Abstract
Innate-like B cells (ILBs) are heterogeneous populations of unconventional B cells with innate sensing and responding properties. ILBs in mice are composed of B1 cells, marginal zone (MZ) B cells and other related B cells. ILBs maintain natural IgM levels at steady state, and after innate activation, they can rapidly acquire immune regulatory activities through the secretion of natural IgM and IL-10. Thus, ILBs constitute an important source of IL-10-producing regulatory B cells (Bregs), which have been shown to play critical roles in autoimmunity, inflammation and infection. The present review highlights the latest advances in the field of ILBs and focuses on their regulatory functions. Understanding the regulatory activities of ILBs and their underlying mechanisms could open new avenues in manipulating their functions in inflammatory, infectious and other relevant diseases.
Keywords: immune regulation, innate-like B cells, interleukin 10, natural antibody, regulatory B cells
Introduction
B cells in mammals are composed of several subsets with different functionalities. In mice, three major B subsets have been identified, namely, follicular B2 cells, B1 cells (including CD5+ B1a and CD5− B1b cells) and marginal zone (MZ) B cells. B2 cells are responsible for immune responses against thymus-dependent antigens, and with help from T cells, B2 cells undergo germinal center reactions and generate high-affinity antibodies with precise antigen specificity. In contrast, B1 and MZ B cells rapidly respond to thymus-independent antigens and produce low-affinity antibodies with broad reactivity. In addition to antibody production, B cells also actively take part in immune regulation by acting as antigen-presenting cells and secreting cytokines. These immune regulatory functions are largely restricted to B1 and MZ B cells, which share many properties with innate immune cells. In this regard, B1 and MZ B cells are also named innate-like B cells (ILBs).
The initial definition of ILBs is based on features of their B-cell receptors (BCRs), which are semi-invariant or germ-line-encoded with limited diversity.1,2 Consequently, antibodies generated from B1 cells and MZ B cells are polyreactive and autoreactive, with the capacity to recognize conserved structures across species. In addition, ILBs generally exhibit the following features: (i) they are the major source of natural antibodies at steady state; (ii) they express high levels of IgM and low levels of IgD on their surfaces and show defective BCR signaling following surface immunoglobulin crosslinking; and (iii) they are promptly activated following innate stimulation. Following triggering by TLR agonists or microbial pathogens, ILBs produce a large amount of natural antibodies (mainly IgM), providing a critical early defense against infections. Innately activated ILBs also produce IL-10, a key regulatory cytokine that plays a crucial role in downmodulating immune responses.3
In this review, we especially focus on natural IgM and IL-10, two effector molecules with regulatory activities that are produced by murine ILBs. Recent advances in the field of human ILBs are presented, including the current understanding of their regulatory functions.
ILBs and natural antibody
Natural antibodies refer to circulating serum antibodies produced at steady state, of which natural IgM occupies a special position. Natural IgM not only acts as a first line of defense against invading microbes, but also plays active roles in maintaining immune homeostasis, such as removing apoptotic cells and decreasing inflammatory responses.4
Natural IgM produced by ILBs at steady state
ILBs, particularly B1 cells, have been reported to be the major producers of natural IgM. Early transfer experiments showed that more than 80% of serum natural IgM is derived from B1 cells.5 The spleen and bone marrow have been identified as the two sites containing spontaneous IgM-secreting cells.6 In adult mice, B1 cells are mainly located in coelomic cavities, but can also be found in the spleen. A recent study indicated that the majority of spontaneous IgM-secreting cells in the spleen were B1a cells. In contrast, peritoneal B1a cells spontaneously produced minimal amounts of IgM in the absence of exogenous stimulation. B1a cells from the bone marrow also contributed to natural IgM production at steady state.7 These B1a cells expressed high levels of surface IgM and CD19 and lacked CD138 expression, thus clearly separating them from plasma cells. B lymphocyte-induced maturation protein-1 (Blimp-1) is a transcriptional repressor identified as a master regulator of plasma differentiation.8 B1a cells did not appreciably upregulate Blimp-1 and related transcriptional factor X-box binding protein 1 expression at steady state.7,9 Thus, the detailed molecular mechanisms controlling spontaneous natural IgM secretion by B1a cells are not currently understood.
Natural IgM produced by ILBs following innate activation
Following stimulation with the TLR4 agonist lipopolysaccharide (LPS) or the TLR9 agonist CpG, B1 and MZ B cells but not B2 cells rapidly produce IgM and concomitantly upregulate Blimp-1 and X-box binding protein 1.10,11,12 In vivo, following intraperitoneal injection of LPS or bacteria, peritoneal B1 cells rapidly migrate into the spleen and differentiate into IgM-secreting cells.13,14 Another study found that following intravenous infection with Streptococcus pneumoniae, both MZ and B1 cells were activated and differentiated into IgM-producing cells within 3 days.15 By neutralizing the pathogens and enhancing complement activation, newly generated natural IgM, together with the natural IgM present at steady state, play a critical role in protecting the host before an adaptive immune response is elicited. In addition, B1 cells are activated in TCRα-deficient mice maintained in a conventional facility, most likely due to microbial stimulation from the environment. Consequently, B1 cell-derived natural antibodies are required to inhibit the development of chronic colitis in these mice.16 Recently, TLR9 expression in peritoneal B1b cells has been shown to be essential for the production of self-reactive IgM, which plays a critical role in controlling Th17 development and severe autoimmunity in lupus-prone mice.17
In contrast to its unclear role at steady state, Blimp-1 is required for IgM secretion by innate-activated ILBs.18 TLR ligation is known to upregulate Blimp-1 expression.19 Murine B cells express high levels of TLR1, 2, 4, 7 and 9, and there is no substantial difference in TLR expression levels among B1, MZ and B2 cells.12 Aside from a lower proliferative response following treatment with the TLR4 agonist LPS, B2 cells show similar or even greater proliferation in response to TLR2, 7 and 9 agonists compared with B1 and MZ B cells.12 B2 cells also respond to LPS, as shown by their normal TLR proximal signaling and their upregulation of the costimulatory and activation molecules CD25, CD86 and MHC II following LPS stimulation.20 In summary, TLR expression and signaling alone is unlikely to explain the differential innate antibody responses between B1, MZ B cells and B2 cells.
