Abstract
AIM
To investigate the effects of hepatocyte growth factor (HGF) on MMP-2 expression in scleral fibroblasts from guinea pig with LIM.
METHODS
Sixty 1-week-old guinea pigs were chosen for the study. The right eyes were treated with -10.0 D lenses as the LIM group; the left eyes remained untreated as the control group. The refraction and axial length were measured by streak retinoscopy and A-scan ultrasonography respectively prior to and 4 weeks after the experiment. Four weeks later, the guinea pigs were sacrificed and primary scleral fibroblasts were taken for tissue culture. The 3rd-5th generation scleral fibroblasts were chosen for the experiments. The expression levels of HGF and MMP-2 protein in the scleral fibroblasts were analyzed by Western blotting. After HGF with different doses acted on the scleral fibroblasts of the control group, MMP-2 protein expression in the scleral fibroblasts was analyzed by Western blotting. HGF siRNA was transfected into the scleral fibroblasts of the LIM group and the protein expressions of HGF and MMP-2 were analyzed by Western blotting.
RESULTS
The LIM group became myopic with a significant increase in axial length (7.97±0.29 mm vs 7.01±0.26 mm, P<0.05), and a significant decrease in refraction (-5.06±0.31 D vs 0.55±0.25 D, P<0.05) compared with the control group. The protein expression of HGF in the scleral fibroblasts of the LIM group was significantly higher compared with the control group ( 1.26±0.04 vs 0.32 ±0.04, P<0.05). The protein expression of MMP-2 in the scleral fibroblasts of the LIM group was significantly higher compared with the control group (0.89±0.06 vs 0.42±0.05, P<0.05). In the scleral fibroblasts of the control group, HGF(0, 0.1, 1, 10 ng/mL) upregulated MMP-2 protein expression in a dose-dependent manner (0.35±0.03, 0.44±0.02, 0.91±0.03, 1.33±0.04, all P<0.05). In the scleral fibroblasts of the LIM group transfected with HGF siRNA, MMP-2 protein expressions were significantly decreased compared with the negative control group (0.29±0.03 vs 0.81±0.05, P<0.05).
CONCLUSION
HGF is a upstream mediator of MMP-2 in scleral fibroblasts from guinea pig.
Keywords: myopia, fibroblasts, hepatocyte growth factor, MMP-2, guinea pig
INTRODUCTION
Myopia is a very common eye disease and its incidence has increased yearly. The pathogenesis of myopia is still unclear. Sclera is a connective tissue forming the outer coat of the eye. In the development of myopia, variations in the mechanical behavior of the sclera will induce active remolding of the tissue, which weakens its elasticity and bearing capacity. As a result, myopia develops as the eye axis is excessively extended [1]. The major cells that form the sclera are fibroblasts and they play important roles in the progression of myopia [2]. Recent studies have shown that an abnormal visual environment can increase the expression of matrix metalloproteinase 2 (MMP-2) in the sclera, leading to excessive degradation of the extracellular matrix (ECM) and axial extension, thus resulting in myopia [3]-[5]. However, the specific mechanism of increased MMP-2 in the development of myopia is unknown. Hepatocyte growth factor (HGF) is a multifunctional growth factor that can regulate a variety of pathological processes [6]-[8]. Studies have shown that HGF can upregulate the expression of MMP-2, which results in ECM degradation in a variety of tissues and cells [9]-[11]. Furthermore, recent research has shown that HGF expression is closely associated with mouse ocular growth[12]. It is well known that the development of myopia is associated with excessive ocular enlargement and myopic refractive error. Therefore, HGF might be involved in the development of myopia. However, whether HGF is expressed in scleral fibroblasts and whether HGF upregulates MMP-2 expression in lens-induced myopia (LIM) remains unknown. In our study, to investigate the possible role of HGF in the development of LIM, we established a guinea pig model of LIM and cultured scleral fibroblasts. We measured the expression of HGF and MMP-2 protein in the scleral fibroblasts and then treated the scleral fibroblasts with HGF in the control group and HGF siRNA in the LIM group. Subsequently, corresponding MMP-2 protein expression was detected.
