Abstract
AIM
To investigate the effect of intravitreal injection administered sorafenib, a multikinase inhibitor, in a rat model of oxygen-induced retinopathy (OIR).
METHODS
Seven-day-old Sprague-Dawley rats (n=144) were randomly assigned to six groups. Group A received normal partial oxygen pressure and groups B, C, D, E and F were exposed to hyperoxia (75±2)% from postnatal 7d (P7) to P12 to induce retinopathy of prematurity. The rats in groups C, D, E and F were received intravitreal injections of either vehicle (DMSO) or sorafenib at P12 (5, 20 and 80 µg, respectively). Then they returned to normoxia after P12. The retinas were whole-mounted and imaged with a confocal microscopy. The vascular branching points were counted to quantify neovascularization at P17. Cross-sections of the retina were stained with hematoxylin and eosin (HE). The nuclei of new vessels breaking the internal limiting membrane were counted to quantify the proliferative neovascular response.
RESULTS
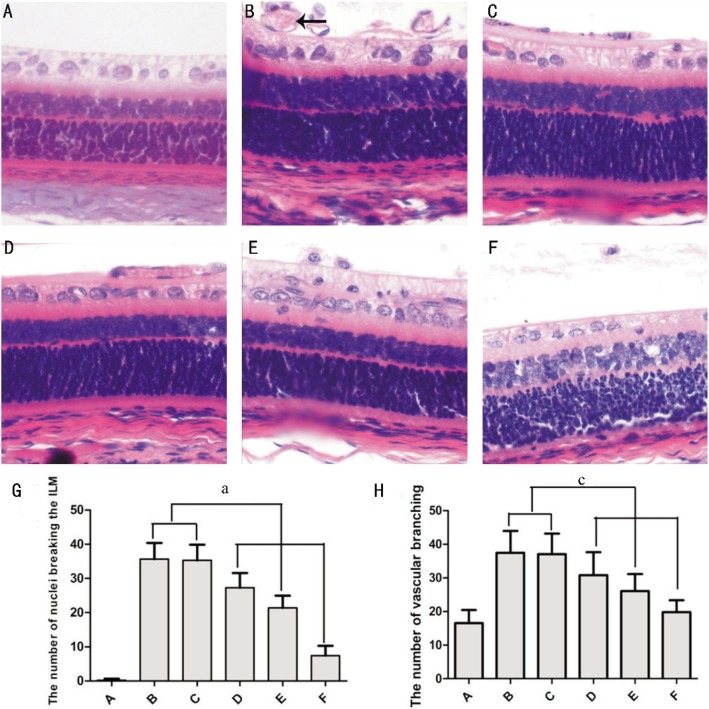
The retinal vessel in groups B and C turned into tortuosity and a great deal of neovascularization were observed. Sorafenib-treated rats had significantly less neovascularization as compared with vehicle-treated and control rats in a dose dependent manner (P<0.05). The number of vascular branching points in A, B, C, D, E and F were 16.50±3.90, 37.44±6.47, 37.08±5.10, 30.80±6.85, 26.08±5.08 and 19.83±3.51, respectively. The number of the nuclei of retinal new vessel in A, B, C, D, E and F were 0.22±0.42, 35.66±4.70, 35.30±4.54, 27.30±4.28, 21.41±3.53, and 7.41±2.87, respectively. There were significant difference between each group (P<0.05) except groups B and C.
CONCLUSION
In the rat OIR model, sorafenib could inhibit retinal neovascularization in a dose dependent manner.
