [Google Scholar]
- Dass S, Vital EM, Emery P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum. 2007;56:2715–2718. doi: 10.1002/art.22811. [DOI] [PubMed] [Google Scholar]
- Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie CH, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood. 2002;100:4537–4543. doi: 10.1182/blood-2002-05-1525. [DOI] [PubMed] [Google Scholar]
- Tu W, Lau YL, Zheng J, Liu Y, Chan PL, Mao H, et al. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood. 2008;112:2554–2562. doi: 10.1182/blood-2008-04-152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- Schneider P, Takatsuka H, Wilson A, Mackay F, Tardivel A, Lens S, et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194:1691–1697. doi: 10.1084/jem.194.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters S, Webster KE, Sutherland A, Gardam S, Groom J, Liuwantara D, et al. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol. 2009;182:793–801. doi: 10.4049/jimmunol.182.2.793. [DOI] [PubMed] [Google Scholar]
- Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci USA. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- Mauri C. Regulation of immunity and autoimmunity by B cells. Curr Opin Immunol. 2010;22:761–767. doi: 10.1016/j.coi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol. 2003;170:5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- Tedder TF. Matsushit, T Regulatory B cells that produce IL-10: a breath of fresh air in allergic airway disease. J Allergy Clin Immunol. 2010;125:1125–1127. doi: 10.1016/j.jaci.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–1124.e8. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Carter NA, Rosser EC, Mauri C. IL-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of Tr1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther. 2012;14:R32. doi: 10.1186/ar3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecki E, Paget C, Fontaine J, Capron M, Trottein F, Faveeuw C. Role of marginal zone B lymphocytes in invariant NKT cell activation. J Immunol. 2009;182:6105–6113. doi: 10.4049/jimmunol.0802273. [DOI] [PubMed] [Google Scholar]
- Sonoda KH, Stein-Streilein J. CD1d on antigen-transporting APC and splenic marginal zone B cells promotes NKT cell-dependent tolerance. Eur J Immunol. 2002;32:848–857. doi: 10.1002/1521-4141(200203)32:3<848::AID-IMMU848>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T. Invariant Valpha19i T cells regulate autoimmune inflammation. Nat Immunol. 2006;7:987–994. doi: 10.1038/ni1370. [DOI] [PubMed] [Google Scholar]
- Bosma A, Abdel-Gadir A, Isenberg DA, Jury EC, Mauri C. Lipid-antigen presentation by CD1d+ B cells is essential for the maintenance of invariant natural killer T cells. Immunity. 2012;36:477–490. doi: 10.1016/j.immuni.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- Alderson MR, Lynch DH. Receptors and ligands that mediate activation-induced death of T cells. Springer Semin Immunopathol. 1998;19:289–300. doi: 10.1007/BF00787226. [DOI] [PubMed] [Google Scholar]
- Anel A, Bosque A, Naval J, Pineiro A, Larrad L, Alava MA, et al. Apo2L/TRAIL and immune regulation. Front Biosci. 2007;12:2074–2084. doi: 10.2741/2212. [DOI] [PubMed] [Google Scholar]
- Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]