Abstract
Osteopontin (OPN), a multifunctional glycoprotein, has three transcripts that have distinct roles in tumors in vitro. Whether OPN transcripts have different functions in tumor processes in vivo is unclear. It has been reported that immune cell-derived OPN can promote tumor formation. We propose a hypothesis that tumor-derived OPN may facilitate tumor immune escape by affecting immune cell differentiation and function. In this study, we constructed lentiviral expression vectors of OPN transcripts and transfected them into the MCF-7 cell line. MCF-7 cells transfected with OPN transcripts were injected into the armpit of nude mice, and tumor growth was monitored. The results showed that all OPN transcripts promoted local tumor formation, but that there was no significant difference among transcripts. We also investigated the effect of the OPN expressed by tumor cells on monocyte differentiation by coculturing monocytes with tumor supernatant. We found OPN-c upregulated CD163 levels compared with OPN-a and OPN-b; however, none of the transcripts affected HLA-DR and CD206 levels. All OPN transcripts significantly inhibited TNF-α and enhanced IL-10 production by monocytes. Furthermore, we found that the overexpression of OPN transcripts significantly upregulated TGF-β1 and MCP-1 production by tumor cells. Using neutralizing antibody and recombinant cytokines, we found that OPN overexpressed by tumor cells regulates the production of TNF-α and IL-10 by monocytes partly via MCP-1 and TGF-β1, respectively. Collectively, our results show that OPN transcripts have no distinct role in breast cancer formation in vivo. We also demonstrate that OPN regulates the alternative activation of monocytes via TGF-β1 and MCP-1, which may represent an additional mechanism for tumor immune escape.
Keywords: alternative activation of monocytes, immune escape, OPN transcripts, MCP-1, TGF-β1
Introduction
Osteopontin (OPN), a multifunctional phosphorylated glycoprotein, has important roles in many pathophysiologic process, including bone remodeling, cancer and inflammation.1,2,3 Various types of cancers express high levels of OPN,2,4 and the overexpression of OPN in a previously benign cell line is sufficient to produce a metastatic phenotype.5,6 Knockdown of OPN with siRNA can attenuate the process of cancer in vitro and in vivo.7,8 In contrast to tumor cells, immune cells are another main source of OPN.9,10,11 It has been reported that immune cell-derived OPN is crucial for the induction of cell-mediated immune responses and thus contributes to the host anti-tumor defense.12,13 Therefore, it has been suggested that OPN may have contrary roles in host defense and tumor process.14 Furthermore, macrophage-derived OPN can restore the metastatic potential of OPN-knockdown tumor cells, which indicates that different sources of OPN may interact with each other.15 We therefore hypothesize that the OPN expressed by tumor cells may participate in tumor immune evasion through regulating the differentiation and function of immune cells.
Alternative RNA splicing of human OPN results in three transcripts: OPN-a (full type), OPN-b (with deletion of exon 5) and OPN-c (with deletion of exon 4). Differing from OPN-a and OPN-b, OPN-c is specifically expressed in breast tumors, and more effectively supports anchorage-independent growth in vitro.16 Clinical data suggest that OPN-c may be a more valuable diagnostic and prognostic marker than the conventional breast cancer markers (i.e., estrogen receptor, progesterone receptor and HER2).17 In non-small-cell lung cancer, OPN transcripts differentially regulate vascular endothelial growth factor secretion and angiogenesis.18 Recently, Tilli and colleagues demonstrated that the isoform OPN-c is specifically expressed in ovarian tumor samples and significantly activates OvCar-3 cell proliferation, migration, invasion, anchorage-independent growth and tumor formation in vivo.19 These results demonstrate that different OPN transcripts have distinct roles that may be associated with their distinct roles in host defense and tumor process. Whether OPN transcripts have different roles in breast cancer formation in vivo and whether OPN transcripts expressed by tumor cells have distinct functions on immune cell differentiation are unknown.
In this study, we investigated the function of different OPN transcripts on breast cancer formation in vivo. We found that tumor-derived OPN transcripts participated in the alternative activation of monocytes, which may represent another essential mechanism of immune evasion mediated by OPN.
