Th17 cells that express lineage-specific transcription factor RORC in human (and RORγt in mice) and produce cytokines IL-17A, IL-17F, IL-21, GM-CSF and IL-22, constitute distinct subset of CD4+ T cells.1 Various reports indicated that Th17 cells have a critical role in the pathogenesis of several inflammatory and autoimmune diseases including rheumatoid arthritis, multiple sclerosis and psoriasis.2 However, all IL-17-producing Th17 cells are not pathogenic. For example, existence of regulatory Th17 cells with immune-suppressive phenotype had been detected in small intestine.3 As such, the factor(s) that govern(s) programming of non-pathogenic versus pathogenic Th17 cells is not completely known. Combination of TGF-β1 and IL-6 is sufficient to induce Th17 differentiation from naïve CD4+ T cells, but IL-23 produced by the activated innate cells is critical for stabilizing Th17 cells and for acquiring pathogenic functions.1,4 Also results from experimental autoimmune encephalomyelitis (EAE) in T-bet-deficient mice demonstrated that encephalitogenic Th17 cells express Th1-transcription factor T-bet and encephalitogenecity of both Th1 and Th17 cells is governed by T-bet and not by T-cell lineage-specific cytokines IFN-γ and IL-17.5 Therefore, in a recent article in Nature mmunology,6 Vijay Kuchroo and his colleagues aimed at identifying the IL-23-dependent effector molecule that imparts pathogenic phenotype to Th17 cells and the role of T-bet in the process.
By using EAE model, the authors first reconfirmed the role of IL-23 in the induction of pathogenic Th17 cells. Adoptive transfer of TGF-β1, IL-6 and IL-23-differentiated MOG-specific CD4+ T cells led to development of severe disease in mice, whereas mild or no disease was observed when TGF-β1 and IL-6-differentiated T cells were transferred. Analysis of molecular changes in IL-23-exposed Th17 cell revealed considerable induction of TGF-β3 and provided the clue for the role of TGF-β3 in the induction of pathogenic Th17 cells.
Further, Th17-polarizing experiments in IL-23R-deficient cells revealed that IL-23 might not be essential for the initial induction of TGF-β3 and it could be induced by IL-6. Nevertheless, IL-23/IL-23R certainly required for further enhancement and maintenance of TGF-β3 expression. Additionally, the in vivo expression and induction of TGF-β3 in response to IL-23 was also confirmed by immunizing TGF-β3-eYFP fate-reporter mice with either MOG or MOG+IL-23. TGF-β3 was found to be endogenously expressed by CD4+, CD8+, γδ+ T cells and B cells, but not by myeloid cells. However, exposure to IL-23 mostly restricted TGF-β3 expression to Th17 cells.
Considering the association of IFN-γ-secreting Th1 cells in the pathogenesis of autoimmune diseases, the authors intended to confirm if TGF-β3 could also be expressed by these cells. Deletion of TGF-β3+ cells in vivo, in MOG-immunized mice led to selective ablation IL-17+ T cells with no apparent changes in IFN-γ+ T cells. These results collectively implicated the nexus between TGF-β3 and pathogenic Th17 cells. Additional experiments also indicated that similar to TGF-β3, pathogenic Th17 cells could also be generated by a combination of IL-1β, IL-6 and IL-23, but both pathways were dependent on IL-23–IL-23R axis.
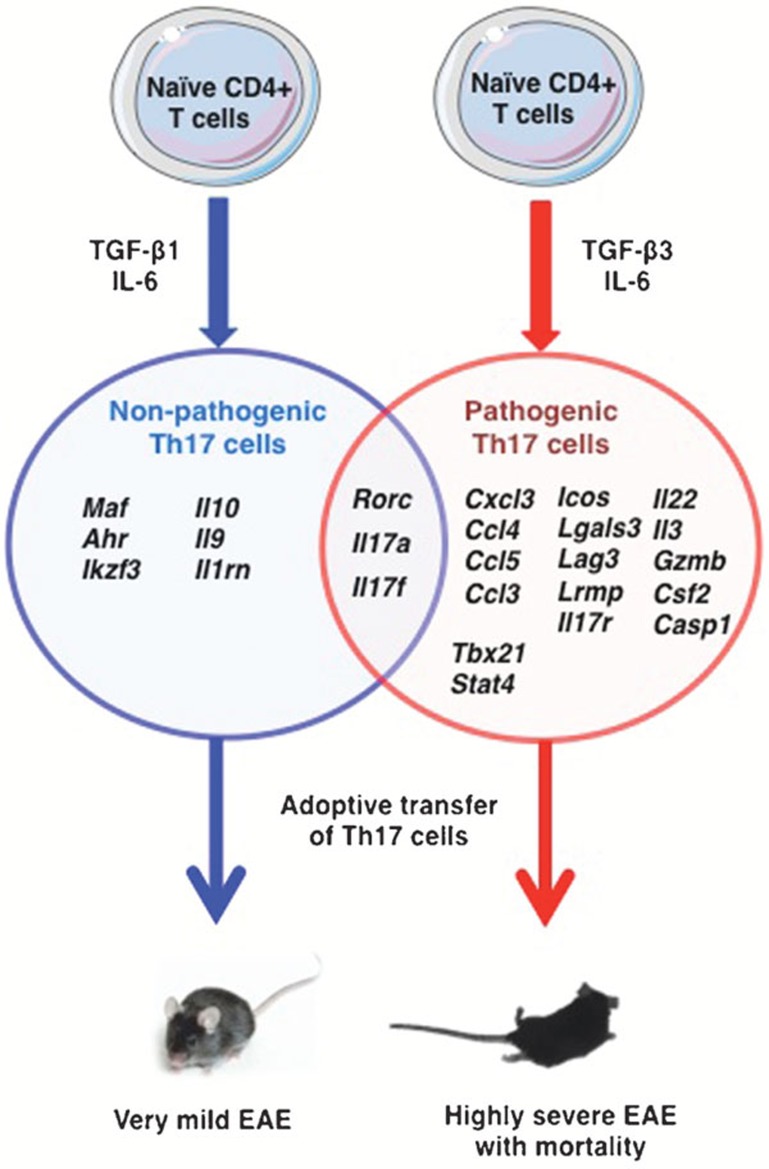
To determine if TGF-β3 has the potential to induce polarization of Th17 cells from naive CD4+ T cells, Lee et al. compared the expression of IL-17 and other Th17-associated molecules in the cells polarized in the presence of IL-6 along with either TGF-β3 or TGF-β1. Th17 cells polarized by TGF-β3 expressed Rorc, Il17a and Il17f similar to the cells polarized by TGF-β1. However, in contrast to TGF-β1-induced cells, TGF-β3-induced Th17 cells showed significantly higher expression of Il22 and Il23r than the former. Furthermore, adoptive transfer of TGF-β3-induced but not TGF-β1-induced Th17 cells induced severe EAE in naïve mice with high mortality.