Keywords: sorafenib, retinopathy of prematurity, neovascularization, tyrosine kinase inhibitors
INTRODUCTION
Retinopathy of prematurity (ROP) is a developmental disorder that occurs in the incompletely vascularized retina of premature infants which have low birth weight and low gestational age. ROP is an important cause of blindness in children, which appears neovascularization and fibrous hyperplasia[1]. Progress in perinatal health care and neonatal intensive care in recent years has led to an increased survival of extremely low birth weight infants weighing ≤1000 g at birth, but subsequently, to an increasing incidence of ROP[1]-[3]. And furthermore, Asian infants had the highest rates of ROP than those of black and white infants[4]-[6]. Despite surgical treatments such as cryotherapy and laser photocoagulation have yielded beneficial and promising therapeutic effects clinically in present, the surgical treatments cause visual field defect, vision loss, incidence of ametropia increase and even blindness[3],[7]. Either laser therapy or cryotherapy needs doctor with an adept indirect ophthalmoscope skill under the scleral top pressure, which were laborious and required special training. Hence, research is on way to find novel treatment modalities which requires less skills and equipments for this blind-causing disease.
Vascular endothelial growth factor (VEGF) is a known promoter of angiogenesis and interacts with endothelial cells via its two membrane bound receptors, VEGFR-1 and VEGFR-2, which belong to the tyrosine kinase receptor family[8]. Of these two receptors, VEGFR-2 is thought to mediate VEGF-induced signals such as vascular hyperpermeability and promote the differentiation and proliferation of endothelial cells[9],[10]. Expression of VEGFR-2 is found to be mainly associated with pathological neovascularisation and potentiated by VEGF[10],[11]. Although VEGF clearly plays a major role in the development of neovascular disease, other growth factor pathways, such as platelet-derived growth factor receptor (PDGFR), tyrosine kinases, have been implicated in ocular neovascularization disease[12],[13]. Platelet-derived growth factor (PDGF) stimulates VEGF transcription by PDGFR and also plays an important function in pericyte recruitment to neovessels. Sprouting endothelial cells express PDGF, and pericytes secrete PDGFR-β. And further more, endothelial cells undergo apoptosis without pericyte support and VEGF signaling[14]-[16]. Thereby, combined inhibition of the VEGF and PDGF signaling pathway may enhance the efficiency of suppression on neovascularization.
Sorafenib is an oral multikinase inhibitor, approved for the treatment of renal cell carcinoma. Sorafenib inhibits VEGFR-2, PDGFR-β and the serine threonine kinase Raf, which acts through the Raf/MEK/ERK kinase signaling pathway[17]. Recent case reports have also suggested possible therapeutic benefits of sorafenib in the treatment of exudative age-related macular degeneration (AMD)[18],[19]. However, there is no study which has examined the effects of sorafenib on ROP.
To enhance the clinical efficacy of angiostatic therapy, it is necessary to inhibit ROP retinal neovascularization by combine VEGFR-2 and PDGFR-β. In this paper, we observed the effect of sorafenib on rat OIR model, in order to provide new ideas and theoretical basis for the treatment of ROP.
MATERIALS AND METHODS
Materials
Pregnant Sprague-Dawley (SD) rats (Laboratory Animal Center of Xinjiang Medical University, Xinjiang, China). Rats were allowed unlimited access to rat chow and water and were exposed to a 12h:12h light-dark cycle. Room temperature was maintained at 24°C in a humidified atmosphere. All animals were cared for in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Methods
Grouping
SD rats were divided into six groups (n=24 per group) randomly, as follows: A) controlled group; B) ROP group; C) vehicle-treated ROP group; D) low doses sorafenib-treated ROP group; E) middle doses sorafenib-treated ROP group; F) high dose sorafenib-treated ROP group (5, 20, and 80 µg, respectively). Rats in group A received normal partial oxygen pressure all the way.
Rat model of oxygen induced retinopathy
OIR model was induced in newborn rats as described previously with some modifications by Smith et al[20]. Briefly, At postnatal day 7 (P7), SD rats and their nursing dams were placed in a controlled atmosphere chamber and exposed to hyperoxia (75±2)% O2 for 5d (P7 to P12) and then returned to normoxia (room air). At P12, the rats were returned to room atmosphere for 5d to allow the development of retinal neovascularization. At the time neonatal rats were dosed once by intravitreal injections at P12 (see below). Litter numbers were between 14 and 16 pups for each experiment to ensure consistency in outcomes. All animals were weighed, and mean body weights of litters were found to be within 15 g of each other at the time of treatment.