Materials and methods
Reagents
RPMI-1640 medium was purchased from Hyclone (Logan, UT, USA) and Fetal Bovine Serum was from Bio International (Auckland, New Zealand). Lipopolysaccharide (LPS) was from Sigma (St Louis, MO, USA). Anti-OPN polyclonal antibody was from Abcam (Cambridge, UK). rhOPN, rhMCP-1, rhTGF-β1, blocking antibody for OPN, CD44, TGF-β1 and MCP-1 were purchased from R&D (Minneapolis, MN, USA). Fluorescently-labeled antibodies CD14, HLA-DR, CD206, CD163, CD44s, CD44v4, CD44v6, CD44v7 and αVβ3 for FACS analysis were purchased from BD PharMingen (San Diego, CA, USA).
Construction of lentiviral OPN expression vectors and infection of MCF-7 cells
The constructs for the expression of the human osteopontin splice variants were obtained by reverse transcription PCR from the malignant breast tumor cell line MDA-MB-435. The coding sequence of osteopontin was amplified with the following primers: opn-Age I-F, CAGGATCCCCGGGTACCGGTCGCCACCATGAGAATTGCAGTGATTTGC and opn- Age I-R, TCACCATGGTGGCGACCGGTACATTGACCTCAGAAGATGCACTA. The amplified products were excised with Age I and subcloned into the pGC-FU vector (Genechem, Shanghai, China). Sequence fidelity and accurate reading frames were verified by DNA sequencing analysis. Lentiviral stocks were generated by the cotransfection of constructed pGC-FU vector with pHelper 1.0 and Helper 2.0 (Genechem) packaging constructs into 293T cells using LipofectAmine 2000 (Invitrogen, Camarillo, CA, USA). Viral supernatants were collected 48 h later. MCF-7 cells (obtained from ATCC) were transfected with lentiviral expression vectors containing Opn (MOI: 100) in the presence of 5 µg/ml polybrene.
Western blot
The cells were lysed in lysis buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 5 mM iodoacetamide and 2 mM phenylmethylsulfonyl fluoride) and equal amounts of total proteins were separated on a 10% SDS–polyacrylamide gel. Proteins were blotted onto a nitrocellulose membrane, which was then blocked by incubating with TBS (Tris-buffered saline, 0.1% Tween 20) containing 5% non-fat dried milk. Subsequently, membranes were incubated with rabbit polyclonal anti-OPN (1∶1000) and washed with TBS. Antigen–antibody complexes were visualized after incubating the membrane with 1∶5000 diluted goat anti-rabbit IgG antibody coupled to horseradish peroxidase and detected by enhanced chemiluminescence.
RNA extraction and reverse transcriptase polymerase chain reaction
Total RNA was isolated with Trizol according to the manufacturer's instructions. The RNA concentration was quantified by UV spectrophotometer at 260 nm and the purity and integrity was determined using the A260/A280 ratio and lab-on-chip assay (Agilent bioanalyzer). Total RNA was treated with DNase, and then reverse-transcribed using RevertAid M-MuLV Reverse Transcriptase (Fermentas) with Oligo dT primers according to the manufacturer's protocol. One µl of single-strand cDNA was used as the template for the PCR reaction with DNA polymerase. PCR amplification was performed after a hot start at 95 °C for 5 min, followed by 30 cycles (94 °C for 30 s, 57 °C for 30 s and 72 °C for 45 s) with a final extension at 72 °C for 7 min. Levels of the housekeeping gene β-actin were used as an internal control for the normalization of RNA quantity and quality differences among the samples. The primer sequences used for the RT-PCR analysis were as follows: OPN (for all transcripts): sense, 5′- TACCAGTTAAACAGGCTGATTC-3′, anti-sense, 5′- CCATATCATCCATGTGGTCA-3′ β-actin: sense, 5′-AGCGAGCATCCCCCAAAGTT-3′, anti-sense, 5′-GGGCACGAAGGCTCATCATT-3′.
In vivo xenograft model
In total 5×106 cells were injected into the armpit of nude mice (n=4/group). Animals were kept in pathogen-free conditions and monitored using a Kodak 2000MM for tumor formation and metastasis by detecting the GFP expression. After 8 weeks, mice were sacrificed by cervical dislocation. Primary tumors were dissected out and photographed. One part of the tumors was fixed in 10% formalin solution and used for histopathology. The lungs and liver were also dissected for a metastasis analysis.
Preparation of conditioned supernatants from tumor cells
MCF-7 cells transfected with different OPN transcripts and pGC control lentivirus were cultured with RPMI-1640 medium at 1×105 cells/ml for 24 h. Debris-free cell supernatant was collected by centrifugation at 10 000 g for 5 min.