Immunoglobulins are synthesized and assembled within the endoplasmic reticulum (ER), and a highly developed ER is a hallmark of antibody-secreting B cells. Resting MZ B cells contain more rough ER and ER resident chaperones and folding enzymes than B2 cells. In addition, LPS stimulation causes a more rapid increase in levels of these factors in MZ B cells, which is correlated with their accelerated kinetics of antibody secretion.10 Thus, MZ B cells are equipped for rapid antibody production following innate stimulation. B1 cells likely share similar ER properties. Recently, an ER-localized protein Mzb1 was found to be preferentially expressed in B1 and MZ B cells. Knockdown of Mzb1 by siRNA in MZ B cells resulted in decreased IgM production following LPS stimulation.21 The role Mzb1 plays in innate antibody responses is hypothesized to be through interaction with Grp94, a master chaperone for TLRs,22 and ERp57, an oxidoreductase important for glycoprotein folding.23
ILBs and regulatory B cells (Bregs)
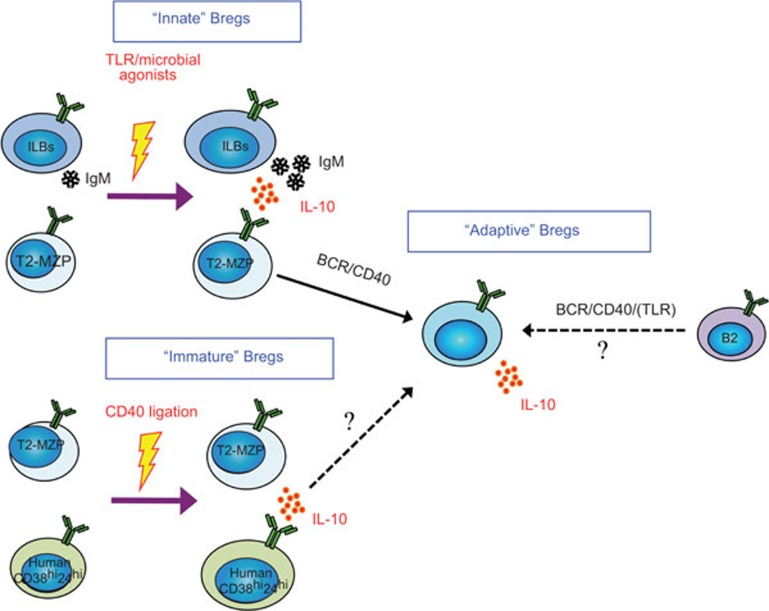
The first piece of evidence that B cells may exert suppressive activity came from early studies in guinea pigs in the mid-1970s. Transferring total but not B cell-depleted splenocytes inhibited delayed-type hypersensitivity reactions.24 The negative role of B cells in downmodulating immune responses has then been demonstrated in several murine models, mainly in autoimmune25,26,27 and inflammatory diseases.28 It was not until 2006 that the name ‘regulatory B cells' was introduced to the scientific community to delineate B-cell populations with suppressive functions.29 While the transcription factor Foxp3 is a universal marker for regulatory T cells (Tregs), there is currently no known master transcription factor that identifies Bregs. Instead, Bregs have been defined based on their functions, particularly by their capacity to produce the suppressive cytokine IL-10. Similar to Tregs, Bregs need to be activated before exerting their functions. Different activation modes can lead to the generation of different types of IL-10-producing regulatory B cells, which have been reviewed recently.30,31 Even though ‘innate type' and ‘adaptive type' Bregs have been proposed by Mizoguchi and Bhan,29 there is currently no unified model that incorporates all types of Bregs. Depending on their origins and responding properties, I propose to modify the classification of Bregs into three main categories: ‘adaptive' Bregs with antigen specificity that are generated through an adaptive immune responses via BCR and CD40 and possibly TLR signaling; ‘immature' Bregs that are developed from a population of immature B cells following direct CD40 ligation; and ‘innate' Bregs that are derived from ILBs that are activated following TLR or innate microbial stimulation (Figure 1).
Figure 1.