Intravitreal injections
The rats in groups C, D, E, and F were received intravitreal injections of either vehicle (DMSO) or sorafenib at P12 (5, 20, and 80 µg, respectively) as described previously with some modifications[21]. Rats were anesthetized with intraperitoneal injection of 4.3% chloral hydrate (0.01 mL/g), and pupils dilated with topical tropicamide (1%), left eye of each animal. Under a Stereo microscope (TOPCON OMS-110, Tokyo, Japan), a sclerotomy was performed about 1.0 mm posterior to the limbus using a syringe (Hamilton Co, Reno, USA) fitted with a 33-gauge needle, with special caution taken to avoid damaging the lens. DMSO (5 µL) or sorafenib (5 µL; 1, 4, and 16 µmol/L, respectively), was injected into the vitreous cavities along the puncture incision. Ofloxacin eye ointment was applied after injection.
Dissecting retinal tissue
Rat pups were anesthetized by 4.3% chloral hydrate (0.01 mL/g) on P17. Rats were intracardially perfused with paraformaldehyde (PFA; 1.0 mL, 4%) in phosphate-buffered saline. Eyes were enucleated and prefixed in PFA for 2h before anterior segments, and vitreous were removed. Retinas with intact ora serratas were dissected, placed into PBS, and flattened onto microscope slides by four right-angle incisions. Eyes were enucleated and prefixed in PFA 24h for Hematoxylin and Eosin (HE) Staining.
Retinal flat mounts
For whole mounts, the retina and lens were dissected and fixed in 4% PFA for 30min at 4°C, and prepared for staining analysis as previously described[22]. The lens was then removed and flattened retinas were permeabilized in ice-cold ethanol (70% vol/vol) for 20min, then in PBS/1% nonionic surfactant (Triton X-100; Sigma) for 30min. Retinas were incubated with Fluorescein labeled GSLI-isolectin B (Vector Laboratories, Peterborough, Britain) in PBS overnight at 4°C, then rinsed three times in PBS and mounted in PBS/glycerol (2:1) with Vectashield (Vector Laboratories, Burlington, CA, USA). Slides were secured with coverslips and sealed with nail varnish. Images of the retinal blood vessels were captured with Laser scanning confocal microscope (Leica TCS SP8, Wetzlar, Germany) and were digitally stored for analysis. Three horizons were selected randomly from each retinal flat mounts in high magnification, and then calculated the average of branching points.
Hematoxylin and eosin staining
Other rats were stained as described by Chu et al[23]. The eyes were enucleated, and fixed in 4% paraformaldehyde for 24h at 4°C prior to paraffin embedding. Serial 5-µm paraffinembedded axial sections that did not contain the optic nerve head were obtained. The sections were stained with hematoxylin and eosin, and ten intact sections, 30 µm apart, were evaluated. Retinal vascular cell nuclei that were anterior to the internal limiting membrane were quantified using a double-masked protocol. The accepted method of quantifying abnormal neovascularization in the OIR model relies on counting such cells. The mean number of vascular cell nuclei in three sections stands for the mean number of neovascular cell nuclei per 5-µm section per eye.