Of note, the authors found that both TGF-β3 and TGF-β1 signal through the same receptor (TGF-βRII) for Th17 differentiation, suggesting that TGF-β3 and TGF-β1 deliver differential downstream signaling via TGF-βRII. In fact, TGF-β3 and IL-6-differentiated Th17 cells had higher expression and phosphorylation of Smad1 and Smad5, and lower expression and phosphorylation of Smad2 and Smad3, which was in contrast to TGF-β1-induced Th17 cells.
Finally, to discover the molecular signature that distinguishes potentially pathogenic and non-pathogenic Th17 cells, Lee et al. differentiated CD4+ naive T cells into Th17 cells under six different polarizing conditions. mRNA expression profiling of these cells by whole-genome microarray and subsequent principal component analysis categorized Th17 populations into three groups based on the differences in their molecular expression profiles.
Group I, consisted of Th17 cells induced by TGF-β1 and IL-6 with or without IL-23 and were mild or not pathogenic in vivo; group II included highly pathogenic cells induced by TGF-β3 and IL-6 or by IL-1β, IL-6; and group III included TGF-β3, IL-6 and IL-23- or IL-1β, IL-6 and IL-23-induced Th17 cells and had high expression of IL-23R similar to group II. In conclusion, the authors demonstrated that Th17 cells induced by TGF-β3, IL-6 or IL-1β, IL-6 have similar molecular profile.6 These pathogenic Th17 cells were marked by enhanced expression of genes encoding for specific cytokines, chemokines and transcription factors and downregulated expression of immunoregulatory genes (Figure 1).
Figure 1.
The molecular signatures of TGF-β1 and IL-6- induced non-pathogenic and TGF-β3 and IL-6-induced pathogenic Th17 cells. EAE, experimental autoimmune encephalomyelitis.
The microarray analysis also identified increased expression of Tbx21 (that encodes T-bet) in TGF-β3, IL-6-polarized pathogenic Th17 cells. The authors found that T-bet indeed has an important role for the induction of pathogenic signatures in Th17 cells. As TGF-β3 was also linked with pathogenic Th17 cells, the authors further investigated the possible link between T-bet and TGF-β3. They observed that T-bet-deficient Th17 cells did not induce EAE and had lower TGF-β3 expression. However, upon providing TGF-β3 exogenously, these cells overcame the necessity of T-bet, gained the pathogenicity and induced EAE upon adoptive transfer. These results indicated that T-bet is an integral part of pathogenic programming of Th17 cells and might regulate endogenous production of TGF-β3.
In conclusion, this study identified TGF-β3 as a ‘driving force' to induce pathogenic Th17 cells. However, certain key issues remain to be answered: Is there any role for TGF-β3 in IL-1β, IL-6-induced pathogenic Th17 cells? As Th17 cells are important for the clearance of fungal infections and extracellular pathogens, do these protective Th17 cells function independent of TGF-β3? Does TGF-β3 play a critical role in driving potentially pathogenic Th17/Th2 phenotype in allergy and asthma?7 Answering these questions and confirming the results in humans should pave the way to target TGF-β3 in several auto-inflammatory diseases.
Acknowledgments
This work is supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Université Pierre et Marie Curie, Université Paris Descartes and European Community's Seventh Framework Programme (FP7/2007-2013) under Grant Agreement No: 260338 ALLFUN and ANR-10-BLAN-1309 HYDROPHOBIN.
References
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Wang Y, Chi H. Regulation of TH17 cell differentiation by innate immune signals. Cell Mol Immunol. 2012;9:287–295. doi: 10.1038/cmi.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, et al. Identification of a novel subset of human circulating memory CD4+ T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222–230. e1–4. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]