MATERIALS AND METHODS
Materials
Animals
Sixty 1-week-old, healthy male guinea pigs were obtained from the Experimental Animal Center at Zhengzhou University. The right eyes were treated with -10.0 D lenses as the LIM group; the left eyes were untreated and served as the control group. The lenses were rigid gas permeable (RGP) lenses (power: -10.0 D, diameter: 12 mm, optical zone: 10.5 mm-11.5 mm, posterior surface radius: 8 mm; Daiwa lens Co., Ltd, Japan). The RGP lenses were mounted onto a ring, then the ring were sutured onto the skin around the eye. All guinea pigs were fed indoors in a standardized way. All procedures were approved by the Institutional Animal Care and Use Committee of Zhengzhou University and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Measurement of refractive parameters
Tropicamide (0.25%) were used to dilate the pupil every 5min for 3 times. Refractive measurements were obtained using streak retinoscopy (Heine Optotechnik GmbH & Co. KG, Herrsching, Germany) 45min after the first mydriasis. Axial length were measured with A-scan ultrasonography (Nidek Co., Ltd., Beijing, China). Refractive data are presented as the mean spherical equivalent refractive error. Since the accommodation of guinea pigs' eyes were very powerful, retinoscopy was conducted in the central zone shortly after the ciliary muscle was completely paralyzed. The refractive parameters above were measured prior to visual manipulations, and 4wk after the manipulations were initiated. All refraction data were presented as mean derived from 5 repeated measures.
Isolation, culture and identification of scleral fibroblasts
Four weeks later, the guinea pigs were sacrificed. Under sterile conditions, both eyes were extracted and the separated clean posterior pole of the scleral tissue was cut into tissue blocks that were 1×1×1-mm3. Primary culture was carried out using a bottom-up adherent method at 37°C in a humidified atmosphere containing 5% CO2. After 2h, the culture flask was turned over and Dulbecco's modified Eagle's medium (DMEM; Gibco, NY, USA) containing 10% fetal bovine serum (FBS, Shanghai Yantuo Biotecnology, Shanghai, China)), 100 IU/mL penicillin and 100 µg/mL streptomycin was added. The medium was replenished twice a week. For about 14d, fibroblasts growing form the scleral tissue were visible. When cells became confluent, passaging was carried out. Fibroblasts between 3 and 5 passages were used for the experiments. The fibroblasts were identified with anti-vimentin antibody by immunocytochemistry staining (ABC method). Rabbit anti-mouse vimentin monoclonal antibody (Santa Cruz, CA, USA) was used as the primary antibody respectively.
Western blotting analysis
(1) HGF and MMP-2 protein expression in both groups of scleral fibroblasts: each group of scleral fibroblasts was harvested and lysed with Harsh RIPA lysis buffer (Gibco, Invitrogen, Guangzhou, China) according to the manufacturer's instructions. The protein concentration was determined with a BCA Protein Assay Kit (HaiGene, Harbin, China). Then, 20 g of the total protein lysate were analyzed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto nitrocellulose (NC) membranes (PALL, New York, USA) for 25min at 15 V. Nonspecific antibody binding was blocked with 5% skim milk for one hour at room temperature. The membranes were incubated overnight at 4°C with primary (rabbit anti-mouse HGF polyclonal antibodies (Santa Cruz, CA, USA), 1:100; rabbit anti-mouse MMP-2 polyclonal antibodies (Santa Cruz, CA, USA), 1:100). and secondary (goat anti-rabbit IgG, 1:1500) antibodies using a Super Signal Western Blotting Kit (HaiGene, Harbin, China). The membranes were developed with Super ECL reagent (Gibco, Invitrogen, Guangzhou, China) and exposed to film. Each protein was normalized to the corresponding values of the β-actin control. (2) MMP-2 protein expression in scleral fibroblasts of the control group treated with HGF: the scleral fibroblasts of the control group were seeded onto sterile 6-well plates for 24h at a concentration of 2×104 /mL. Cells were washed with sterile PBS and treated with serum-free DMEM for 24h. Then cells were washed with sterile PBS and treated with either serum-free DMEM (control) or serum-free DMEM with 0.1 ng/mL, 1 ng/mL, or 10 ng/mL HGF (Sigma, St. Louis, MO, USA) for 24h. Western blotting was used to detect MMP-2 protein expression. (3) MMP-2 protein expression in scleral fibroblasts of the LIM group mediated by HGF siRNA: scleral fibroblasts of the LIM group were transfected with HGF siRNA (Invitrogen, Carlsbad, CA, USA), silencing HGF (AAGCCUUGCAAGUGAA-UGGAAGUCC) and control siRNA (Invitrogen, Carlsbad, CA, USA), (GGAACCUACUUGUUCGAACGUCCUU) using 22.5 µL Lipofectamine 2000 and 50 nM siRNA, according to the manufacturer's instructions. Twenty-four hours later, the cells were seeded onto culture plates and Western blotting was used to detect HGF and MMP-2 protein expressions.