Statistical Analysis
SPSS 17.0 for windows statistical software was used in this study. The quantification data are expressed as means±standard deviation (SD). Comparisons between the mean variables of multiple groups were performed using one-way ANOVAs, and further assessed by SNK-q test for comparisons between each dose group and the vehicle group. P values <0.05 were considered statistically significant. The
RESULTS
Analysis of the Retinal Flat Mounts
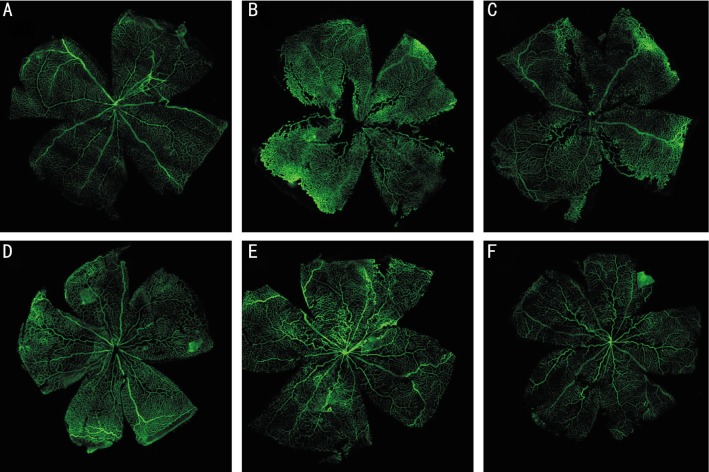
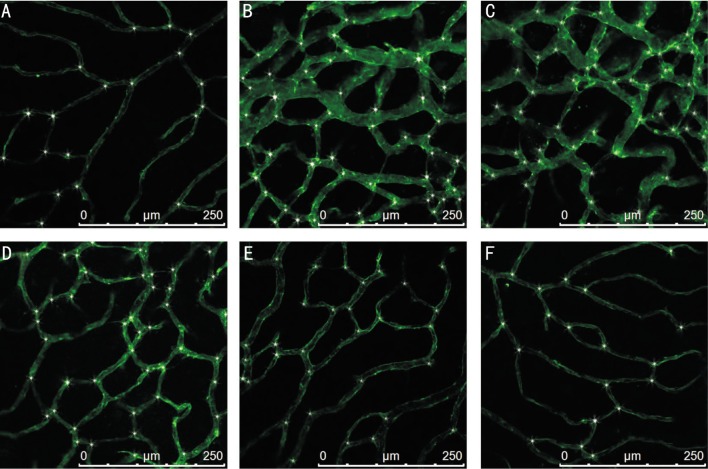
Centripetal growths of superficial vessels emerge from the optic disc and reach the far periphery approximately in controlled group (Figure 1A). However, abnormal preretinal neovascular tufts were seen throughout the retina, especially in the mid-periphery, at the interface between the hypovascular central retina and the more vascularized periphery in ROP group and vehicle-treated ROP group (Figure 1B, 1C). These neovascular tufts protruded above the inner limiting membrane of the retina into the vitreous. In addition, the retinal vessels were tortuosity and expansion. Compared with vehicle-treated group, sorafenib-treated rats demonstrated markedly reduced neovascularization (Figure 1D, 1E, 1F). Image of retinal flat mounts under a higher magnification version: ROP group and vehicle-treated group: Retevasculosum were disordered, superficial and deep vessels were not clear. The vascular branches were complex and irregular. Micrangium were tortuous and dilated. Vascular structures were abnormal (Figure 2B, 2C). Sorafenib-treated rats demonstrated markedly reduced on vessels tortuousity and dilation. Additionally, vascular density was of degressive (Figure 2D, 2E, 2F). ROP treated with high dose sorafenib rats shows that retinal vascular returned to normality compared with controlled group (Figure 2F, 2A). There was an average of 16.50±3.90, 37.44±6.47, 37.08±5.10, 30.80±6.85, 26.08±5.08, and 19.83±3.51 vessel branch points respectively (Figure 3H).
Figure 1. Rat overall retinal flat mount. At P17, rat retinal flat mounts were stained with fluorescein labeled GSL I–isolectin B4 and scanned with laser scanning confocal microscope.
A: Controlled group; B: ROP group; C: Vehicle-treated ROP group; D: Low doses sorafenib-treated ROP group; E: Middle doses sorafenib-treated ROP group; F: High dose sorafenib-treated ROP group (×100).
Figure 2. Rat retinal flat mount in high magnification. At P17, rat retinal flat mounts were stained with fluorescein labeled GSL I–isolectin B4 and scanned with laser scanning confocal microscope.