Breg classification and their developmental pathways. Bregs can be divided into three types: ‘Innate', ‘Immature' and ‘Adaptive'. Following stimulation with TLR or microbial agonists, murine and human ILBs are converted into ‘Innate' Bregs, which produce high amounts of IL-10 and IgM. ‘Immature' Bregs are generated from murine T2-MZP cells or human immature CD38hiCD24hi B cells following direct CD40 triggering. Both ‘Innate' and ‘Immature' Bregs are quick effector cells, secreting IL-10 within hours. In contrast, ‘Adaptive' Bregs are generated via an adaptive immune response, and the origins of ‘Adaptive' Bregs are not well defined. It is plausible that these Bregs are developed from B2 cells, but recent experimental evidence has suggested that T2-MZP cells could be an important source.31 Solid lines are supported experimentally, while dotted lines are speculative. BCR, B-cell receptor; Breg, regulatory B cell; ILB, innate-like B cell; T2-MZP, transitional type 2 marginal zone precursor.
‘Adaptive' Bregs
The evidence for the existence of IL-10-producing adaptive Bregs comes from early studies in murine autoimmune disease models. Fillatreau et al. used the murine experimental autoimmune encephalomyelitis (EAE) model and found B cells from recovered mice produced IL-10 following autoantigen restimulation. These IL-10-producing B cells were critical for the recovery of diseased mice.26 CD40 was also implicated in the protective role of these Bregs, as chimeras with selective CD40 deficiency exhibited a more severe EAE than controls.26 In a murine arthritis model using the DBA/1-TCR-β-Tg mice, Mauri et al. found that B cells from arthritic mice produced IL-10 following cognate antigen and CD40 cosignaling. These in vitro-stimulated B cells can protect recipient mice from developing arthritis.27 In both cases, signaling through both the BCR and CD40 is required for the differentiation of adaptive Bregs. Calcium signaling is a critical downstream event following BCR triggering, and a recent study demonstrated that conditional deficiency of the calcium sensors stromal interaction molecules 1 and 2 in B cells resulted in the loss of IL-10-producing adaptive Bregs and led to an exacerbation in EAE disease scores compared with wild-type mice.32 This study further emphasized that antigen recognition is the defining feature for adaptive Bregs. However, the sources of these Bregs were not clearly defined in the study.
‘Immature' Bregs
By using lupus-prone MRL/lpr mice, Mauri's group found that an agonistic anti-CD40 antibody could directly stimulate CD19+CD1dhiCD21hiCD23+ transitional type 2 marginal zone precursor (T2-MZP) B cells to produce substantial amounts of IL-10. An intracellular staining assay indicated that T2-MZP B cells were the major IL-10 producers under this experimental condition.33 In contrast to the adaptive Bregs induced in arthritic DBA/1-TCR-β-Tg mice that require both CD40 and BCR signaling,27 the induction of IL-10 in MRL/lpr B cells by the anti-CD40 antibody was independent of BCR signaling, and BCR stimulation actually decreased IL-10 production.33 Thus, it is clear that CD40 stimulation alone is sufficient to induce immature B cells to produce IL-10, and consequently, these B cells have been named ‘immature' Bregs. It will be interesting to check whether other types of immature B cells have similar IL-10-producing capacity following sole CD40 stimulation.
‘Innate' Bregs
In contrast to B2 cells, a distinct feature of ILBs (including B1,34,35 MZ B cells36 and related B cells37,38) is their rapid capacity to produce high amounts of IL-10 following innate activation. In this sense, these IL-10-producing ILBs have been classified as ‘innate' Bregs. Numerous studies have demonstrated a regulatory role for these ILB-derived Bregs in autoimmunity, inflammation and infection.
Innate Bregs and autoimmunity
Fillatreau et al. demonstrated the regulatory role of adaptive IL-10-producing Bregs in a murine EAE model26 and later found that IL-10-producing innate Bregs also participated in the negative regulation of autoimmune-mediated inflammation in this model. The innate stimulation was provided by the components of Mycobacterium tuberculosis in the complete Freund's adjuvant used to induce the disease. MyD88 and TLR signaling in B cells were required for their regulatory activities, but the phenotype of these Bregs were not explored in the study.39 In another study, the regulatory effect of Bregs in EAE was attributed to a population of MZ B cell-related CD1dhiCD5+ B cells. Interestingly, these CD1dhiCD5+ Bregs mainly played regulatory roles during the initiation phase of EAE, while Tregs were dominant in the control of immunopathogenesis during the late phase.40
Complete Freund's adjuvant is also used to generate collagen or methylated bovine serum albumin-induced arthritis in mice. In two arthritic models, T2-MZP B cells have been identified as the major IL-10-producing Bregs.37,41 These Bregs inhibit Th1 and Th17 development and promote the generation of Foxp3+ Tregs.41 B cell-activating factor (BAFF) is a member of TNF family of cytokines and plays a role in the regulation of B-cell maturation and function. BAFF levels are increased in patients with autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus. Studies have shown that BAFF can stimulate murine splenic B cells to produce IL-10, and these IL-10-producing B cells share this phenotype with MZ B cells and CD1dhiCD5+ Bregs.42 BAFF also potently expands CD1dhiCD5+ Bregs in vitro, and adoptive transfer of these Bregs ameliorates collagen-induced arthritis via suppressing the Th17 response.43
Innate Bregs and inflammation
In the early 1990s, O'Garra et al. reported that mouse peritoneal CD5+ (Ly-1+) B1a cells were the main source of IL-10 following LPS stimulation, raising the possibility of these cells having immunoregulatory activities.34 As CD5+ B1a cells constitute the first wave of murine B cell development, it would be interesting to investigate their immunoregulatory roles during the neonatal period.
