Abstract
AIM
To investigate the healing process after severe corneal epithelial damage in rats treated with mesenchymal stem cells (MSCs) cultured with or without keratinocyte growth factor (KGF-2) and autologous serum (AS) on amniotic membrane (AM). Many patients are blind and devastated by severe ocular surface diseases due to limbal stem cell deficiency. Bone marrow-derived MSCs are potential sources for cell-based tissue engineering to repair or replace the corneal tissue, having the potential to differentiate to epithelial cells.
METHODS
The study included 5 groups each including 10 female “Sprague Dawley” rats in addition to 20 male rats used as bone marrow donors. Group I rats received AM+MSCs, Group II rats AM+MSCs cultured with KGF-2, Group III rats AM+MSCs cultured with KGF-2+AS, Group IV rats only AM and Group V rats, none. AS was derived from blood drawn from male rats and bone marrow was obtained from the femur and tibia bones of the same animals. Therapeutic effect was evaluated with clinical, histopathological and immunohistochemical assessment. MSC engraftment was demonstrated via detection of donor genotype (Y+) in the recipient tissue (X) with polymerase chain reaction.
RESULTS
Corneal healing was significantly better in Groups I-III rats treated with MSC transplantation compared to Group IV and Group V rats with supportive treatment only. The best results were obtained in Group III rats with 90% transparency, 70% lack of neovascularization, and 100% epithelium damage limited to less than 1/4 of cornea.
CONCLUSION
We suggest that culture of MSCs with KGF-2 and AS on AM is effective in corneal repair in case of irreversible damage to limbal stem cells.
Keywords: corneal wound healing, mesenchymal stem cells, keratinocyte growth factor-2, autologous serum, amniotic membrane
INTRODUCTION
Many patients are blind and devastated by severe ocular surface diseases due to limbal stem cell (LSC) deficiency. Several pathological conditions such as chemical or thermal injury, Stevens-Johnson syndrome, contact lens-induced kerathopathy, genetic disease of aniridia, and multiple endocrine deficiency-associated keratitis can lead to LSC deficiency. Bone marrow-derived mesenchymal stem cells (MSCs) are potential sources for cell-based tissue engineering to repair or replace the corneal tissue because they are easily obtained and propagated in large amounts in culture with self-renewal capacity and plasticity, having the potential to differentiate to epithelial cells[1]-[3].
Corneal alkali burns can cause stem cell loss and subsequent epithelial erosion, ulceration, stromal inflammation and neovascularization. In severe injury, both corneal limbus and central epithelia can be destructed, accompanied by the invasion of the corneal surface by conjunctival epithelial cells, resulting in abnormal conjunctivalization and permanent visual impairment[4],[5]. Amniotic membrane (AM) transplantation can serve as substrate for LSCs depending on their presence remaining on the corneal surface. Although AM transplantation is proven to be effective in promoting re-epithelization and reducing inflammation, it does not alone suppress neovascularization and conjunctivalization in complete LSC deficiency. When limbal deficiency is total and diffuse, allograft limbal transplantation needs to be performed[6],[7]. LSCs cultured on AM can effectively reconstruct the injured corneal surface[6]-[8].
Adult MSCs isolated from bone marrow are multipotent cells that are able to differentiate into a number of mesenchymal phenotypes of various tissues and have earned considerable clinical attention in regenerative medicine due to their plasticity. MSCs lack tissue-specific characteristics but under the influence of appropriate signals can differentiate into specialized cells with a phenotype distinct from that of precursor[9]. Furthermore, MSCs secrete large quantities of bioactive factors that are both immunomodulatory and trophic, inhibiting ischemia-caused apoptosis and scarring while stimulating angiogenesis[10],[11].
Autologous serum (AS) and keratinocyte growth factor (KGF) are effective agents in the development of epithelial tissue and regeneration during corneal damage repair[12],[13]. The paracrine interaction of two fibroblast-derived epithelial mitogens, hepatocyte growth factor (HGF) and KGF is well-known in cornea: KGF and HGF are expressed differentially by limbal and corneal fibroblasts and are modulated by cytokines, suggesting their roles in modulating corneal epithelial stem cells and transient amplifying cells[14]. KGF-2 (FGF-10) -a member of the FGF family- is produced by mesenchymal tissues, induces the proliferation of epithelial cells, and plays a role in the regeneration of the damaged epithelial tissue[13],[15]. Based on these data, it can be hypothesized that KGF-2 may contribute to the corneal epithelial healing provided by MSCs.
The aim of this study was to investigate the healing process after total corneal epithelial damage in rats treated with bone marrow derived MSCs, KGF-2, AS, and AM.
MATERIALS AND METHODS
This study was prospectively designed in the Department of Medical Biology and Genetics, Ondokuz Mayis University, Faculty of Medicine. The study was approved by the Human and Animal Research Ethics Committee of the University. All animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory animals with a protocol approved by the Institutional Animal Ethic Committee of Ondokuz Mayis University. The pathological, molecular and ocular assessments were blindly performed by two different investigator groups.
Materials
The study included 5 groups each including 10 female “Sprague Dawley” rats with additional 20 male rats to be used as bone marrow donors. Group I rats received AM+MSCs, Group II rats AM+MSCs cultured with KGF-2, Group III rats AM+MSCs cultured with KGF-2+AS, Group IV rats only AM and Group V rats no treatment other than antibiotics and corticosteroids.
Methods
Preparation of human amniotic membrane and derivation of mesenchymal stem cells, and autologous serum
AM was obtained at the time of cesarean sectioning from voluntary pregnant women with female fetuses, who gave consent for the process. The AMs were washed with phosphate-buffered saline containing penicillin and streptomycin and stored at -80°C in low-glucose (1 g/L) Dulbeccos's modified Eagle's medium (DMEM-LG/Biological Industry) including 50% glycerol after separation of epithelium and basement membrane from bulk tissues[1],[4].