A: Controlled group; B: ROP group; C: Vehicle-treated ROP group; D: Low doses sorafenib-treated ROP group; E: Middle doses sorafenib-treated ROP group; F: High dose sorafenib-treated ROP group. Asterisks: Vascular branching.
Figure 3. Hematoxylin and Eosin staining. At P17, cross-sections of the retina were stained with HE (×400).
A: Controlled group; B: ROP group; C: Vehicle-treated ROP group; D: Low doses sorafenib-treated ROP group; E: Middle doses sorafenib-treated ROP group; F: High dose sorafenib-treated ROP group; G: The number of nuclei breaking the internal limiting membrane (ILM); H: The number of vascular branching points. Arrows: lumen. aP<0.05, cP<0.05.
Analysis of the Retina Stained with Hematoxylin and Eosin
The controlled group did not show any ocular pathology (Figure 3A). In the ROP group and vehicle-treated ROP group, there were a large amount of nuclei extending into the internal limiting membrane (Figure 3B, 3C), occurring either singularly or scattered in clusters, part of which formed lumen (Figure 3B, Arrows). In addition, retina had a great deal of neovascular tufts, extending beyond the internal limiting membrane into the vitreous (figure and data not shown). The number of vascular cell nuclei that penetrated the retinal internal limiting membrane were quantified and evaluated. The mean number of nuclei that extended into the internal limiting membrane was notably decreased in sorafenib-treated ROP pups, especially in high doses group, compared with ROP and vehicle-treated ROP group rats. Further, the degree of neovascularization in sorafenib-treated rats was on the decrease (5, 20 and 80 µg, respectively). There was an average of 0.22±0.42, 35.66±4.70, 35.30±4.54, 27.30±4.28, 21.41±3.53, and 7.41±2.87 nuclei extending into the internal limiting membrane respectively (Figure 3G), F=566.471, P=0.000.
Discussion
It is considered that the pathophysiologic mechanism of ROP consists two phases. Exposure of the rats to hyperoxia causes suppression of VEGF and its receptor VEGFR, vaso-obliteration and cessation of normal retinal blood vessel development, which mimics Phase I of ROP. When rats return to room air, the nonperfused portions of the retina become relatively hypoxic, which in turn causes hypoxia-induced VEGF and VEGFR production up-regulation markedly and retinal neovascularization, similar to Phase II of ROP[24]-[27]. Thus, we explore sorafenib on ROP via OIR model.
Sorafenib the molecular of which contains bond, hydrophilic amide groups, and lipophilic pyridine, has good biological activity. Sorafenib is characterized by good absorbability because of its small molecular weight and the strong tissue[27]. Furthermore, its long half-life could reduce intraocular injection times[28]. Additionally, in contrast with the use of bevacizumab and ranibizumab, compounds that are derived from human recombinant monoclonal antibody, sorafenib is a synthetic urea derivative and the risk of increased immunogenicity is low. Rats exposured to hyperoxia, superficial and deep vessel layer were vague. Retinal vessels turned to tortuosity and expansion; branches were complex and irregular. A great deal of vascular tufts and extensive neovascularization could be seen. The whole retinal neovascularization density increased. In the preparation of retinal flat mounts, vascellum fragility increased and was prone to hemorrhage. Peripheral strongly staining isolectin GS-positive clumps and strongly staining positive spots scattered around show bleeding points. However after treated with sorafenib, bleeding reduced greatly. Besides, bleeding is little in controlled group. These endothelial cells formed lumen, in which large number of red blood cells could be seen. These misdirected vascular elements are also quantified in HE-stained. HE staining demonstrated that, there were a large amount of nuclei protruding into the internal limiting membrane, and further more, extending into the vitreous in ROP group.
After intravitreal injection sorafenib of three concentration gradients, 1, 4, and 16 µmol/L, most neovascularization markedly reduced, vascular tortuosity and expansion attenuated to varying degrees, neovascularization density decreased. In addition, the extent of neovascularization suppression with injection 16 µmol/L sorafenib was greatest, the structure almost returned to normal. In short, sorafenib decreased the neovascularization of ROP in a dose-dependent manner.