Flow cytometric analysis
Cells were collected and washed with cold phosphate-buffered saline (PBS) three times and labeled with appropriate fluorescence conjugated monoclonal antibody for 20 min away from light. The cells were also stained with the corresponding isotype-matched control antibodies. Cells were then washed with cold PBS three times, and the expression of surface molecules was assayed by flow cytometer (FACSCalibur; Becton Dickinson, CA, USA). A minimum of 10 000 events per sample were collected for phenotypic analysis.
Monocyte subset purification and coculture with tumor supernatant
The use of buffy coats from healthy donors was approved by the Institutional Review Board of Shandong University. CD14+ cells were isolated from human peripheral blood mononuclear cells using microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany), according to the manufacturer's instructions. The purity of CD14+ monocytes was >94%, as determined by flow cytometry (FACSCalibur). The isolated monocytes (2×105 cells/ml) were cocultured with corresponding supernatant from tumor cells for 40 h, subsequently washed with PBS and treated with 100 ng/ml LPS for another 6 h.
Cytokine measurement
OPN and TGF-β1 levels were measured using the Quantikine ELISA kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. The other cytokines were determined by the Bio-Plex Protein Array system (Bio Rad, Hercules, CA, USA), following the manufacturer's instructions.
Statistical analysis
Data are presented as the mean±s.d. Student's t-test and one-way ANOVA were used for statistical analysis, and P values less than 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 11.5 for Windows (SPSS, Chicago, IL, USA).
Results
Construction of lentiviral expression vectors of OPN transcripts
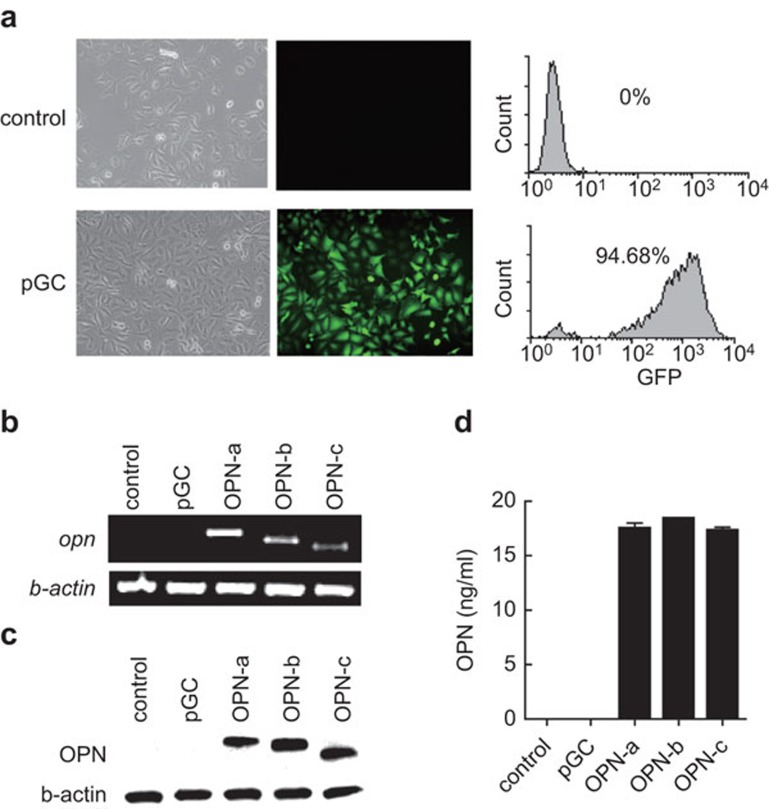
OPN has three transcripts, i.e., OPN-a, -b and -c, generated via alternative splicing. OPN-c is specifically expressed in tumors.16 To further investigate the function of OPN transcripts in the tumor process, we constructed lentiviral expression vectors for the transcripts and transfected them into the MCF-7 breast cancer cell line that does not express endogenous OPN, as described in the section on ‘Materials and methods'.16 By detecting GFP compared to the pGC group, we confirmed that the transfection efficiency was roughly 95% (Figure 1a). By RT-PCR, we verified the successful overexpression of 3 OPN transcripts at the mRNA level (Figure 1b). We further confirmed the protein level in cell lysate and supernatant by western blotting (Figure 1c) and ELISA (Figure 1d).
Figure 1.
Stable expression of OPN transcripts in MCF-7 cells. (a) The transfection efficiency was determined by detecting GFP via FACS. The overexpression of OPN transcripts in MCF-7 was determined at the mRNA level by RT-PCR. (b) Protein level in cell lysates (c) and supernatants (d) were determined by western blotting and ELISA. The results are representative of four independent experiments. OPN, osteopontin.