AS was derived from blood drawn from anesthetized male rats by external intracardiac puncture. In addition, bone marrow was drawn from the femur and tibia bones of the same animals and mononuclear cells were separated by the density gradient method[1]. Mononuclear cells collected from the interphase were identified with flow cytometry [FACSCalibur TM (Becton, Dickinson and Company, Vancouver, Canada)] using the hematopoietic and mesenchymal stem cell determinants [CD11b/c (+), CD45 (+), CD90 (-), CD106 (-), CD44 (-) (BD Pharmingen, San Diego, USA)]. Identified cells were cultivated on 25-cm2 -T25- tissue culture plate with a concentration of 106/mL with MSC medium (200 mL/L FBS, 100 U/mL penicillin, 100 Ug/mL streptomycin and 20 mL/L L-Glutamin added into 5 mL DMEM-LG). Then the cell culture plates were incubated at 37°C with 50 mL/L CO2 in a humidified atmosphere of 95% air. The medium was changed daily for 3d and every 3-4d afterward during incubation. The cells were propagated with subcultures after colony forming unit fibroblasts (CFU-F) became visible (Figure 1). Some of the MSCs produced were suspended and were identified once more with flow cytometry using bone marrow derived hematopoietic and mesenchymal stem cell surface determinants [CD11b/c (-), CD45 (-), CD90 (+), CD106 (+), CD44 (+)]. Before use, AM was pretreated with 0.25% trypsin in 0.02% EDTA for 15-30min to remove the amniotic epithelium. The denuded AM was prepared as discs with 1.2 cm diameters which were placed into the cell culture plates. Cells were then plated at a density of 1×105 cells/cm2 on AM with MSC medium (200 mL/L FBS, 100 U/mL penicillin 100 Ug/mL streptomycin and 20 mL/L L-Glutamin added into 5 mL DMEM-LG). Then the cell culture plates were expanded until 90% confluence at 37°C with 50 mL/L CO2 in a humidified atmosphere of 95% air. The medium was changed every 3-4d. The membranes were separated into 3 groups after 1wk incubation. These Groups I, II and III consisted of MSCs seeded on denuded AMs incubated with previously described medium for 3wk. Groups II and III membranes included additionally 1 µg/mL KGF-2 only and 1 µg/mL KGF-2+20 mL/L autologous serum, respectively. The KGF-2 used in the study is recombinant human KGF-2 (Bioscience, Germany). Group IV consisted of AMs only, without any intervention or MSCs. In Groups II and III, it was observed that MSCs on amnion discs started to show epitheloid formation in invert microscope. The steps of MSCs on AM are shown in Figure 2.
Figure 1. Colony forming unit fibroblasts (CFU-Fs -arrows) on 14d of bone marrow cultivation (inverted microscope, zeiss-axiovert ×40) bar 100 µm.
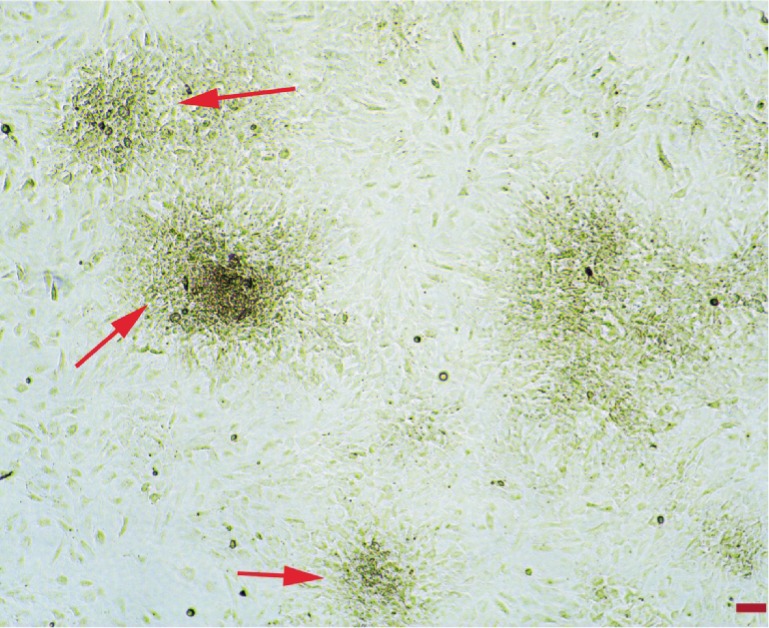
Figure 2. Steps of MSCs preparation on AM.
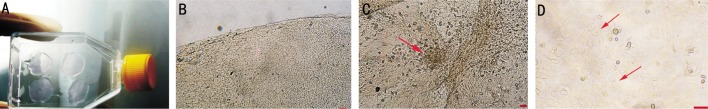
A: AM in cell culture plate as discs; B: Empty AM (zeiss-axiovert ×40) bar 100 µm; C: Colony forming unit-fibroblasts (CFU-F) on AM at 7d (arrow) (zeiss-axiovert ×40) bar 100 µm; D: The beginning of partial epithelial formation of MSCs cultured with KGF-2 in Groups II and III (arrows) (zeiss-axiovert ×400) bar 100 µm.
Cell viability analysis
Induced (with KGF-2) mesencyhmal stem cells on the amnion membran were rinsed 3 times, incubated in 2.4 U/mL Dispase II (Roche, Indianapolis, IN, USA) in DMEM for 2h at 37°C. The induced MSCs were removed under a dissecting microscope and treated with 0.25% trypsin+0.02% EDTA for 15-30min at 37°C. Single cell suspensions were stained with 0.4% trypan blue for the quantification of cell viability with the Countess Automated Cell Counter (Invitrogen). Cell viability was found to be 85%.
Morphological analysis of cell culture on denuded amniotic membrane
Human AMs were treated with 0.25% trypsin+0.02% EDTA at 37°C for 15-30min and scraped gently with a cell scraper (Nunc International, Rochester, NY, USA) to remove their amniotic epithelial cells. MSC single-cell suspensions were seeded on the denuded AM at a density of 1×105 cells/cm2 in T25 well plate. The cells were cultured with previously described medium until confluence (21d) and fixed and sectioned for H&E an immun staining with pancytokeratin (Klon AE1/AE3, LabVision) and cytokeratin K19 (Klon Ks19.1, LabVision) (Figure 3).