Recently, research combined with VEGFR and PDGFR is mostly confined to the field of anti-tumor, but very little to the anti-neovascularization in ophthalmology. In a recent case report, Kernt et al[18] described a patient with a renal cell carcinoma who had exudative AMD and used a standard dose of sorafenib for the cancer. In addition, Diago et al[19] described two patients with exudative AMD who needed frequent ranibizumab injections received sorafenib as off-label treatment to reduce the number of intraocular injections. Patients above experienced improvement of macular oedema based on the use of optical coherence tomography. Marked improvement of the patient's visual acuity were noted[18],[19]. Kernt et al[29],[30] described that sorafenib protected human optic nerve head astrocytes and retinal pigment epithelium cells from light-induced over-expression of VEGF, PDGF, and placenta growth factor. Meanwhile, oral administration of the sorafenib significantly suppressed the development of laser-induced choroidal neovascularization (CNV) and caused regression of established CNV in mice and rats[31],[32]. All of these are consistent with our experimental results.
In this study, we investigated the effect of intravitreal injection sorafenib through the establishment of OIR rat model. Retinal flat mount was stained with fluorescein labeled GSL I-isolectin B. Whatever new vessels perfused or not, isolectin B could label the abnormal hyperplastic endothelial cells specifically, and the line between new and normal vessels was clear. Staining with isolectin-GS allowed vascular obliteration and tufts could be seen, compared with FITC-dextran angiography[33]. This method could reflect the actual situation of retinal neovascularization more fully, and was a better method to quantify the retinal neovascularization currently[34]. With the optical sectioning and 3D reconstruction to retinal flat mount through laser scanning confocal microscope and selection the horizontal plane to export the complete picture, each layer of vessels and neovascularization conditions were shown more clear and comprehensive. What's more, by the local administeration of intravitreal injection could make it soon to achieve effective drug concentrations as it did in the case of reducing the blood concentration thereby decrease side effects of drugs compared with systemic administration.
The profiles of adverse events occur with continuous systemic administration of sorafenib. The most common side effects in patients treated for renal cell carcinoma are dermatological symptoms such as rash or hand and foot skin reactions, diarrhoea and lowgrade hypertension. Because of being a small sample experiment, the research about appropriate dosage and side effects such as the toxic to retina did not undergo further. Hence, it needs further investigation on the optimal dose and the maximum safe dose of sorafenib in wider samples with longer follow-up periods.
In conclusion, we investigated the significant suppression effect of intravitreal injection administered sorafenib, a novel multitargeted receptor tyrosine kinase inhibitor, on ROP progression in a rat OIR model. It showed that the drug significantly inhibited the neovascularization of ROP in a dose-dependent manner. These provide new ideas and theoretical basis for the treatment of ROP. Naturally, these results mandate further clinical investigation of sorafenib for ROP.
Acknowledgments
Conflicts of Interest: Tian LL, None; Ren B, None; Gao XW, None; Luo Y, None; Cai Y, None; Zhou K, None; Du AJ, None; ZhaoY, None.