All OPN transcripts promoted tumor formation in vivo
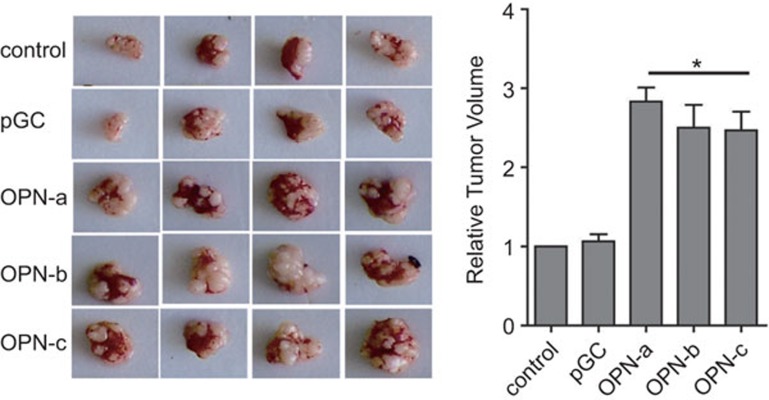
Although it has been reported that OPN-c can more effectively support the anchorage-independent growth of breast tumors in vitro, whether OPN transcripts have different functions in tumor formation in vivo is unclear. We injected MCF-7 cells transfected with different OPN transcripts into nude mice subcutaneously and monitored the subsequent tumor growth in these recipients. As shown in Figure 2, the overexpression of OPN transcripts promoted local tumor formation compared with control and pGC vector groups. However, the splice variants OPN-b and OPN-c did not show superior effects to the wide-type OPN-a in this process.
Figure 2.
All OPN transcripts promote tumor formation in vivo. In total 5×106 cells were injected into the armpit of nude mice (n=4/group). Animals were kept in pathogen-free conditions for tumor formation. After 8 weeks, mice were sacrificed by cervical dislocation. Primary tumors were dissected out and photographed. *P<0.05, compared with control and pGC groups. OPN, osteopontin.
Tumor-derived OPN regulates the activation of monocytes
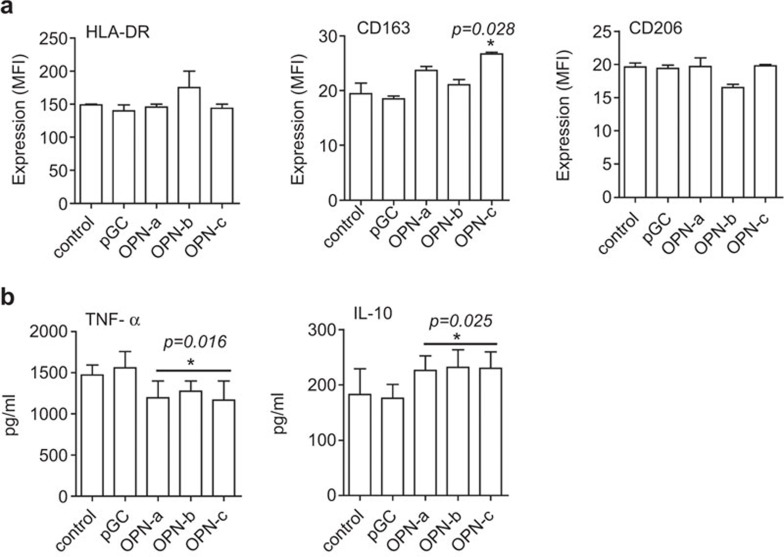
Immune cell-derived OPN can restore the metastatic potential of OPN-knockdown tumor cells. Whether the OPN expressed by tumor cells is involved in the differentiation and activation of immune cells and further regulates tumor immune evasion is unclear. In this study, we cocultured human CD14+ monocytes with MCF-7 supernatant from control, pGC and OPN transcript overexpression groups to detect whether OPN overexpressed by MCF-7 regulates monocyte activation. After 40 h, cells were collected for flow cytometry. As shown in Figure 3a, coculture with supernatants from cells overexpressing all three OPN transcripts did not regulate HLA-DR and CD206 levels in monocytes (Figure 3a). Interestingly, supernatant from cells overexpressing OPN-c significantly upregulated CD163 levels compared with OPN-a and OPN-b (Figure 3a) supernatants. We then investigated the ability of tumor supernatant-treated monocytes to respond to LPS. Compared with groups of control or pGC, overexpression of OPN inhibited TNF-α and increased IL-10 levels (Figure 3b). However, there was no difference between OPN transcripts in the regulation of TNF-α and IL-10. The overexpression of OPN had no significant effects on the IL-8, IL-12 and IL-6 levels (data not shown). Taken together, these results indicate that OPN overexpressed by MCF-7 cells induces the alternative activation of monocytes.