Figure 3. Section staining with H&E and immunohistochemical staining in induced (with KGF-2 and AS) MSCs on denuded AM transplanted on Group III (light microscope, olympus BX51).
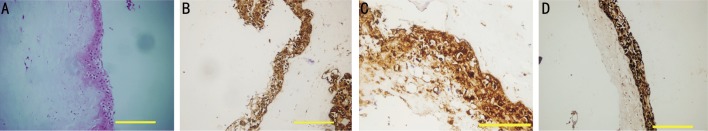
A: Induced MSCs stratified into multilayer structure on AM, H&E (×100) bar 200 µm; B: Cytokeratin 19 positivity (×200) bar 200 µm; C: Cytokeratin 19 positivity (×1000) bar 50 µm. D: Pancytokeratin positivity (×200) bar 100 µm.
Injury of rat corneas, transplantation of amniotic membrane and mesenchymal stem cells
The corneas of female rats were injured by 1 mol/L NaOH during 30s using a method modified from Ma et al[1]. Anesthesia was induced with intramuscular injections of ketamine hydrochloride, 50 mg/kg and xylazine, 10 mg/kg. Proparacaine hydrochloride, 0.5% was used for topical anesthesia and a wire lid speculum was inserted in the right eye. Eight millimeter Whatman #3 filter paper discs were soaked for 10-20s in 1 mol/L NaOH solution. The discs were drained, and excess moisture absorbed on tissue paper, with reproducible wetting of ±5% by weight. The alkali-soaked discs were applied to the central cornea and limbus and held gently in position with forceps for 30s. The cornea was finally irrigated over 20s with 15 mL saline. The remaining limbus and central epithelial region was surgically debrided. The rats were followed up for corneal epithelial defect. Four rats with infection, hyphema, corneal fistula, phthisis or hypopion of the injured eye were excluded from the study. After the documentation that there is no healing on the corneas over a week, the rats were divided to five groups for different regimes of treatment. In each group, the eyes were treated with gentamicin and dexamethazone q.i.d during 4wk. The groups were as follows:
Group I: MSC cultured on AM
Group II: MSC cultured with KGF-2 on AM
Group III: MSC cultured with KGF-2 and AS on AM
Group IV: AM alone
Group V: No intervention (control group)
The pre-prepared AMs were transplanted to the cornea and limbus of 10 female rats (Group IV) and temporal blepharoraphy was performed with 4/0 nylon suture. After 14d, the transplanted AMs were removed and the corneas were washed with an abundant amount of saline in order to wash out the non-implanted cells, if there is any. After 24h, the rats were examined under white light for corneal clarification and neovascularization; and under blue light with diluted fluoroescein solution to determine epithelial defects. Then the rats were sacrificed and their cornea layer was excised and divided into two parts: one for histopathological examination and the other for DNA extraction to detect stem or epithelial cells of male origin.
For Groups I-III, MSCs were inoculated on the AM to yield a cell distribution of 1×105cells/cm2 at the time of corneal burn, and were incubated for 3wk. The membranes were transplanted to Groups I, II and III rats when the incubation period was over. While the MSC medium included no supplement in Group I rats, it included 1 µg/mL KGF-2 in Group II rats and an additional 20 mL AS in Group III rats. After the transplantation, Groups I-III rats were subject to the processes described above for Group IV. Group V rats had no intervention other than gentamicin and dexamethazone.
Parameters assessed under blue light with diluted topical fluoroescein
The therapeutic effect of each treatment regimen was evaluated based on transparency, neovascularization, and epithelial integrity, according to the method used by Ma et al[1]. Corneal transparency was categorized as: 1) completely transparent; 2) iris blurred; 3) Pupil could not be visualized. The assessment of neovascularization was performed as follows: 1) No neovascularization; 2) neovascularization detected within 2 mm of the limbus; 3) neovascularization extending to >2 mm of limbus. The integrity of corneal epithelial cells was checked under blue light after a drop of fluoroescein and the extent of damage was classified as: 1) less than 1/4 of the cornea; 2) between 1/4 and 1/2 of the cornea; 3) more than 1/2 of the cornea.
Histopathological and immunohistochemical assessment
The collected corneas were fixed with 4% formaldehyde after sampling for DNA extraction and embedded in paraffin blocks for histopathological and immunohistochemical assessment. The slides were refixed, washed and stained with hematoxylin and eosin (H&E) and examined with Leica HMLB45 (Leica, Germany) light microscope. The corneal epithelium status was classified as follows: 1) completely intact epithelium; 2) epithelium damage <50%; 3) epithelium damage >50% as described by Ma et al[1].
For immunohistochemical staining, the tissues were fixed for 24 h with buffered neutral formalin 10% before the preparation of paraffin-embedded serial sectioning at 4 µm. Paraffin sections were deparaffinized, rehydrated, dipped in 3% hydrogen peroxide solution for 10min, and then processed with a labelled streptavidin-biotin kit (DAKO, Carpinteria, USA). Antibodies examined included pancytokeratin (Klon AE1/AE3, LabVision) and cytokeratin K19 (Klon Ks19.1, LabVision). The slides were boiled in 10 mmol/L sodium citrate buffer pH 6.0 then maintained at a sub-boiling temperature for 10min and cooled on bench top for 30 min. Unfortunately, the cytokeratin K14 (Klon Ks14.1, LabVision) couldn't be worked out despite performing appropriate antibody diluent and antigen unmasking procedures according to the data sheet.
The 3,3′-diaminobenzidine (DAB) kit (DAKO, Carpinteria, USA) were used for colorizing agent. Finally the slides were stained with Mayer hematoxylin for 60s. The slides were washed with buffer phosphate pH 7.6 in all steps until DAB process and with distilled water thereafter. All procedures were performed in room temperature. The results were assessed with Leica HMLB45 (Leica, Germany) light microscopy.