CD5+ B1a cells have a different tissue distribution in neonates and adults. They account for more than 30% of splenic B cells in 7-day-old neonatal mice compared to approximately 2% in adults.35 CD5+ B1a cells are also abundant in the liver and blood, and CD5+ B1a cells represent most ILBs during the neonatal period. In the context of TLR9 agonist CpG stimulation, neonatal CD5+ B1a cells produce high amounts of IL-10, which inhibit IL-12 production by conventional dendritic cells and consequently suppresses Th1 priming in vivo.35 In following studies, other TLR agonists (targeting TLR2, 4 and 7) and certain viruses (influenza virus and baculovirus) were also found to efficiently stimulate neonatal CD5+ B1a cells to produce IL-10. Interestingly, type I interferons (IFNs) derived from activated innate cells strongly promote IL-10 production from neonatal CD5+ B1a cells. Additionally, both type I IFN signaling and IL-10 are required for neonatal CD5+ B1a cells to control the lethal inflammation induced by CpG in IL-10−/− neonatal mice.44 The regulatory role of neonatal innate Bregs was independently confirmed by another group.45 Collectively, these studies uncovered a critical role for CD5+ B1a cells in the regulation of neonatal inflammation and immunity. Neonates are highly susceptible to microbial infections, and in this context, neonatal innate Bregs could play a paradoxical role in immune regulation. On the one hand, IL-10 produced by CD5+ B1a cells can impair the ability of the neonates to generate sufficient immunity against invading pathogens; on the other hand, IL-10 could be beneficial for them to avoid overwhelmingly lethal inflammation. In addition, activated CD5+ B1a cells also secrete high amount of natural IgM, which could provide an additional way for the neonates to neutralize invading pathogens and regulate inflammatory responses.
ILBs also exhibited IL-10-dependent regulatory functions in a murine contact hypersensitivity model. Contact hypersensitivity is a cutaneous immune reaction mediated mainly by antigen-specific Th1/Tc1 cells. Splenic MZ B cells46 and related CD1dhiCD5+ B cells38 inhibited the immune reaction at the acute phase, while peritoneal CD5+ B1a cells47 played suppressive roles during the late remission phase. In both situations, Bregs exerted their regulatory roles in an IL-10-dependent manner, and specifically, the identification of CD1dhiCD5+ B cells as Bregs was based on their IL-10-producing capacity following LPS stimulation.38 However, the degree that innate signals contribute to the regulatory activities of these Bregs is not clear in this model.
Innate Bregs and infectious disease
Microbes such as parasites and bacteria can directly stimulate ILBs to produce IL-10, and these Bregs can exert a regulatory function on disease outcome. This can be either protective or deleterious, depending on the infectious model utilized.
Parasitic infections
Schistosoma mansoni helminth infection in mice is characterized by a Th1 to Th2 shift and granuloma pathology induced by schistosome eggs. Lacto-N-fucopentaose III, an oligosaccharide from schistosome eggs, can stimulate B cells to produce IL-10, and IL-10-producing B cells have been implicated in the Th1 to Th2 shift that takes place during the course of infection.48 Later, this group found that B1 cells produced vast amounts of IL-10 following Lacto-N-fucopentaose III stimulation, and B1 cells were preferentially expanded in S. mansoni-susceptible mouse strains.49 Interestingly, IL-10-producing B cells generated during S. mansoni infection suppressed Th2-mediated severe allergic reactions, namely, anaphylaxis and airway hyperinflammation.50,51 By using IL-10-EGFP reporter mice, the dominant IL-10-producing B cells in the spleen of infected mice were CD1dhi, composed of MZ and related B cells. These Bregs also promoted the infiltration of foxp3+ Tregs to the disease site, which provided an additional means to control the inflammation.51 These studies indicated that several types of Bregs could be induced during S. mansoni infection and play regulatory roles through diverse ways.
Bregs were also shown to perform important regulatory activities in murine Leishmania infection models. L. major can directly stimulate MZ or related T2-MZP/CD1dhiCD5+ B cells to produce IL-10. These Bregs downregulate IL-12 production from L. major-stimulated dendritic cells and skew the T-cell responses toward the non-protective Th2 pathway.52 Similarly, L. donovani stimulates MZ and MZ-related B cells (identified as CD1dhi) to produce IL-10 in a MyD88-depedent manner, which dampens Th1 responses in vivo.53
Bacterial infections
Thus far, only a few studies have directly addressed the role of Bregs in bacterial infections. Neves et al. found that Salmonella typhimurium can directly stimulate B cells to produce IL-10, which was dependent on MyD88 and TLR2/4. Following S. typhimurium intravenous infection, IL-10-producing B cells were detected in the spleen within 1 day. The rapidness of this response indicated that S. typhimurium activated a population of ILBs, even though their phenotype was not detailed in this study. By using chimeric mice in which B cells were selectively deficient for MyD88 or IL-10, IL-10 from innately-activated B cells was demonstrated to suppress protective immunity against S typhimurium, including both the innate (neutrophil and natural killer cell functions) and adaptive (Th1 response) arms.54 Recently, Listeria monocytogenes was found to stimulate MZ B cells to produce large amounts of IL-10 through TLR2 and 4 engagements. MZ B cell-derived IL-10 inhibited innate immunity and increased the host's susceptibility to Listeria infection.55
Viral infections
Viruses can also stimulate the generation of IL-10-producing Bregs. Polyoma virus is a highly oncogenic virus in certain susceptible mouse strains, such as PERA/Ei mice. Polyoma virus stimulates peritoneal B cells from PERA/Ei mice to produce large amounts of IL-10 in a TLR4-dependent manner. Consequently, PERA/Ei mice develop an ineffective CD8+ CTL response, which is not sufficient to control tumor development.56 In a murine cytomegalovirus infectious model, B cells were found to represent the dominant IL-10-producing leukocytes in the spleen using the IL-10-EGFP reporter. Using the mice with B cell-specific deficiency in IL-10, B cell-derived IL-10 was found to play a non-redundant role in restraining the amplitude of virus-specific CD8+ T-cell responses and downregulating plasma cell expansion.57 As type I IFNs are potently induced during viral infections and type I IFNs enhance IL-10 production by Bregs in a neonatal inflammation model,44 it is very likely that this regulatory loop also functions in viral infectious disease.