Figure 3.
OPN overexpressed by tumor cells induces the alternative activation of monocytes. CD14+ cells (n=5) were isolated using CD14+ microbeads, as described in the materials and methods section. 2×105 cells/ml of isolated monocytes were cocultured with corresponding supernatant from tumor cells for 40 h. (a) HLA-DR, CD163 and CD206 levels on the cell surface were determined by FACS. *P<0.05, compared with control and pGC groups. The ability of tumor supernatant-treated monocytes to respond to LPS was assayed by detecting TNF-α and IL-10 (b). Data represent the mean±s.d. of five independent experiments. *P<0.05, compared with the control and pGC groups. LPS, lipopolysaccharide; MFI, mean fluorescence intensity; OPN, osteopontin.
OPN transcripts regulate immunoregulatory cytokine expression by tumor cells
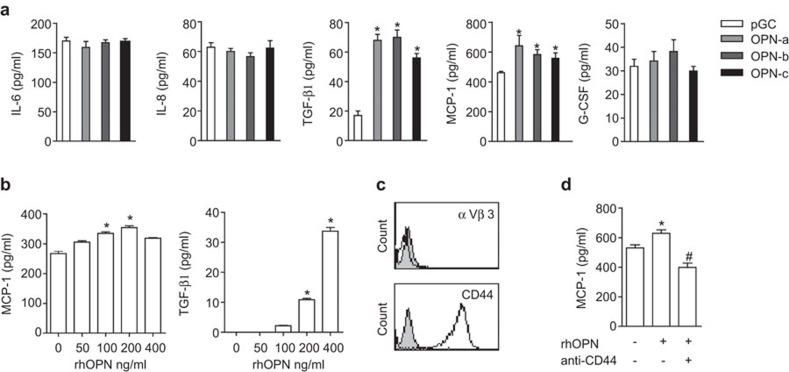
Based on the roles of OPN in host immunity, we proposed that the OPN expressed by tumor cells may indirectly regulate alternative activation of monocytes via certain immunoregulatory cytokines. To test this hypothesis, we analyzed cytokine expression profiles of MCF-7 cells transfected with OPN transcripts using Bio-plex and ELISA. We did not detect measurable levels of IL-2, IL-4, IL-10, GM-CSF, TNF-α, IL-1β, IL-5, IL-7, IL-12, IL-13, IL-17 or MIP-1β (data not shown). As shown in Figure 4a, the overexpression of OPN transcripts did not change IL-6, IL-8 or G-CSF levels, but it significantly enhanced TGF-β1 and MCP-1 levels. The three OPN transcripts showed no difference in TGF-β1 and MCP-1 regulation. Using rhOPN, we further confirmed the regulatory effect of OPN on TGF-β1 and MCP-1 expression (Figure 4b). OPN (100 ng/ml) significantly induced MCP-1 levels, and the maximum level was reached with 200 ng/ml OPN. Stimulation with 400 ng/ml OPN restored MCP-1 to basal levels. In contrast, rhOPN induced TGF-β1 expression in a dose-dependent manner, which may be due to the distinct characteristics of the different cytokines.
Figure 4.
Cytokine expression profiles of MCF-7 cells regulated by OPN transcripts. (a) MCF-7 cells transfected with different OPN transcripts and pGC lentivirus were cultured in RPMI-1640 medium at 1×105 cells/ml for 48 h. The supernatant was collected by centrifugation, and the cytokine levels were assayed by Bio-plex and ELISA (for TGF-β1). *P<0.05, compared with pGC. (b) The regulation of MCP-1 and TGF-β1 was further confirmed by rhOPN. 1×105 cells/ml of MCF-7 cultured with different concentrations of OPN for 48 h and MCP-1 and TGF-β1 levels in supernatant were determined. *P<0.05, compared with group absence of rhOPN. (c) MCF-7 cells (1×105 cells/ml) were plated in six-well plates with RPMI-1640 medium plus 10% FBS for 24 h. The surface expression of CD44 and αvβ3 was detected by FACS. The gray solid line represents isotype control. (d) MCF-7 cells (1×105 cells/ml) were plated in 48-well plates and cultured overnight for adherence. Cells were washed with PBS, pretreated with 40 µg/ml anti-CD44 blocking antibody for 30 min and stimulated with 100 ng/ml rhOPN for 48 h. The level of MCP-1 in supernatant was assayed by ELISA. *P<0.05, compared with control; #P<0.05, compared with the rhOPN-stimulated group. The results are representative of three independent experiments. Each sample was performed in triplicate wells. OPN, osteopontin; PBS, phosphate-buffered saline.