DNA isolation and detection of SRY gene
The genomic DNA was obtained from corneal tissue with DNA DNeasy kit- FavorPrep (Favorgen, Vienna, Austria). The SRY gene presence on the Y chromosome in corneas of the recipient female rats was checked with polymerase chain reaction (PCR). The DNA presences in all tissues were assessed with a house-keeping gene, β-actin, as internal control. The primary sequences of SRY and β-actin genes were determined according to the methods of Ise et al[16] and Bryja and Konecny[17]. Rat SRY gene PCR was performed using the SRY primers (sense 5′-CAGAGATCAGCAAGCATCTGG-3′; antisense 5′-TCTGGTTCTTGGAGGACTGG-3′) with 5min initial denaturation at 95°C followed by 37 cycles of denaturationat 94°C for 30s, 57°C for 30s, and 72°C for 1min at thermal cycling. The rat β-actin gene was performed using the β-actin primers (sense 5′-AGAGAAGCTGTGCTATGTTG3′; anti-sense 5′-GTACTCCTGCTTGCTGATCC-3′) with 5min initial denaturation at 95°C followed by 37 cycles of denaturationat 94°C for 30s, 57°C for 30s, and 72°C for 1min at thermal cycling. The SRY gene product of 288 base pairs and the β-actin gene product of 488 base pairs were therefore amplified. The amplification reaction was performed in 25 µL total volume, adding 1× reaction buffer [10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 0.001% gelatin], 3.0 mmol/L MgCl2, 200 µm dNTP mixture, 0.2 µm SRY primary, 0.15 µm β-actin primary, 200 ng genomic DNA and 1.5 unit Taq polymerase. The mixture was electrophoresed at 2% micropore Nu gel electrophoresis and gels were stained with ethidium bromide. The pUC19/MspII was used as DNA marker. The analyses of the samples which are not positive for SRY gene despite transplantation of MSCs obtained from male rats were performed three times. Each analysis was done with positive (DNA from healthy male rat cornea) and negative controls.
Statistical Analysis
Statistical analyses were performed using SPSS version 18.0. All data were shown as frequency and percentage. Chi-square tests were used for all comparisons that are corneal transparency, neovascularization, fluorescein staining, H&E staining, limbal K19 (+), SRY gene (+), corneal K19 (+) and conjunctival K19 (+). P<0.05 was accepted as statistically significant in comparisons.
RESULTS
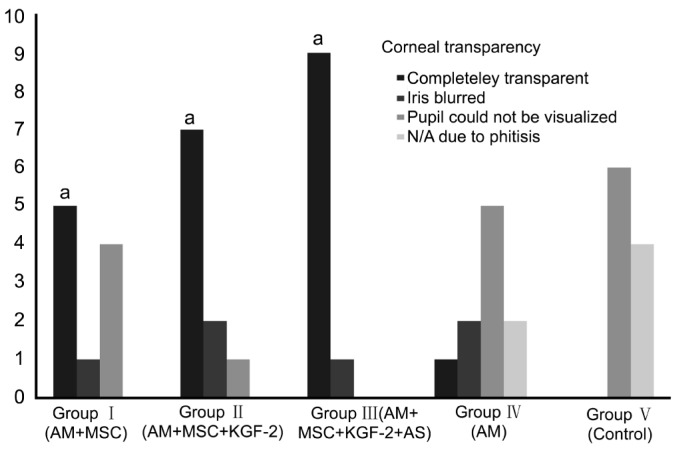
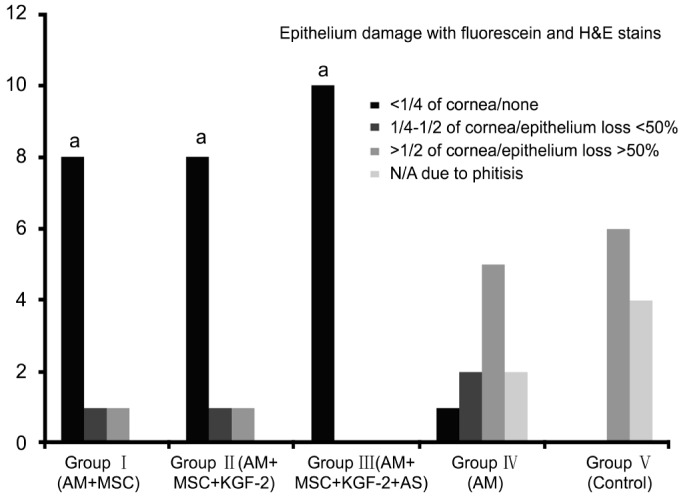
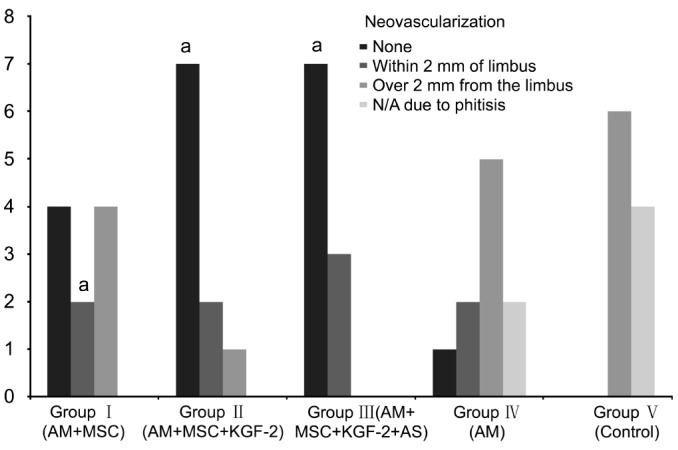
The therapeutic effect was evaluated based on transparency, lack of neovascularization and epithelial integrity. The presence of epithelium on the corneal surface was demonstrated with pancytokeratin positivity. Pancytokeratin was found to be negative in rats with severe corneal damage or eye phthisis. The results obtained in the Groups I-V rats were given in Table 1. The corneas of the rats in Groups I, II and III (groups with MSCs transplantation) showed significantly more transparency with less epithelium damage and less neovascularization compared to the corneas of the Groups IV and V (groups without MSCs transplantation) (P<0.05) (Figures 4–6). Furthermore, there was no rat with eye phthisis in these groups with MSC transplantation whereas 2 rats in Group IV and 4 rats in Group V developed eye phthisis. There was no rat healed in Group V and only one rat showed completely transparent iris with limbal K19 positivity in Group IV. When the MSCs transplanted groups were compared with each other, there was also significant difference between Group I, Group II and Group III for the transparency and neovascularization (χ2=10.1818, P=0.037) whereas it is concluded that there is no significant difference between these groups for epithelium damage (χ2=0.6243, P=0.7319). The best results were obtained in Group III rats with 90% transparency, 70% lack of neovascularization, and 100% epithelium damage limited to less than 1/4 of cornea.