A close look at T2-MZP and CD1dhiCD5+ Bregs
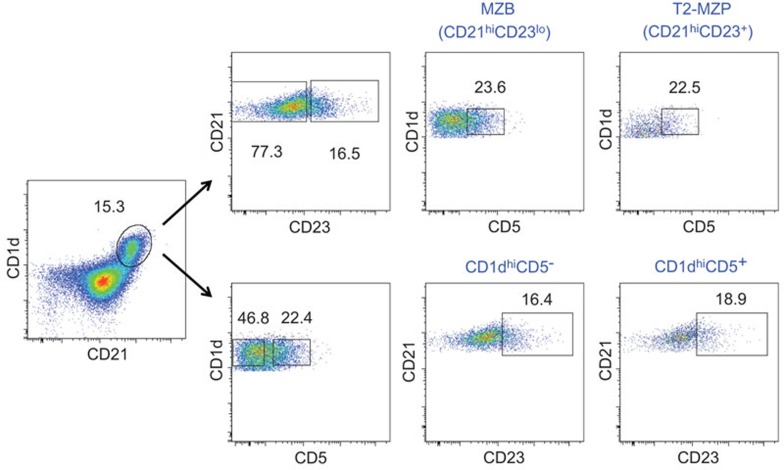
Recent studies in the field of Bregs have focused on the functions of T2-MZP and CD1dhiCD5+ B cells, led by Mauri's group31 and Tedder's group,30 respectively. Both groups emphasized the roles of the TLR, BCR and CD40 pathways in the development of Bregs. Particularly, T2-MZP B cells were shown to independently respond to the TLR, BCR and CD40 pathways.37,58 This feature may place T2-MZP B cells in a special position in terms of Breg development and activity (Figure 1). Phenotypically, both T2-MZP and CD1dhiCD5+ B cells from naive mice express high amounts of CD21 and CD1d, but the expressions of CD23 and CD5 partially overlap (Figure 2). Thus, only a small population of B cells can be simultaneously identified as both T2-MZP and CD1dhiCD5+ B cells with the phenotype of CD21hiCD23+CD1dhiCD5+. Whether this small B cell population harbors higher regulatory activity than CD21hiCD23+CD1dhiCD5− or CD1dhiCD5+CD21hiCD21− B cell subsets is not yet known.
Figure 2.
Phenotypic relationships between murine T2-MZP and CD1dhiCD5+ B cells. The CD1dhiCD21hi population was gated from CD19+ B cells from a naive C57/BL6 mouse spleen. The CD1dhiCD21hi population was then further gated on CD21 and CD23 to identify CD21hiCD23lo MZ B cells and CD21hiCD23+ T2-MZP cells, and the percentage of CD1dhiCD5+ cells was analyzed from each B-cell subset. Alternatively, a CD1dhiCD21hi population was subdivided into CD1dhiCD5+ and CD1dhiCD5− cells, and the expression of CD23 on both B subsets was analyzed. The results indicate that only a small population of B cells can be simultaneously identified as both T2-MZP and CD1dhiCD5+ B cells. MZ, marginal zone; T2-MZP, transitional type 2 marginal zone precursor.
Novel types of IL-10-producing innate B cells
In addition to conventional ILBs (B1a, B1b, MZ and related T2-MZP and CD1dhiCD5+ B cells), several groups have reported novel types of IL-10-producing B cells following innate stimulation. Hao et al. found that there was an accumulation of a population of CD19+CD43−AA4.1−CD21−CD23− B cells in aged mice. These B cells were refractory to BCR and anti-CD40 stimulations but produced IL-10 following stimulation with TLR7 or TLR9 agonists.59 In adult mice, B220−/loCD19+ B cells from the spleen constituted a distinct population of B cells that preferentially produced IL-10 following LPS stimulation.60 Qian et al. reported that regulatory dendritic cells can induce murine splenic B cells to differentiate into a distinct population of IL-10-producing CD19hiFcγIIbhi Bregs. This type of Bregs were also found to exist in vivo and were expanded following immunization.61 These studies enlarged the scope of Bregs and indicated that new types of Bregs could be generated under some specific conditions.