Statistical Analysis
Variables are expressed as mean±standard deviation. Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA); the two groups were compared with a paired t-test. A P value less than 0.05 was considered statistically significant.
RESULTS
Refractive Error
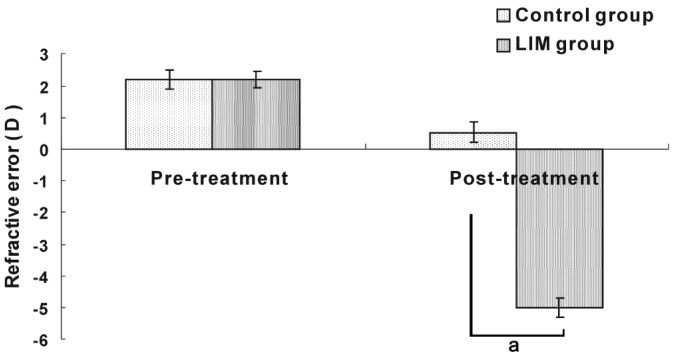
Prior to the experiment, there was no significant difference in refraction between the LIM group (2.2±0.3) D and control group (2.2±0.3) D. Four weeks after the treatment, the LIM group became myopic with a significant decrease in refraction compared with the control group. (-5.06±0.31 D vs 0.55±0.25 D, P<0.05; Figure 1).
Figure 1. Refractive error in the LIM and control groups.
aP<0.05 vs control group.
Axial Length
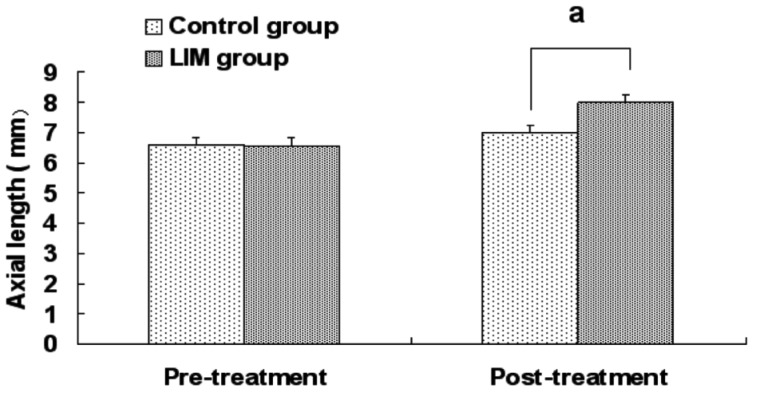
Eyes in both groups exhibited continuous axial length growth throughout the experiment, with the LIM group growing faster. Four weeks after the treatment, the axial length in the LIM group was significantly greater than in the control group (7.97±0.29 mm vs 7.01±0.26 mm, P<0.05; Figure 2).
Figure 2. Axial length in the LIM and control groups.
aP<0.05 vs control group.
Scleral Fibroblasts
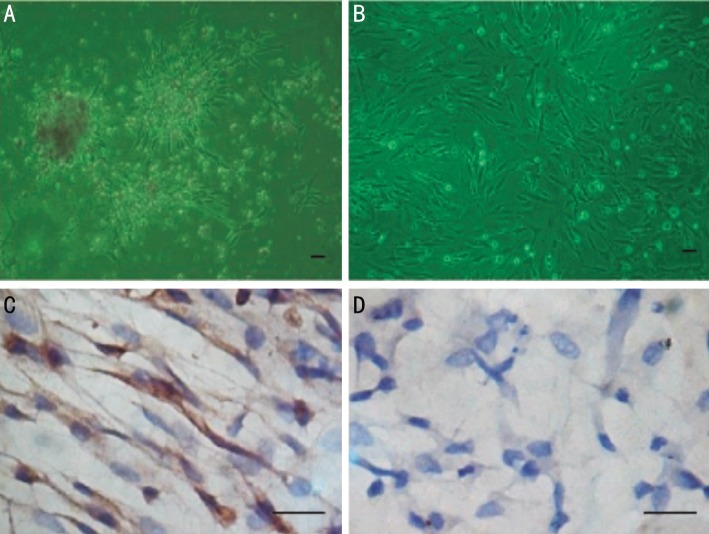
For about 14d, fibroblasts growing form the scleral tissue were visible. When cells became confluent, passaging was carried out. Fibroblasts between 3 and 5 passages were used for the experiments. The fibroblasts were identified with anti-vimentin antibody by immunocytochemistry (Figure 3).