Table 1. Results of the parameters assessed in Groups I-V rats.
| Parameters | Group I AM+MSC | Group II AM+MSC +KGF-2 | Group III AM+MSC +KGF-2+AS | Group IV AM | Group V none (control) |
| Corneal transparency | |||||
| Completely transparent | 5 (50) | 7 (70) | 9 (90) | 1 (10) | 0 (0) |
| Iris blurred | 1 (10) | 2 (20) | 1 (10) | 2 (20) | 0 (0) |
| Pupil could not be visualized | 4 (40) | 1 (10) | 0 (0) | 5 (50) | 6 (60) |
| N/A due to phthisis | 0 (0) | 0 (0) | 0 (0) | 2 (20) | 4 (40) |
| Neovascularization | |||||
| None | 4 (40) | 7 (70) | 7 (70) | 1 (10) | 0 (0) |
| Within 2 mm of limbus | 2 (20) | 2 (20) | 3 (30) | 2 (20) | 0 (0) |
| Over 2 mm from the limbus | 4 (40) | 1 (10) | 0 (0) | 5 (50) | 6 (60) |
| N/A due to phthisis | 0 (0) | 0 (0) | 0 (0) | 2 (20) | 4 (40) |
| Epithelium damage with fluorescein stain | |||||
| <1/4 of cornea | 8 (80) | 8 (80) | 10 (100) | 1 (10) | 0 (0) |
| 1/4-1/2 of cornea | 1 (10) | 1 (10) | 0 (0) | 2 (20) | 0 (0) |
| >1/2 of cornea | 1 (10) | 1 (10) | 0 (0) | 5 (50) | 6 (60) |
| N/A due to phthisis | 0 (0) | 0 (0) | 0 (0) | 2 (20) | 4 (40) |
| Epithelium damage with H&E stain | |||||
| None | 8 (80) | 8 (80) | 10 (100) | 1 (10) | 0 (0) |
| Epithelium loss <50% | 1 (10) | 1 (10) | 0 (0) | 2 (20) | 0 (0) |
| Epithelium loss >50% | 1 (10) | 1 (10) | 0 (0) | 5 (50) | 6 (60) |
| N/A due to phthisis | 0 (0) | 0 (0) | 0 (0) | 2 (20) | 4 (40) |
| Corneal immunohistochemistry K19 | |||||
| Negative | 7 (70) | 9 (90) | 9 (90) | 8 (80) | 6 (60) |
| Positive | 3 (30) | 1 (10) | 1 (10) | 0 (0) | 0 (0) |
| N/A due to phthisis | 0 (0) | 0 (0) | 0 (0) | 2 (20) | 4 (40) |
| Limbal immunohistochemistry K19 | |||||
| Negative | 1 (10) | 1 (10) | 0 (0) | 7 (70) | 6 (60) |
| Positive | 9 (90) | 9 (90) | 10 (100) | 1 (10) | 0 (0) |
| N/A due to phthisis | 0 (0) | 0 (0) | 0 (0) | 2 (20) | 4 (40) |
| SRY gene status | |||||
| Negative | 1 (10) | 1 (10) | 0 (0) | 8 (80) | 6 (60) |
| Positive | 9 (90) | 9 (90) | 10 (100) | 0 (0) | 0 (0) |
| N/A due to phthisis | 0 (0) | 0 (0) | 0 (0) | 2 (20) | 4 (40) |
AM: Amniotic membrane; MSC: Mesenchymal stem cell; KGF-2: Keratinocyte growth factor-2; AS: Autologous serum; H&E: Hematoxylin-eosin.
n=10 (%)
Figure 4. Bar chart showing corneal transparency in Groups I-V rats.
The corneas of the rats in Groups I, II and III (groups with MSCs transplantation) showed significantly more transparency compared to the corneas of the Groups IV and V (groups without MSCs transplantation) (n=10) (aP<0.05).
Figure 6. Bar chart showing epithelium damage with H&E and fluoroescein in Groups I-V rats.
The corneas of the rats in Groups I, II and III (groups with MSCs transplantation) showed significantly less epithelium damage compared to the corneas of the Groups IV and V (groups without MSCs transplantation) (n=10) (aP<0.05).
Figure 5. Bar chart showing neovascularization in Groups I-V rats.
The corneas of the rats in Groups I, II and III (groups with MSCs transplantation) showed significantly less neovascularization compared to the corneas of the Groups IV and V (groups without MSCs transplantation) (n=10) (aP<0.05).
In our study, the 288 base pair SRY gene and 488 base pair β-actin gene (internal control) was positive with PCR in the corneal tissue in all female rats with MSCs transplantation except two rats (one in Group I, the other in Group II). In Group IV rats treated with AM only and Group V rats (control) both without MSCs transplantation, only 488 base pair β-actin gene was positive with PCR. Limbal K19 status was determined for the appearance of undifferentiated epithelium in rats with excised limbus. Except for two rats described above, limbal epithelium K19 was positive in all rats treated with MSCs while it was negative in rats with AM only and in controls.