By binding to receptors such as CD44 and αvβ3, OPN stimulates cell adhesion, chemotactic migration and specific signaling. We thus investigated which receptor is required for the effects of OPN on cytokine production in MCF-7 tumor cells. Using FCM, we found that MCF-7 cells expressed a high level of CD44 but an undetectable level of αvβ3 (Figure 4c). As shown in Figure 4d, blocking CD44 with anti-CD44 antibody substantially reduced the upregulation of MCP-1 by rhOPN, confirming a partial role of the CD44 signaling pathway in cytokine expression. In addition, we did not detect significant effects of OPN transcripts on the expression of the standard form of CD44 (CD44s) or variant forms CD44v4, CD44v6 and CD44v7 (data not shown).
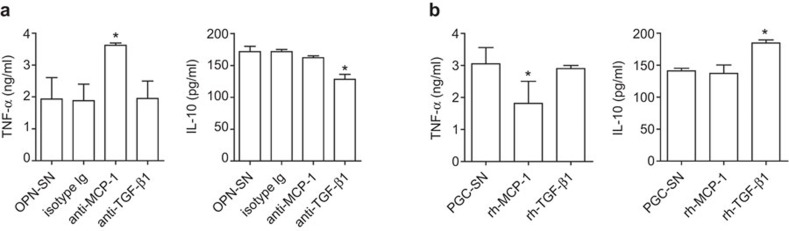
OPN transcripts regulate monocyte activation partially through TGF-β1 and MCP-1
Because OPN transcripts have similar effects on monocyte differentiation and have no effect on the regulation of TGF-β1 and MCP-1 expression, we use only OPN-a to study whether TGF-β1 and MCP-1 participate in the regulation of monocyte differentiation in the following mechanistic experiments. As shown in Figure 5a, we found that the OPN-mediated suppression of TNF-α secretion by monocytes was partially reversed when MCP-1 was blocked by its neutralizing antibody. However, blocking TGF-β1 had no effect on TNF-α expression. Interestingly, blocking experiments showed that the IL-10 level was partially regulated by TGF-β1. Moreover, we found that rhMCP-1 directly inhibited the secretion of TNF-α from monocytes stimulated with pGC supernatant (Figure 5b), but did not change the IL-10 level. Instead, rhTGF-β1 was involved in the regulation of IL-10 expression. Taken together, these data demonstrate that OPN suppresses TNF-α via MCP-1 and increased IL-10 through TGF-β1, respectively.
Figure 5.
MCP-1 and TGF-β1 regulated by OPN are involved in alternative activation of monocytes. (a) 2×105 monocytes (n=5) were cocultured with OPN-SN (supernatant from the group overexpressing OPN-a), pretreated with neutralizing antibody or isotype control for 40 h, washed with PBS and treated with 100 ng/ml LPS for another 6 h. TNF-α and IL-10 were assayed. *P<0.05, compared with the OPN-SN and isotype control groups. (b) 2×105 monocytes (n=5) were cocultured with pGC-SN (supernatant from the pGC group) in the presence of rhMCP-1 and rhTGF-β1 for 40 h, washed with PBS and treated with 100 ng/ml LPS for another 6 h. TNF-α and IL-10 were assayed. *P<0.05, compared with the pGC-SN group. LPS, lipopolysaccharide; OPN, osteopontin; PBS, phosphate-buffered saline.
Discussion
It has been reported that OPN-c can more effectively support anchorage-independent growth of breast tumors in vitro. Courter et al. have shown that the overexpression of either OPN-a or OPN-b promoted local tumor growth and lung metastasis in SCID mouse xenografts.20 However, in that study, they did not examine the function of OPN-c. Most recently, it has been reported that OPN-c specifically activated ovarian tumor cell proliferation, migration, invasion, anchorage-independent growth and tumor formation in vivo.19 With consideration of the high level of OPN expressed by tumor cells, we constructed lentiviral expression vectors driven by a strong constitutive promoter and compared the function of all three OPN transcripts in breast cancer in vivo. We found that the overexpression of OPN transcripts significantly induced tumor growth possibly by promoting proliferation and preventing apoptosis of tumor cells; however, there was no significant difference among the three transcripts. Moreover, we did not detect metastasis to the lung and/or liver, which may result from the different methods of generating xenografts.