Figure 3. Guinea pig scleral fibroblasts under microscope.
A: Growth of scleral fibroblasts from the scleral tissue; B: The third generation of scleral fibroblasts; C: The scleral fibroblasts were identified with anti-vimentin antibodies by immunocytochemistry; D: Negative control, anti-vimentin antibody was replaced by PBS. The scale bar represents 50 µm.
Protein expressions of HGF and MMP-2 in the scleral fibroblasts
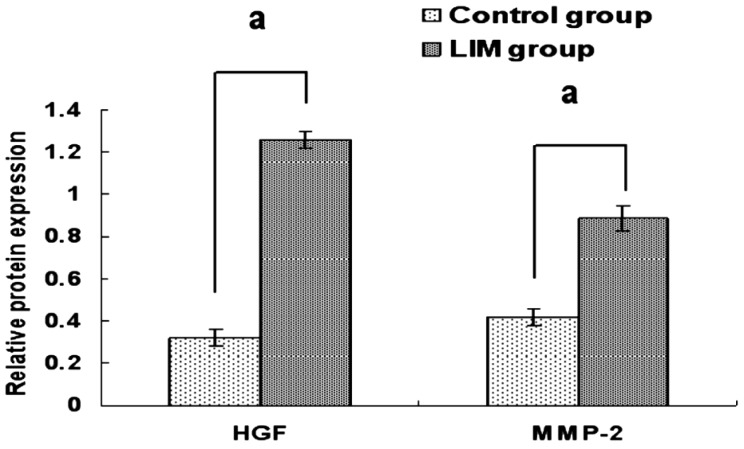
HGF and MMP-2 protein expressions were detected in the scleral fibroblasts of the two groups. The protein expression of HGF in the scleral fibroblasts of the LIM group was significantly higher compared with the control group (1.26±0.04 vs 0.32±0.04 P<0.05). The protein expression of MMP-2 in the scleral fibroblasts of the LIM group was significantly higher compared with the control group (0.89±0.06 vs 0.42±0.05, P<0.05; Figures 4 and 5).
Figure 4. Protein expressions of HGF and MMP-2 in the scleral fibroblasts of the LIM and control eyes.
aP<0.05 vs control group.
Figure 5. Protein expressions of HGF and MMP-2 in the scleral fibroblasts of the LIM and control eyes.
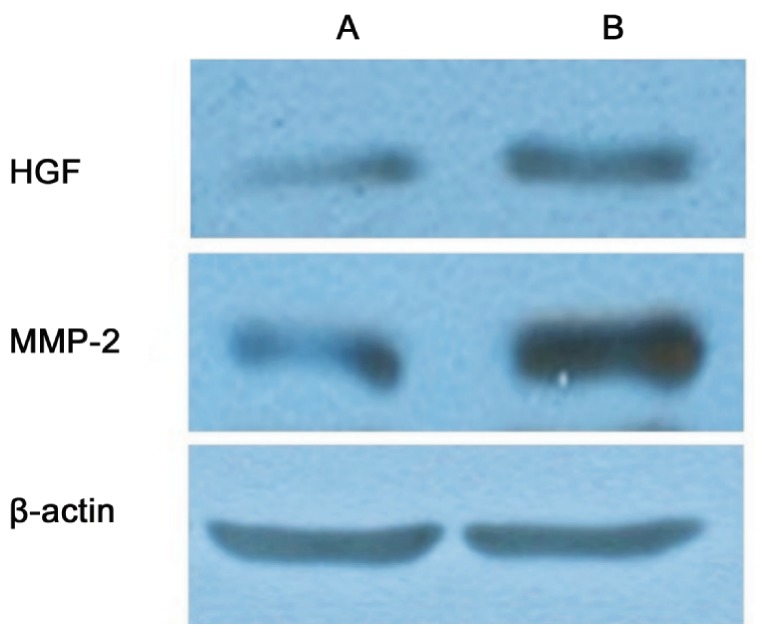
(A: Control group; B: LIM group).