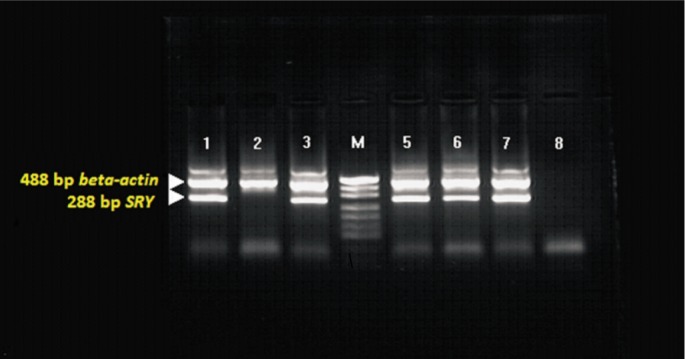
The images of corneas with fluorescein staining under blue light after transplantation in different groups are shown in Figure 7. The histopathological and immunohistochemical findings of damaged and repaired corneas are shown in Figure 8. The PCR findings showing chimerism are given in Figure 9.
Figure 7. Images of corneas with fluorescein staining after transplantation.
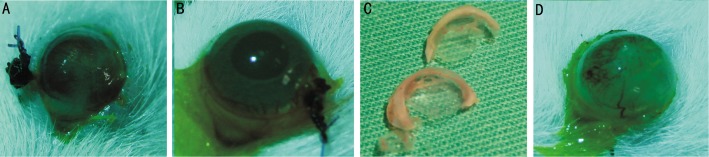
A: Group I, partial epithelium reconstruction; B: Group III, complete healing with intact epithelium; C: Macroscopic appearance of the undamaged cornea (above) and Group III (AM+MSCs+KGF-2+AS) cornea with complete healing (below); D: Group IV, showing total epithelium damage and neovascularization.
Figure 8. Histopathological and immunohistochemical images of the cornea epithelium (light microscope, Leica HMLB45, Leica, Germany).
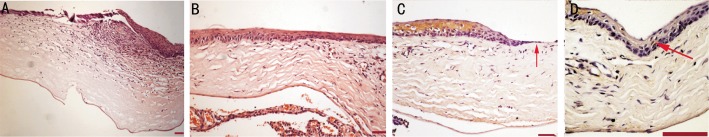
A: H&E staining of the damaged cornea epithelium bar 100 µm (×4); B: Group III Rat: H&E staining of the repaired cornea epithelium bar 100 µm (×10); C: Group IV rat: Limbal K19 (-) immunohistochemical staining of the unrecovered limbal epithelium (arrow) bar 100 µm (×20); D: Group II rat: Limbal K19 (+) immunohistochemical staining of the repaired limbal. Epithelium with induced MSC (with KGF-2) transplantation (arrow) bar 100 µm (×40).
Figure 9. PCR image showing chimerism. [M (pUC19)] in wells 1, 3, 5 and 6; no chimerism in 2nd well; positive and negative controls in 7th and 8th wells, respectively.
DISCUSSION
In cases with severe permanent cornea damage, allo-transplantation and auto-transplantation of LSCs are applied to restore ocular surface integrity. The difficulties met with allo- and auto-transplantation cases led to search for other cell sources than LSCs[18]-[20]. Bone marrow-derived MSCs are multipotent adult stem cells that have the potential to differentiate not only to lineages of mesenchymal tissues, but also to cells with visceral mesoderm, neuroectoderm and endoderm characteristics including retinal neuronal cells and corneal cells[2],[9],[21]-[24]. In our research, we have considered MSCs as a potential source for ocular surface reconstruction because they are readily isolated and relatively easily expanded, proliferate extensively without loss of differentiation capacity, and have the potential to differentiate into epithelial cells.
Keratins (cytokeratins and hair keratins) are a family of cytoskeletal component proteins of epithelial cells. Yoshida et al[25] investigated the expression pattern of cytokeratins K15, K19, K14, and K12 in human and mouse ocular surface epithelium as putative markers of epithelial phenotype. In their study, conjunctival epithelium was found to be K15 (+), K19 (+), K12 (-); limbal epithelium K15 (+), K19 (+), K12 (+); and central corneal epithelium K15 (-), K19 (-), K12 (+) in rats. Both K15 and K19 can be used to demonstrate conjunctivalized epithelium in rats as they were not expressed in centralized corneal epithelium. We also demonstrated limbal K19 positivity and squamous non-keratinized 5 layers epithelium of the corneal epithelium in H&E stains, especially in Group III.
There are currently few studies in the literature about MSCs transplantation in severe cornea damage[1],[2],[26]. Ma et al[1] transplanted human MSCs from healthy donors, human limbal stem cells and human fibroblasts after growth and expansion on AM into three separate groups of rat corneas 7d after chemical burns. However, they could detect neither human cytokeratin 3 nor human pancytokeratin in the rat eyes transplanted with human MSCs and speculated that the therapeutic effect did not come from epithelial differentiation of MSCs but may be a result of inhibition of neovascularization and inflammation. Ye et al[2] also showed that bone marrow-derived MSCs can engraft to injured cornea after alkali burn to promote wound healing by differentiation, proliferation, and synergizing with hematopoietic stem cells in rabbits with normal bone marrow function. They stated that tissue injury-induced homing of systemically transplanted MSCs to particular sites with subsequent differentiation in response to local tissue microenvironment promotes wound healing. Oh et al[26] stated that anti-inflammatory and anti-angiogenic actions of MSCs might be mediated through paracrine pathways involving soluble factors such as IL-10, TGF-β1, IL-6 and TSP-1. The main difference of our work from these studies is the use of KGF-2 and autologous serum investigating the contribution of these substances to corneal healing process.