While this review is centered on the IL-10-producing property of Bregs, it should be noted that Bregs could exert some regulatory roles independent of IL-10, such as TGF-β-dependent effects from LPS-stimulated murine B cells.62,63 In other cases, splenic CD5+ B1a cells have been found to constitutively express FasL and induce CD4+ T-cell apoptosis. Interestingly, IL-10 can enhance the expression of FasL by B1a cells and strengthen this type of regulatory activity.64
Human ILBs and IL-10-producing Bregs
Human ILBs
While the existence of MZ B and B1 cells is firmly established in mice, the question of whether similar types of ILBs exist in humans remains unanswered. There are several obvious differences between mouse and human B cell studies. First, mice are easy to manipulate, with easy access to the different organs, while human B cell studies are mainly restricted to the peripheral blood. Second, naive laboratory mice normally contain a few memory B cells, while human memory B cells are accumulated from birth and constitute a large fraction of the B-cell pools in adults. In 2004, human blood IgM+IgD+CD27+ B cells (called ‘IgM memory B cells') were proposed to be circulating splenic MZ B cells,65 but others hypothesized that these IgM memory B cells could be real ‘memory' B cells.66 In fact, both IgM memory B cells and isotype-switched memory B cells show much greater responses to innate stimuli than naive B cells, reflecting the properties of ILBs in mice.67 In addition, human IgG+ memory B cells, and to a lesser extent IgM memory B cells, responded more robustly following BCR triggering than naïve B cells. IgG+ memory B cells also show heightened constitutive BCR signaling.68 These features may contribute to the pre-activated state and innate-responding properties of human memory B cells. It is plausible that human memory B cells could be regarded as a kind of ILBs, but whether there are specialized ILB subsets within memory B cells is currently unknown.
There are also controversies concerning the existence of human B1 cells. CD5 is a useful marker to identify B1a cells in mice, but CD5 is broadly expressed on immature and transitional B cells in humans.69 Recently, Rothstein's group reported a population of CD20+CD27+CD43+CD70− ‘human B1 cells' in both cord and adult blood, based on their properties of spontaneous IgM secretion, efficient T-cell stimulation and tonic BCR signaling.70 Later, this group further divided ‘human B1 cells' into CD11b+ and CD11b− subsets and found that the CD11b+ subset expressed high levels of CD14, a monocyte marker.71 However, several groups raised concerns about the fidelity of ‘human B1 cells' and showed that these ‘human B1 cells' could be contaminated with CD3+ T cells, isotype-switched memory B cells and (pre-)plasmablasts.72,73 Thus, further investigation is required to clarify the issue of human B1 cells.
Human IL-10-producing Bregs
Numerous studies have demonstrated that human B cells can produce IL-10 following stimulation, indicating that IL-10-producing Bregs also exist in humans. Referring to the classification of murine Bregs, current literature supports that ‘immature' and ‘innate' types of Bregs are present in humans. The ‘adaptive' type of Bregs could also exist, but scientific evidence is lacking for the time being.
Human immature Bregs
By using CD40-ligation as the stimulus, Duddy et al. found that adult blood CD27− non-memory B cells were major producers of IL-10, while coligation of BCR decreased IL-10 secretion.74 Mauri's group has previously reported that murine T2-MZP B cells produce IL-10 following CD40 stimulation.33 This group extended their study to human B cells and demonstrated that human CD19+CD38hiCD24hi immature B cells produced the highest levels compared with naive and memory B cells of IL-10 following CD40 ligation and consequently suppressed Th1 differentiation. Particularly, this type of regulatory activity by immature B cells was impaired in systemic lupus erythematosus patients.75 B cells from multiple sclerosis patients also produce much less IL-10 than those from healthy controls following CD40 ligation,74 implicating either a reduction in their numbers or compromised functionality of the immature Bregs in multiple sclerosis patients. Interestingly, multiple sclerosis patients infected with helminths generate a population of CD40-responding, IL-10-producing CD1dhi Bregs, which could be beneficial for the resolution of inflammation.76 However, immature B cell markers such as CD38 and CD24 were not used to define the phenotype of this type of CD1dhi Bregs in this study.
A potential role for immature Bregs and disease outcomes has also been reported in other diseases. Newell et al. found that after renal transplantation, tolerant patients showed significantly increased numbers of IL-10-producing immature Bregs compared to patients requiring the immunosuppressive regimen.77 IL-10-producing immature Bregs are also enriched in chronic hepatitis B virus-infected patients, and these Bregs suppress hepatitis B virus-specific CD8+ T-cell responses.78 Collectively, the above studies provide strong evidence that immature Bregs can play suppressive roles in different disease situations in an IL-10-dependent manner.
Human innate Bregs
While murine B cells express most of the TLRs, human B cells have a more restricted TLR expression profile. Human peripheral B cells mainly expressed TLR7 and 9, and higher expression of the two TLRs were found in CD27+ memory B cells than in naive B cells.79 In contrast to IL-10-producing immature Bregs stimulated by CD40 ligation, stimulation with the TLR9 agonist CpG identified another population of IL-10-producing B cells predominantly located within the CD27+ memory B-cell compartment. These innate-activated Bregs can decrease TNF-α production by LPS-stimulated monocytes in an IL-10-depdendent manner80 In addition, human CD27+ memory B cells also respond to DNA-bearing apoptotic cells by secreting IL-10, indicating that this type of Bregs could play an important role in maintaining tolerance.81 While TLR activation is the prime force driving IL-10 expression in human Bregs, costimulation through BCR82 or CD4080 could provide a synergistic effect for IL-10 secretion in CD27+ Bregs.