Effect of HGF on MMP-2 protein expression in the scleral fibroblasts of the control group
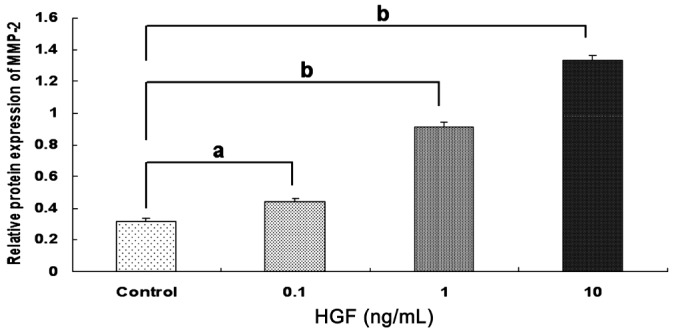
HGF increased MMP-2 protein expression in a dose-dependent manner. HGF ranged from 0.1 ng/mL to 10 ng/mL and there was significantly increased MMP-2 protein expression compared with the controls (0.35±0.03, 0.44±0.02, 0.91±0.03, 1.33±0.04, all P<0.05; Figures 6 and 7).
Figure 6. Protein expression of MMP-2 with different concentrations of HGF in the scleral fibroblasts of the control group.
HGF increased MMP-2 protein expression in a dose-dependent manner aP<0.05 vs control group, bP<0.001 vs control group.
Figure 7. Protein expression of MMP-2 with different concentrations of HGF in the scleral fibroblasts of the control group.
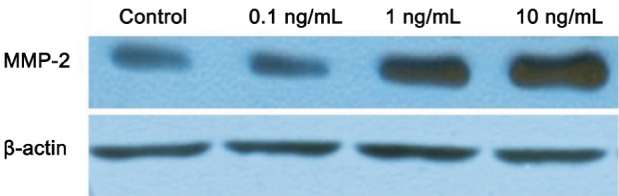
HGF increased MMP-2 protein expression in a dose-dependent manner.
Effect of HGF siRNA on MMP-2 protein expression in the scleral fibroblasts of the LIM group
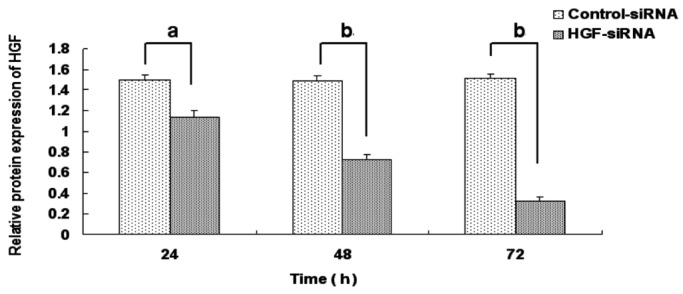
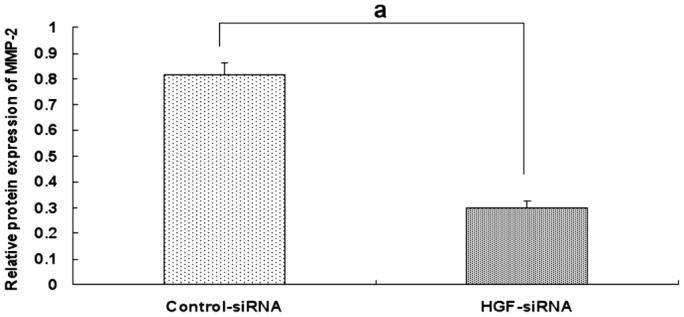
HGF siRNA suppressed HGF expression effectively in the scleral fibroblasts of the LIM group. The protein expression of HGF was decreased with extension of the transfection time compared with the negative control group and the inhibition reached the most significant stage at 72h (24h: 1.14±0.06 vs 1.50±0.05, P <0.05; 48h: 0.72±0.05 vs 1.49±0.05, P<0.05; 72h: 0.324±0.042 vs 1.52±0.04, P<0.05; Figures 8 and 9). Therefore, 72h of transfection was chosen as the time to observe the changes in MMP-2 expression. MMP-2 protein expression was significantly decreased after 72h of HGF siRNA transfection compared with the negative control group (0.29±0.03 vs 0.81±0.05, P<0.05; Figures 10 and 11).