In addition to the direct function of OPN on tumor cells,20 we demonstrate for the first time that the OPN expressed by tumor cells can regulate immune cell activation. By coculture system, we found that supernatant from tumors overexpressing OPN significantly inhibited the proinflammatory cytokine TNF-α and increased anti-inflammatory IL-10 levels from monocytes (Figure 3). Furthermore, we detected the typical AAM markers. The results demonstrated that none of the three OPN transcripts affect HLA-DR or CD206 levels. Interestingly, OPN-c upregulated the CD163 levels compared with OPN-a and OPN-b. It has been reported that IL-10 induced AAM to express high levels of CD163 but not CD206.21,22 Our results showed that OPN-c induced a M2 macrophage subset similar to the IL-10 induced phonotype. Whether OPN-c-induced M2 depends on IL-10 warrants further investigation. These results indicate that the OPN expressed by tumor cells may be associated with the alternative activation of monocytes/macrophages, which is connected to tumor-associated macrophages (TAMs). High numbers of TAMs are associated with poor survival prognosis for patients with solid human tumors, including breast, prostate, ovarian and cervical cancers.23,24 It has been reported that TAMs not only support tumor growth, but also contribute to metastasis, tumor angiogenesis, and immune evasion.24,25 The tumor microenvironment, including tumor-derived molecules such as IL-4, IL-10, TGF-β, and tumor hypoxia are associated with alternative activation and TAM polarization.24,26 In this study, we found that tumor-derived OPN induced the alternative activation of monocytes, which may have an essential role in tumor process.
Based on the roles of OPN in host defense, we presume that OPN indirectly induces the alternative activation of monocytes. We detected the cytokine profiles of MCF-7 cells overexpressing OPN or stimulated with rhOPN. We found that OPN dramatically induced MCP-1 and TGF-β1 levels among 17 cytokines tested. MCP-1 and TGF-β1 are two major immunological cytokines.27,28 It has been reported that OPN regulates MCP-1 expression through the NF-kappaB and MAPK pathways in rheumatoid arthritis.29 Furthermore, the fact that there is no difference in the ability of the three OPN transcripts to regulate MCP-1 and TGF-β1 suggests that OPN transcripts may regulate both cytokines through the common receptor binding domain. The data that OPN transcripts regulate MCP-1 via CD44 (a common receptor present in all OPN transcripts) further support our hypothesis. It has been reported that only a small number of genes (20 of 525) may be uniquely induced by OPN-c versus OPN-a according to microarray analysis.16 However, lung cancer OPN transcripts exhibited angiogenic functional heterogeneity and differentially regulated vascular endothelial growth factor.18 Thus, whether the alternative splicing of OPN mRNA engenders novel receptors mediating special functions of OPN needs to be further elucidated. Furthermore, it has been reported that OPN stimulates CD44 expression via αvβ3;30,31 however, we found that the overexpression of OPN transcripts did not change the CD44s and CD44v levels in MCF-7 cells.
Based on our results, we propose an alternative pathway for immune tolerance induced by the OPN expressed by tumor cells, in which upregulation of the immunosuppressive cytokines MCP-1 and TGF-β1 participates in the induction of alternative activation of monocytes and creates an immunoregulatory milieu facilitating tumor escape from immune surveillance.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 30671902 and 30872321), and the Natural Science Foundation of Shandong province (No. Y2008C02). We would like to thank Professor Wanjun Chen (National Institutes of Health, Bethesda, MD, USA) for assistance in writing this paper.