The data showing stem cell engraftment in direct local transplantation can be obtained via detection of donor genotype (Y+) in the recipient tissue (X) with PCR, FISH or immunohistochemical staining[27]. It is possible to detect 1 ng male DNA (0.1%) in 1 µg female DNA and 0.01% male cell (1:10 000) in male/female mixture with PCR[21]. In our study, we used the multiplex PCR method based on previous studies to detect transplanted bone marrow-derived MSCs of male rats in the female rat corneas[16],[27]-[30]. The SRY gene obtained from the corneal tissue DNA was negative in two rats with MSCs transplantation (one in Group I, the other in Group II) and without cornea recovery, suggesting the failure of MSCs engraftment. This failure may be related to MSCs death due to inappropriate environmental conditions or to wash out of MSCs with eye secretion as well as the lack of AS resulting insufficient cell proliferation on AM. The 288 base pair SRY gene was positive with PCR in the corneal tissue of all recovered female rats and only 488 base pair β-actin gene (internal control) was positive with PCR in Group IV and Group V rats both without MSCs transplantation. Also, limbal epithelium K19 was positive in all recovered rats treated with MSCs while it was negative in rats with AM only and in controls. Based on these findings, we suggest that MSCs transplantation with or without KGF-2 and AS was successful and seriously contributed to the healing of Groups I-III rats.
The role of human AM to support the expansion of limbal epithelial cells can be related to its content of growth factors (NGF, KGF, HGF, bFGF, TGF) and basal membrane components and integrins. We used MSCs in our study by first propagating them on AM without epithelial tissue, and used the AM to transfer cells while benefiting from the preservative effect of its growth factors.
The results of our study showed that corneal healing was significantly better in Group I (AM+MSCs), Group II (AM+MSCs+KGF-2) and Group III (AM+MSCs+KGF-2+AS) rats than in Group IV rats (AM) and Group V rats used as controls. K19 antibody which is also an undifferentiated epithelial marker was negative in the limbus of the rats with limbal excision and without MSC transplantation. Surprisingly, one of the rats in the Group IV (AM) which MSC transplantation was not performed recovered completely. However, the corneal and limbal K19 were (+) in this rat, suggesting that the residual limbal epithelium recovered with the supporting effect of AM. The development of the phthisis in two rats in Group IV shows that the protective effect of the AM was not sufficient for healing or there was additional intraocular or stromal damage. The development of eye phthisis in these rats and in 4 rats in Group V (both without MSC transplantation) with the lack of phthisis in Groups I, II and III (all with MSC transplantation) show MSCs contribution to stromal healing in deep corneal injuries.
Our results were less successful in terms of corneal transparency and neovascularization in Group I rats (without KGF-2) compared with Group II and Group III rats (both with KGF-2). Keratinocyte growth factor (KGF, FGF-7) plays a role in the development of epithelial tissue and regeneration of the damaged epithelial tissue. KGF receptor mRNA is markedly up regulated in the corneal epithelium following scrape injury, suggesting its prominent role in corneal epithelial wound healing[13]. KGF is produced by mesenchymal tissues and with its paracrine effect specifically induces the proliferation of epithelial cells[15]. These findings constitute the reason of our decision to use KGF-2 on MSCs to examine their effect on corneal epithelial healing in vitro. The epithelium damage detected with fluoroescein and H&E staining was also found to be low in Group I rats (without KGF-2) as in Group II and III rats both with KGF-2. However, the corneal K19 positivity of 30% in Group I suggests that the epithelium consists of conjunctivalization and there is no complete healing. The conjunctivalization was found to be only 10% in Group II and Group III, suggesting that it may frequently occur in the absence of KGF-2. Autologous serum was used as a topical agent for cornea damage repair and its effects on fibroblast migration and differentiation is well-established[12]. The best results in terms of corneal transparency and neovascularization are obtained in Groups II and III, in which KGF-2 was used with and without AS, respectively. We hypothesize that the benefits of MSCs transplantation for corneal damage can be augmented by the addition of autocrine and/or paracrine effect of KGF-2 with the proliferative support of AS.
To our knowledge, this is the first study in the literature investigating the effects of MSCs cultured with KGF-2 and AS on AM. In conclusion, we suggest that MSCs may be potential sources for corneal repair in irreversible damage to limbal stem cells and that culture with KGF-2 may possibly aid this process by increasing epithelial proliferation with its autocrine and/or paracrine effect. Also, culture with human AS may contribute to corneal wound-healing increasing cell migration and proliferation. AM alone has not yielded any regenerative role on the corneal epithelium. The effects of KGF-2 and AS on MSCs and corneal epithelium are promising but should be further evaluated.
Acknowledgments
Conflicts of Interest: Pınarlı FA, None; Ökten G, None; Beden Ü, None; Fışgın T, None; Kefeli M, None; Kara N, None; Duru F, None; Tomak L, None.