Conclusion and perspectives
ILBs represent an unconventional and heterogeneous population of B cells in mice. These B cells adopt different developmental pathways from conventional B2 cells and exert immune regulatory effects under physiological and pathological conditions. Particularly, ILBs constitute an important source of IL-10-producing Bregs in multiple models of autoimmunity, inflammation and infection. However, the developmental pathways of human ILBs are largely unknown, and the study of IL-10 producing human Bregs is just in its infancy. Recently, the atypical chemokine receptor D683 and T-cell Ig domain and mucin domain protein 184 were used to identify murine ILBs and IL-10-producing Bregs, respectively. Whether there are any similar markers that could characterize corresponding human B-cell subsets is an interesting question to be addressed in the future.
This review has proposed classifying Bregs into three types. However, in some cases, the boundaries are not distinct, and more studies are needed to clarify their relationships. Another important question is how innate-activated ILBs coordinate the expression of their two key effector molecules: IgM and IL-10. Is there any cross-regulation between them? Do they play synergistic or antagonistic roles in the context of infection?
Finally, given the important roles of murine Bregs in immune regulation, how can we manipulate their functions to develop novel intervention or immunization strategies? More importantly, how can we translate these findings from murine models to human applications?
Acknowledgments
This work was supported in part by grants from National Natural Science Foundation of China (31270961) and Shanghai Science and Technology Development Funds (12QA1403600). The author would like to thank Mr Yiyuan Fang for his help with figure preparation and Mr Brian Mozeleski for his critical reading.
References
- Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- Kearney JF. Innate-like B cells. Springer Semin Immun. 2005;26:377–383. doi: 10.1007/s00281-004-0184-0. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol. 2012;188:939–945. doi: 10.4049/jimmunol.1102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oudenaren A, Haaijman JJ, Benner R. Frequencies of background cytoplasmic Ig-containing cells in various lymphoid organs of athymic and euthymic mice as a function of age and immune status. Immunology. 1984;51:735–742. [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol. 2012;42:120–129. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–169. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- Tumang JR, Frances R, Yeo SG, Rothstein TL. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005;174:3173–3177. doi: 10.4049/jimmunol.174.6.3173. [DOI] [PubMed] [Google Scholar]
- Gunn KE, Brewer JW. Evidence that marginal zone B cells possess an enhanced secretory apparatus and exhibit superior secretory activity. J Immunol. 2006;177:3791–3798. doi: 10.4049/jimmunol.177.6.3791. [DOI] [PubMed] [Google Scholar]
- Fairfax KA, Corcoran LM, Pridans C, Huntington ND, Kallies A, Nutt SL, et al. Different kinetics of blimp-1 induction in B cell subsets revealed by reporter gene. J Immunol. 2007;178:4104–4111. doi: 10.4049/jimmunol.178.7.4104. [DOI] [PubMed] [Google Scholar]
- Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178:7779–7786. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tung JW, Ghosn EE, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci USA. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Mizoguchi E, Sugimoto K, Kibe R, Benno Y, Mizoguchi A, et al. Regulatory role of B-1 B cells in chronic colitis. Int Immunol. 2008;20:729–737. doi: 10.1093/intimm/dxn031. [DOI] [PubMed] [Google Scholar]
- Stoehr AD, Schoen CT, Mertes MM, Eiglmeier S, Holecska V, Lorenz AK, et al. TLR9 in peritoneal B-1b cells is essential for production of protective self-reactive IgM to control Th17 cells and severe autoimmunity. J Immunol. 2011;187:2953–2965. doi: 10.4049/jimmunol.1003340. [DOI] [PubMed] [Google Scholar]
- Savitsky D, Calame K. B-1 B lymphocytes require Blimp-1 for immunoglobulin secretion. J Exp Med. 2006;203:2305–2314. doi: 10.1084/jem.20060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calame K. Activation-dependent induction of Blimp-1. Curr Opin Immunol. 2008;20:259–264. doi: 10.1016/j.coi.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg A, Bandaranayake AD, Andrews SF, Rawlings DJ. Reduced c-myc expression levels limit follicular mature B cell cycling in response to TLR signals. J Immunol. 2009;182:4065–4075. doi: 10.4049/jimmunol.0802961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach H, Rosenbaum M, Duchniewicz M, Kim S, Zhang SL, Cahalan MD, et al. Mzb1 protein regulates calcium homeostasis, antibody secretion, and integrin activation in innate-like B cells. Immunity. 2010;33:723–735. doi: 10.1016/j.immuni.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, et al. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe H, Michalak M. ERp57, a multifunctional endoplasmic reticulum resident oxidoreductase. Int J Biochem Cell B. 2010;42:796–799. doi: 10.1016/j.biocel.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974;251:550–551. doi: 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann NY Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Fujii Y, Baba A, Hikida M, Kurosaki T, Baba Y. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity. 2011;34:703–714. doi: 10.1016/j.immuni.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity. 2005;22:467–477. doi: 10.1016/j.immuni.