References
- Denhardt DT, Noda M. Osteopontin expression and function: role in bone remodeling. J Cell Biochem Suppl. 1998;30–31:92–102. [PubMed] [Google Scholar]
- Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68:152–161. doi: 10.1158/0008-5472.CAN-07-2126. [DOI] [PubMed] [Google Scholar]
- Barraclough R, Chen HJ, Davies BR, Davies MP, Ke Y, Lloyd BH, et al. Use of DNA transfer in the induction of metastasis in experimental mammary systems. Biochem Soc Symp. 1998;63:273–294. [PubMed] [Google Scholar]
- Chen H, Ke Y, Oates AJ, Barraclough R, Rudland PS. Isolation of and effector for metastasis-inducing DNAs from a human metastatic carcinoma cell line. Oncogene. 1997;14:1581–1588. doi: 10.1038/sj.onc.1200993. [DOI] [PubMed] [Google Scholar]
- Gong M, Lu Z, Fang G, Bi J, Xue X. A small interfering RNA targeting osteopontin as gastric cancer therapeutics. Cancer Lett. 2008;272:148–159. doi: 10.1016/j.canlet.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Wai PY, Mi Z, Guo H, Sarraf-Yazdi S, Gao C, Wei J, et al. Osteopontin silencing by small interfering RNA suppresses in vitro and in vivo CT26 murine colon adenocarcinoma metastasis. Carcinogenesis. 2005;26:741–751. doi: 10.1093/carcin/bgi027. [DOI] [PubMed] [Google Scholar]
- Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol. 2008;181:7480–7488. doi: 10.4049/jimmunol.181.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkl AC, Wussler J, Ahrens T, Thoma K, Kon S, Uede T, et al. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106:946–955. doi: 10.1182/blood-2004-08-3228. [DOI] [PubMed] [Google Scholar]
- Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- O'Regan AW, Hayden JM, Berman JS. Osteopontin augments CD3-mediated interferon-gamma and CD40 ligand expression by T cells, which results in IL-12 production from peripheral blood mononuclear cells. J Leukoc Biol. 2000;68:495–502. [PubMed] [Google Scholar]
- Crawford HC, Matrisian LM, Liaw L. Distinct roles of osteopontin in host defense activity and tumor survival during squamous cell carcinoma progression in vivo. . Cancer Res. 1998;58:5206–5215. [PubMed] [Google Scholar]
- Cheng J, Huo DH, Kuang DM, Yang J, Zheng L, Zhuang SM. Human macrophages promote the motility and invasiveness of osteopontin-knockdown tumor cells. Cancer Res. 2007;67:5141–5147. doi: 10.1158/0008-5472.CAN-06-4763. [DOI] [PubMed] [Google Scholar]
- He B, Mirza M, Weber GF. An osteopontin splice variant induces anchorage independence in human breast cancer cells. Oncogene. 2006;25:2192–2202. doi: 10.1038/sj.onc.1209248. [DOI] [PubMed] [Google Scholar]
- Mirza M, Shaughnessy E, Hurley JK, Vanpatten KA, Pestano GA, He B, et al. Osteopontin-c is a selective marker of breast cancer. Int J Cancer. 2008;122:889–897. doi: 10.1002/ijc.23204. [DOI] [PubMed] [Google Scholar]
- Blasberg JD, Goparaju CM, Pass HI, Donington JS. Lung cancer osteopontin isoforms exhibit angiogenic functional heterogeneity. J Thorac Cardiovasc Surg. 2010;139:1587–1593. doi: 10.1016/j.jtcvs.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilli TM, Franco VF, Robbs BK, Wanderley JL, da Silva FR, de Mello KD, et al. Osteopontin-c splicing isoform contributes to ovarian cancer progression. Mol Cancer Res. 2011;9:280–293. doi: 10.1158/1541-7786.MCR-10-0463. [DOI] [PubMed] [Google Scholar]
- Courter D, Cao H, Kwok S, Kong C, Banh A, Kuo P, et al. The RGD domain of human osteopontin promotes tumor growth and metastasis through activation of survival pathways. PLoS ONE. 2010;5:e9633. doi: 10.1371/journal.pone.0009633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40:1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Zheng W, Li R, Pan H, He D, Xu R, Guo TB, et al. Role of osteopontin in induction of monocyte chemoattractant protein 1 and macrophage inflammatory protein 1beta through the NF-kappaB and MAPK pathways in rheumatoid arthritis. Arthritis Rheum. 2009;60:1957–1965. doi: 10.1002/art.24625. [DOI] [PubMed] [Google Scholar]
- Chellaiah MA, Biswas RS, Rittling SR, Denhardt DT, Hruska KA. Rho-dependent Rho kinase activation increases CD44 surface expression and bone resorption in osteoclasts. J Biol Chem. 2003;278:29086–29097. doi: 10.1074/jbc.M211074200. [DOI] [PubMed] [Google Scholar]
- Chellaiah MA, Kizer N, Biswas R, Alvarez U, Strauss-Schoenberger J, Rifas L, et al. Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression. Mol Biol Cell. 2003;14:173–189. doi: 10.1091/mbc.E02-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]