Figure 8. Effect of HGF siRNA on HGF protein expression in scleral fibroblasts of the LIM group.
aP<0.05 vs negative control group, bP<0.001 vs negative control group.
Figure 9. Effect of HGF siRNA on HGF protein expression in scleral fibroblasts of the LIM group.
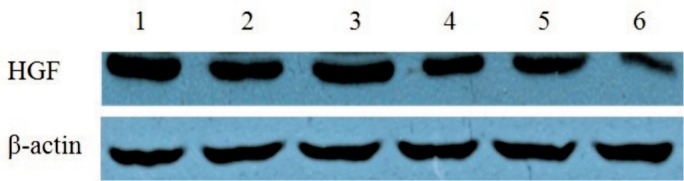
HGF knockdown decreased HGF protein expression in a time-dependent manner and HGF protein expression was significantly decreased after 72h compared with the negative control group (1: Control-siRNA 24h; 2: HGF-siRNA 24h; 3: Control-siRNA 48h; 4: HGF-siRNA 48h; 5: Control-siRNA 72h; 6: HGF-siRNA 72h).
Figure 10. Effect of 72 h of HGF siRNA on MMP-2 protein expression in the scleral fibroblasts of the LIM group.
aP<0.05 vs negative control group.
Figure 11. Effect of 72h of HGF siRNA on MMP-2 protein expression in the scleral fibroblasts of the LIM group.
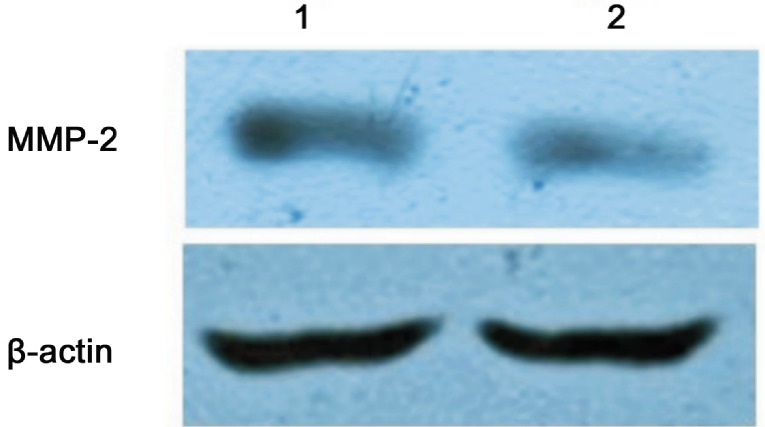
MMP-2 protein expression was significantly decreased compared with the negative control group (1: Control-siRNA; 2: HGF-siRNA).
DISCUSSION
The concave lenses used in our experiment successfully induced myopia in the guinea pigs. Over the 4-week experiment, there were myopic changes in refractive error and ocular elongation in both the control group and the LIM group. The changes in the LIM group were significantly greater than those in the control group, suggesting that the guinea pigs responded as predicted to the concave lenses and became markedly myopic. Our results suggested that the visual environment plays an important role in the occurrence of myopia. Recent studies have shown that an abnormal visual environment can lead to excessive degradation of the ECM, and thus the sclera is re-shaped and the eye becomes myopic [3]-[5]. Christensen et al[13]suggested that the sclera participates directly in the synthesis of the ECM and collagen and plays a very important role in the formation of myopia. Fibroblasts are the major cells in the sclera and are also the major source of the ECM. Therefore, the biological properties of scleral fibroblasts and the effect of external factors on fibroblasts were studied, which may provide insight into the mechanisms of myopia.