REFERENCES
- 1.Ma Y, Xu Y, Xiao Z, Yang W, Zhang C, Song E, Du Y, Li L. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cell. Stem Cells. 2006;24(2):315–332. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- 2.Ye J, Yao K, Kim JC. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model, engraftment and involvement in wound healing. Eye (Lond) 2006;20(4):482–490. doi: 10.1038/sj.eye.6701913. [DOI] [PubMed] [Google Scholar]
- 3.Kan I, Melamed E, Offen D. Autotransplantation of bone-marrow derived stem cells as therapy for neurodegenerative diseases. Handb Exp Pharmacol. 2007;180:219–242. doi: 10.1007/978-3-540-68976-8_10. [DOI] [PubMed] [Google Scholar]
- 4.Du Y, Chen J, Funderburgh J, Zhu X, Li L. Functional reconstruction of rabbit corneal epithelium by human limbal cells cultured on amniotic membrane. Mol Vis. 2003;9:635–643. [PMC free article] [PubMed] [Google Scholar]
- 5.Charukamnoetkanok P. Corneal stem cells: bridging the knowledge gap. Semin Ophthalmol. 2006;21(1):1–7. doi: 10.1080/08820530500501322. [DOI] [PubMed] [Google Scholar]
- 6.Utheim TP, Lyberg T, Ræder S. The culture of limbal epithelial cells. Methods Mol Biol. 2013;1014:103–129. doi: 10.1007/978-1-62703-432-6_7. [DOI] [PubMed] [Google Scholar]
- 7.Ricardo JR, Cristovam PC, Filho PA, Farias CC, de Araujo AL, Loureiro RR, Covre JL, de Barros JN, Barreiro TP, dos Santos MS, Gomes JA. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea. 2013;32(3):221–228. doi: 10.1097/ICO.0b013e31825034be. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Sheha H, Fu Y, Giegengack M, Tseng SC. Oral mucosal graft with amniotic membrane transplantation for total limbal stem cell deficiency. Am J Ophthalmol. 2011;152(5):739–747. doi: 10.1016/j.ajo.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36(4):568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Müller I, Kustermann-Kuhn B, Holzwarth C, Isensee G, Vaegler M, Harzer K, Krägeloh-Mann I, Handgretinger R, Bruchelt G. In vitro analysis of multipotent mesenchymal stromal cells as potential cellular therapeutics in neurometabolic diseases in pediatric patients. Exp Hematol. 2006;34(10):1413–1419. doi: 10.1016/j.exphem.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Caplan A. Why are MSCs therapeutic? New data: new insight. J Pathol. 2008;217(2):318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson SL, Secker GA, Daniels JT. The effect of therapeutic human serum drops on corneal stromal wound-healing activity. Curr Eye Res. 2008;33(8):641–652. doi: 10.1080/02713680802254790. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Zhou X, Ma J, Tian H, Jiao Y, Zhang R, Huang Z, Xiao J, Zhao B, Qian H, Li X. Effects of keratinocyte growth factor-2 on corneal epithelial wound healing in a rabbit model of carbon dioxide laser injury. Biol Pharm Bull. 2010;33(6):971–976. doi: 10.1248/bpb.33.971. [DOI] [PubMed] [Google Scholar]
- 14.Chen TC, Chang SW. Effect of mitomycin C on IL-1R expression, IL-1-related hepatocyte growth factor secretion and corneal epithelial cell migration. Invest Ophthalmol Vis Sci. 2010;51(3):1389–1396. doi: 10.1167/iovs.09-3494. [DOI] [PubMed] [Google Scholar]
- 15.Cheng CC, Wang DY, Kao MH, Chen JK. The growth-promoting effect of KGF on limbal epithelial cells is mediated by upregulation of DeltaNp63alpha through the p38 pathway. J Cell Sci. 2009;122(Pt 24):4473–4480. doi: 10.1242/jcs.054791. [DOI] [PubMed] [Google Scholar]
- 16.Ise H, Nikaido T, Negishi N, Sugihara N, Suzuki F, Akaike T, Ikeda U. Effective hepatocyte transplantation using rat hepatocytes with low asialoglycoprotein receptor expression. Am J Pathol. 2004;165(2):501–510. doi: 10.1016/S0002-9440(10)63315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryja J, Konecny A. Fast sex identification in wild mammals using PCR amplification of the Sry gene. Folia Zool. 2003;52(3):269–274. [Google Scholar]
- 18.Menzel-Severing J, Kruse FE, Schlötzer-Schrehardt U. Stem cell-based therapy for corneal epithelial reconstruction: present and future. Can J Ophthalmol. 2013;48(1):13–21. doi: 10.1016/j.jcjo.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Lal I, Panchal BU, Basu S, Sangwan VS. In-vivo expansion of autologous limbal stem cell using simple limbal epithelial transplantation for treatment of limbal stem cell deficiency. BMJ Case Rep. 2013:2013. doi: 10.1136/bcr-2013-009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauklin M, Steuhl KP, Meller D. Characterization of the corneal surface in limbal stem cell deficiency and after transplantation of cultivated limbal epithelium. Ophthalmology. 2009;116(6):1048–1056. doi: 10.1016/j.ophtha.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Rao NA, Kimoto T, Zamir E, Giri R, Wang R, Ito S, Pararajasegaram G, Read RW, Wu GS. Pathogenic role of retinal microglia in experimental uveoretinitis. Invest Ophthalmol Vis Sci. 2003;44(1):22–31. doi: 10.1167/iovs.02-0199. [DOI] [PubMed] [Google Scholar]
- 22.Castanheira P, Torquetti L, Nehemy MB, Goes AM. Retinal incorporation and differentiation of mesenchymal stem cells intravitreally injected in the injured retina of rats. Arq Bras Oftalmol. 2008;71(5):644–650. doi: 10.1590/s0004-27492008000500007. [DOI] [PubMed] [Google Scholar]
- 23.Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106(6):984–991. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 24.Reinshagen H, Auw-Haedrich C, Sorg RV, Boehringer D, Eberwein P, Schwartzkopff J, Sundmaher R, Reinhard T. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2011;89(8):741–748. doi: 10.1111/j.1755-3768.2009.01812.x. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida S, Shimmura S, Kawakita T, Miyashita H, Den S, Shimazaki J, Tsubota K. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47(11):4780–4786. doi: 10.1167/iovs.06-0574. [DOI] [PubMed] [Google Scholar]
- 26.Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26(4):1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 27.Albini TA, Wang RC, Reiser B, Zamir E, Wu GS, Rao NA. Microglial stability and repopulation in the retina. Br J Ophthalmol. 2005;89(7):901–903. doi: 10.1136/bjo.2004.060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills RAD, Coster DJ, Williams KA. Effect of Immunosuppression on outcome measures in a model of rat limbal transplantation. Invest Ophthalmol Vis Sci. 2002;43(3):647–655. [PubMed] [Google Scholar]
- 29.Song IK, Joo CK. Morphological and functional changes in the rat cornea with an ethanol-mediated epithelial flap. Invest Ophthalmol Vis Sci. 2004;45(2):423–428. doi: 10.1167/iovs.03-0947. [DOI] [PubMed] [Google Scholar]
- 30.Mi S, Yang X, Zhao Q, Qu L, Chen S, M Meek K, Dou Z. Reconstruction of corneal epithelium with cryopreserved corneal limbal stem cells in a goat model. Mol Reprod Dev. 2008;75(11):1607–1616. doi: 10.1002/mrd.20900. [DOI] [PubMed] [Google Scholar]