References
- 1.Fleck BW, McIntosh N. Pathogenesis of retinopathy of prematurity and possible preventive strategies. Early Hum Dev. 2008;84(2):83–88. doi: 10.1016/j.earlhumdev.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 2.O'Connor AR, Spencer R, Birch EE. Predicting long-term visual outcome in children with birth weight under 1 001g. J AAPOS. 2007;11(6):541–545. doi: 10.1016/j.jaapos.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Simpson JL, Melia M, Yang MB, Buffenn AN, Chiang MF, Lambert SR. Current role of cryotherapy in retinopathy of prematurity: a report by the American Academy of Ophthalmology. Ophthalmology. 2012;119(4):873–877. doi: 10.1016/j.ophtha.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Aralikatti AK, Mitra A, Denniston AK, Haque MS, Ewer AK, Butler L. Is ethnicity a risk factor for severe retinopathy of prematurity? Arch Dis Child Fetal Neonatal Ed. 2010;95(3):F174–F176. doi: 10.1136/adc.2009.160366. [DOI] [PubMed] [Google Scholar]
- 5.Holmström G, van Wijngaarden P, Coster DJ, Williams KA. Genetic susceptibility to retinopathy of prematurity: the evidence from clinical and experimental animal studies. Br J Ophthalmol. 2007;91(12):1704–1708. doi: 10.1136/bjo.2007.117283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husain SM, Sinha K, Bunce C, Arora P, Lopez W, Mun KS, Reddy MA, Adams GG. Relationships between maternal ethnicity, gestational age, birth weight, weight gain, and severe retinopathy of prematurity. J Pediatr. 2013;163(1):67–72. doi: 10.1016/j.jpeds.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6(4):511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 9.Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15(7):4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromberg-White JL, Boguslawski E, Hekman D, Kort E, Duesbery NS. Persistent inhibition of oxygen-induced retinal neovascularization by anthrax lethal toxin. Invest Ophthalmol Vis Sci. 2011;52(12):8979–8992. doi: 10.1167/iovs.11-7651. [DOI] [PubMed] [Google Scholar]
- 11.McLeod DS, Taomoto M, Cao J, Zhu Z, Witte L, Lutty GA. Localization of VEGF receptor-2 (KDR/Flk-1) and effects of blocking it in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2002;43(2):474–482. [PubMed] [Google Scholar]
- 12.Kinose F, Roscilli G, Lamartina S, Anderson KD, Bonelli F, Spence SG, Ciliberto G, Vogt TF, Holder DJ, Toniatti C, Thut CJ. Inhibition of retinal and choroidal neovascularization by a novel KDR kinase inhibitor. Mol Vis. 2005;11:366–373. [PubMed] [Google Scholar]
- 13.Rousseau B, Larrieu-Lahargue F, Bikfalvi A, Javerzat S. Involvement of fibroblast growth factors in choroidal angiogenesis and retinal vascularization. Exp Eye Res. 2003;77(2):147–156. doi: 10.1016/s0014-4835(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 14.Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes H, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18(2):338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 15.Hasumi Y, Kłosowska-Wardęga A, Furuhashi M, Östman A, Heldin C, Hellberg C. Identification of a subset of pericytes that respond to combination therapy targeting PDGF and VEGF signaling. Int J Cancer. 2007;121(12):2606–2614. doi: 10.1002/ijc.22999. [DOI] [PubMed] [Google Scholar]
- 16.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111(9):1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rini BI. Vascular endothelial growth factor-targeted therapy in renal cell carcinoma: current status and future directions. Clin Cancer Res. 2007;13(4):1098–1106. doi: 10.1158/1078-0432.CCR-06-1989. [DOI] [PubMed] [Google Scholar]