The scleral fibroblasts of the guinea pigs were cultured in vitro in this study. It was found that the cells needed 2-3wk to grow from the tissue block and, after 1wk of further culture, the cells were fused and grew faster. The primary culture time of the scleral fibroblasts was significantly longer than fibroblasts from other parts of the body (such as lung fibroblasts and skin fibroblasts), which might be related to the relatively small amount of cell components in the sclera. When the scleral fibroblasts were passaged to the 3rd-5th generation, they showed strong vitality and were suitable for the study in vitro.
During the formation of myopia, MMP-2 is considered to be a major factor in the degradation of the ECM in the posterior sclera [14], [15]. In our study, it was found that MMP-2 protein expression in scleral fibroblasts of the LIM group was higher than that of the control group (P<0.05). The formation of LIM in guinea pigs was associated with an increase in the expression of MMP-2 protein in scleral fibroblasts, which was similar to the findings from Dai et al[16], which were studies on the formation of deprivation myopia. However, the upstream factor leading to the increase in MMP-2 protein expression was unknown.
HGF has been reported to upregulate the expression of MMP-2 and enhance its activity in various tissues, resulting in ECM degradation and the inhibition of tissue fibrosis [17]-[19]. Furthermore, recent research has shown that HGF expression is closely associated with mouse ocular growth [20]. It is well known that the development of myopia is associated with excessive ocular enlargement and myopic refractive error. Therefore, HGF might be related to the development of myopia. Further study of HGF expression with Western blotting was carried out and it was found that HGF protein expression in scleral fibroblasts of the control group was low, indicating that HGF might play a role in the regulation of normal physiological functions in the sclera. HGF protein expression in scleral fibroblasts of the LIM group was increased and had the same expression trend of MMP-2. This suggests that, in the LIM group, the abnormal visual environment may stimulate the retina and ultimately play a role in the synthesis of HGF in scleral fibroblasts, which in turn upregulate MMP-2. However, it has not been reported whether HGF could increase MMP-2 expression in scleral fibroblasts. So, we stimulated the scleral fibroblasts of control eyes with HGF with different doses, and the expression of MMP-2 protein was detected by Western blotting. The results showed that different concentrations of HGF induced MMP-2 protein expression of scleral fibroblasts in the control group in a dose-dependent manner, suggesting that HGF exerts its biological effect by upregulating the expression of MMP-2. To further verify the relationship between HGF and MMP-2, the expression of HGF in scleral fibroblasts was reduced with a siRNA transfection method, thus determining whether the decreased expression of HGF could reduce MMP-2 expression. Given the relatively high expression level of endogenous HGF in scleral fibroblasts in the LIM group, it was selected for HGF siRNA transfection. The expressions of HGF and MMP-2 protein were detected by Western blotting. Experimental results showed that HGF siRNA showed a time-dependent inhibition of HGF protein expression. After 72h of transfection, HGF protein expression inhibition was significant. Therefore, 72h transfected HGF siRNA was chosen to observe the expression changes in MMP-2 protein. After 72h of HGF siRNA transfection, HGF protein expression was significantly decreased, while MMP-2 protein expression was significantly decreased at the same time. The findings suggested that the increase and decrease of MMP-2 expression were closely related to HGF, thus the upstream and downstream relationship between HGF and MMP-2 in scleral fibroblasts was identified.
This study was a preliminary investigation into the relationship between HGF and MMP-2 in scleral fibroblasts of guinea pigs with LIM. However, the exact pathway through which HGF effects MMP-2 expression remains to be explored.
Acknowledgments
Foundation: Supported by Projects of Henan Health Department, China (No.201304007); Henan Science and Technology Department, China (No.142102310110)
Conflicts of Interest: Li XJ, None; Yang XP, None, Wan GM, None; Wang YY, None; Zhang JS, None.