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie CH, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184:3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- Yang M, Deng J, Liu Y, Ko KH, Wang X, Jiao Z, et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol. 2012;180:2375–2385. doi: 10.1016/j.ajpath.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang X, Deriaud E, Jiao X, Braun D, Leclerc C, Lo-Man R. Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J Exp Med. 2007;204:1107–1118. doi: 10.1084/jem.20062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WE, Goldstein DR. Neonatal B cells suppress innate Toll-like receptor immune responses and modulate alloimmunity. J Immunol. 2007;179:1700–1710. doi: 10.4049/jimmunol.179.3.1700. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Fujimoto M, Ishiura N, Kuwano Y, Nakashima H, Yazawa N, et al. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol. 2007;171:560–570. doi: 10.2353/ajpath.2007.061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H, Hamaguchi Y, Watanabe R, Ishiura N, Kuwano Y, Okochi H, et al. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J Immunol. 2010;184:4637–4645. doi: 10.4049/jimmunol.0901719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velupillai P, Harn DA. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: a mechanism for regulation of CD4+ T-cell subsets. Proc Natl Acad Sci USA. 1994;91:18–22. doi: 10.1073/pnas.91.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velupillai P, Secor WE, Horauf AM, Harn DA. B-1 cell (CD5+B220+) outgrowth in murine schistosomiasis is genetically restricted and is largely due to activation by polylactosamine sugars. J Immunol. 1997;158:338–344. [PubMed] [Google Scholar]
- Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004;173:6346–6356. doi: 10.4049/jimmunol.173.10.6346. [DOI] [PubMed] [Google Scholar]
- Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immun. 2010;125:1114–1124.e8. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Ronet C, Hauyon-La Torre Y, Revaz-Breton M, Mastelic B, Tacchini-Cottier F, Louis J, et al. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol. 2010;184:886–894. doi: 10.4049/jimmunol.0901114. [DOI] [PubMed] [Google Scholar]
- Bankoti R, Gupta K, Levchenko A, Stager S. Marginal zone B cells regulate antigen-specific T cell responses during infection. J Immunol. 2012;188:3961–3971. doi: 10.4049/jimmunol.1102880. [DOI] [PubMed] [Google Scholar]
- Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Lee CC, Kung JT. Marginal zone B cell is a major source of IL-10 in Listeria monocytogenes susceptibility. J Immunol. 2012;189:3319–3327. doi: 10.4049/jimmunol.1201247. [DOI] [PubMed] [Google Scholar]
- Velupillai P, Garcea RL, Benjamin TL. Polyoma virus-like particles elicit polarized cytokine responses in APCs from tumor-susceptible and -resistant mice. J Immunol. 2006;176:1148–1153. doi: 10.4049/jimmunol.176.2.1148. [DOI] [PubMed] [Google Scholar]
- Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava B, Quinn WJ, 3rd, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andres B, Prado C, Palacios B, Alia M, Jagtap S, Serrano N, et al. Dynamics of the Splenic Innate-like CD19+CD45Rlo cell population from adult mice in homeostatic and activated conditions. J Immunol. 2012;189:2300–2308. doi: 10.4049/jimmunol.1200224. [DOI] [PubMed] [Google Scholar]
- Qian L, Qian C, Chen Y, Bai Y, Bao Y, Lu L, et al. Regulatory dendritic cells program B cells to differentiate into CD19hiFcgammaIIbhi regulatory B cells through IFN-beta and CD40L. Blood. 2012;120:581–591. doi: 10.1182/blood-2011-08-377242. [DOI] [PubMed] [Google Scholar]
- Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol. 2003;170:5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- Lundy SK, Boros DL. Fas ligand-expressing B-1a lymphocytes mediate CD4+-T-cell apoptosis during schistosomal infection: induction by interleukin 4 (IL-4) and IL-10. Infect Immun. 2002;70:812–819. doi: 10.1128/iai.70.2.812-819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or “memory” B cells. J Immunol. 2007;179:13–19. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182:890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- Davey AM, Pierce SK. Intrinsic differences in the initiation of B cell receptor signaling favor responses of human IgG+ memory B cells over IgM+ naive B cells. J Immunol. 2012;188:3332–3341. doi: 10.4049/jimmunol.1102322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DO, Rothstein TL. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011;208:2591–2598. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descatoire M, Weill JC, Reynaud CA, Weller S. A human equivalent of mouse B-1 cells. J Exp Med. 2011;208:2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Andres M, Grosserichter-Wagener C, Teodosio C, van Dongen JJ, Orfao A, van Zelm MC. The nature of circulating CD27+CD43+ B cells. J Exp Med. 2011;208:2565–2566; author reply 2566–2569. doi: 10.1084/jem.20112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008;64:187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–3935. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles K, Heaney J, Sibinska Z, Salter D, Savill J, Gray D, et al. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc Natl Acad Sci USA. 2012;109:887–892. doi: 10.1073/pnas.1109173109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, et al. IL-10 produced by activated human B cells regulates CD4+ T-cell activation in vitro. Eur J Immunol. 2010;40:2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- Hansell CA, Schiering C, Kinstrie R, Ford L, Bordon Y, McInnes IB, et al. Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood. 2011;117:5413–5424. doi: 10.1182/blood-2010-11-317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]