- 18.Kernt M, Staehler M, Stief C, Kampik A, Neubauer AS. Resolution of macular oedema in occult choroidal neovascularization under oral Sorafenib treatment. Acta Ophthalmol. 2008;86(4):456–458. doi: 10.1111/j.1600-0420.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- 19.Diago T, Pulido JS, Molina JR, Molina JR, Collet LC, Link TP, Ryan EH., Jr Ranibizumab combined with low-dose sorafenib for exudative age-related macular degeneration. Mayo Clin Proc. 2008;83(2):231–234. doi: 10.1111/j.1600-0420.2007.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 21.Chen F, Xie Z, Wu X, Du W, Wang J, Zhu J, Ji HQ, Wang YK. Intravitreal injection of soluble erythropoietin receptor exacerbates photoreceptor cell apoptosis in a rat model of retinal detachment. Curr Eye Res. 2012;37(12):1156–1164. doi: 10.3109/02713683.2012.713156. [DOI] [PubMed] [Google Scholar]
- 22.Byfield G, Budd S, Hartnett ME. The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model. Invest Ophthalmol Vis Sci. 2009;50(7):3360–3365. doi: 10.1167/iovs.08-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu Z, Dou G, Wang Y, Wu XJ, Zhang Y. Preliminary study of retinal pathological features in preterm birth pups exposed to an animal model of oxygen-induced retinopathy in mice. Graef Arch Clin Exp Ophthalmol. 2013;251(8):1937–1943. doi: 10.1007/s00417-013-2366-8. [DOI] [PubMed] [Google Scholar]
- 24.Moravski CJ, Kelly DJ, Cooper ME, Gilbert RE, Bertram JF, Shahinfar S, Skinner SL, Wilkinson-Berka JL. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000;36(6):1099–1104. doi: 10.1161/01.hyp.36.6.1099. [DOI] [PubMed] [Google Scholar]
- 25.Stone J, Chan-Ling T, Pe'er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1996;37(2):290–299. [PubMed] [Google Scholar]
- 26.Suzuma K, Takagi H, Otani A, Suzuma I, Honda Y. Increased expression of KDR/Flk-1 (VEGFR-2) in murine model of ischemia-induced retinal neovascularization. Microvas Res. 1998;56(3):183–191. doi: 10.1006/mvre.1998.2111. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, Tomczak P, Lyulko O, Alyasova A, Harza M, Kogan M, Alexeev BY, Sternberg CN, Szczylik C, Zhang J, Strahs A, Esteves B, Slichenmyer W, Berkenblit A, Hutson TE. Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: results from a phase III trial. J Clin Oncol. 2013;31(30):3791–3799. doi: 10.1200/JCO.2012.47.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strumberg D, Richly H, Hilger R A, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, Schwartz B, Awada A, Voigtmann R, Scheulen ME, Seeber S. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23(5):965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 29.Kernt M, Liegl RG, Rueping J, Neubauer AS, Haritoglou C, Lackerbauer CA, Eibl KH, Ulbig MW, Kampik A. sorafenib protects human optic nerve head astrocytes from light-induced overexpression of vascular endothelial growth factor, platelet-derived growth factor, and placenta growth factor. Growth Factors. 2010;28(3):211–220. doi: 10.3109/08977191003604505. [DOI] [PubMed] [Google Scholar]
- 30.Kernt M, Thiele S, Hirneiss C, Neubauer AS, Lackerbauer CA, Wolf A, Eibl KH, Haritoglou C, Ulbig MW, Kampik A. Zytoprotektive und antiangiogene Wirkung des Multikinase inhibitors sorafenib im retinalen Pigmentepithel. Der Ophthalmologe. 2011;108(5):445–451. doi: 10.1007/s00347-010-2304-7. [DOI] [PubMed] [Google Scholar]
- 31.Chung EJ, Yoo S, Lim HJ, Byeon SH, Lee JH, Koh HJ. Inhibition of choroidal neovascularisation in mice by systemic administration of the multikinase inhibitor, sorafenib. Br J Ophthalmol. 2009;93(7):958–963. doi: 10.1136/bjo.2008.149187. [DOI] [PubMed] [Google Scholar]
- 32.Park YH, Roh SY, Lee YC. Effect of sorafenib on experimental choroidal neovascularization in the rat. Clin Exp Ophthalmol. 2010;38(7):718–726. doi: 10.1111/j.1442-9071.2010.02328.x. [DOI] [PubMed] [Google Scholar]
- 33.Banin E, Dorrell MI, Aguilar E, Ritter MR, Aderman CM, Smith AC, Friedlander J, Friedlander M. T2-TrpRS inhibits preretinal neovascularization and enhances physiological vascular regrowth in OIR as assessed by a new method of quantification. Invest Ophthalmol Vis Sci. 2006;47(5):2125–2134. doi: 10.1167/iovs.05-1096. [DOI] [PubMed] [Google Scholar]
- 34.Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest. 2006;116(12):3266–3276. doi: 10.1172/JCI29683. [DOI] [PMC free article] [PubMed] [Google Scholar]