REFERENCES
- 1.Chen BY, Wang CY, Chen WY, Ma JX. Altered TGF-β2 and bFGF expression in scleral desmocytes from an experimentally-induced myopia guinea pig model. Graefes Arch Clin Exp Ophthalmol. 2013;251(4):1133–1144. doi: 10.1007/s00417-013-2269-8. [DOI] [PubMed] [Google Scholar]
- 2.Hu D. Progress in the study of myopic etiology and pathogenesis. Chin J Optometry Ophthalmol. 2004;6(1):1–5. [Google Scholar]
- 3.Guggenheim JA, McBrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996;37(7):1380–1395. [PubMed] [Google Scholar]
- 4.Liang YH, Li P, Zhao JX, Dai MK, Huang QF. Arsenic trioxide regulates the production and activities of matrix metalloproteinases-1, -2, and -9 in fibroblasts and THP-1. Chin Med J. 2012;125(24):4481–4187. [PubMed] [Google Scholar]
- 5.Wu DG, Wang YY, Fan LG, Luo H, Han B, Sun LH, Wang XF, Zhang JX, Cao L, Wang XR, You YP, Liu N. MicroRNA-7 regulates glioblastoma cell invasion via targeting focal adhesion kinase expression. Chin Med J. 2011;124(17):2616–2621. [PubMed] [Google Scholar]
- 6.Lim YC, Han JH, Kang HJ, Kim YS, Lee BH, Choi EC, Kim CH. Overexpression of c-Met promotes invasion and metastasis of small oral tongue carcinoma. Oral Oncol. 2012;48(11):1114–1119. doi: 10.1016/j.oraloncology.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Chang RA, Chen Z, Li ZF. Role of HGF-loaded nanoparticles in treating rat acute hepatic failure. Hepatogastroenterology. 2013;60(127):1720–1725. [PubMed] [Google Scholar]
- 8.Nurwidya F, Takahashi F, Murakami A, Kobayashi I, Kato M, Shukuya T, Tajima K, Shimada N, Takahashi K. Acquired resistance of non-small cell lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Respir Investig. 2014;52(2):82–91. doi: 10.1016/j.resinv.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(6):588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signalling pathway in cancer. Expert Opin Ther Targets. 2012;16(6):553–572. doi: 10.1517/14728222.2012.680957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui Q, Wang Z, Jiang D, Qu L, Guo J, Li Z. HGF inhibits TGF-β1-induced myofibroblast differentiation and ECM deposition via MMP-2 in Achilles tendon in rat. Eur J Appl Physiol. 2011;111(7):1457–1463. doi: 10.1007/s00421-010-1764-4. [DOI] [PubMed] [Google Scholar]
- 12.Zhou G, Williams RW. Eye1 and Eye 2: Gene loci that modulate eye size, lens weight, and retinal area in the mouse. Invest Ophthalmol Vis Sci. 1999;40(5):817–825. [PubMed] [Google Scholar]
- 13.Christensen AM, Wallman J. Evidence that increased scleral growth underlies visual deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 1991;32(7):2143–2150. [PubMed] [Google Scholar]
- 14.Yang SR, Ye JJ, Long Q. Expressions of collagen, matrix metalloproteases-2, and tissue inhibitor of matrix metalloproteinase-2 in the posterior sclera of newborn guinea pigs with negative lens-defocused myopia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2010;32(1):55–59. doi: 10.3881/j.issn.1000-503X.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Siegwart JT, Jr, Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005;46(10):3484–3492. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai SZ, Zeng JW, Wang LY. Effect of pirenzepine on form deprivation myopia in chicks and its possible mechanism. Zhonghua Yan Ke Za Zhi. 2006;42(1):42–47. [PubMed] [Google Scholar]
- 17.Lee WJ, Park SE, Rah DK. Effects of hepatocyte growth factor on collagen synthesis and matrix metalloproteinase production in keloids. J Korean Med Sci. 2011;26(8):1081–1086. doi: 10.3346/jkms.2011.26.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willems S, Verleden SE, Vanaudenaerde BM, Wynants M, Dooms C, Yserbyt J, Somers J, Verbeken EK, Verleden GM, Wuyts WA. Multiplex protein profiling of bronchoalveolar lavage in idiopathic pulmonary fibrosis and hypersensitivity pneumonitis. Ann Thorac Med. 2013;8(1):38–45. doi: 10.4103/1817-1737.105718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signalling pathway in cancer. Eur J Cancer. 2010;46(7):1260–1270. doi: 10.1016/j.ejca.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G, Wang Y, Zhang Y, Wan X, Li J, Liu K, Wang F, Liu K, Liu Q, Yang C, Yu P, Huang Y, Wang S, Jiang P, Qu Z, Luan J, Duan H, Zhang L, Hou A, Jin S, Hsieh TC, Wu E. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr Mol Med. 2012;12(2):163–176. doi: 10.2174/156652412798889063. [DOI] [PMC free article] [PubMed] [Google Scholar]