Abstract
AIM
To study the efficacy difference between form-deprived myopia (FDM) and lens-induced myopia (LIM), the degree of myopia, axial length and pathological changes of the posterior sclera from guinea pigs were evaluated.
METHODS
Four-week pigmented guinea pigs were randomly assigned into 3 groups, including normal control (n=6), FDM group with monocular cover (n=11) and LIM group with monocular -7D lens treatment (n=11). FDM group was form-deprived while LIM group was lens-induced for 14 d. Refractive error and axial length were measured prior to and post treatment, respectively. Morphological changes of sclera were examined using both light and electronic microscopes.
RESULTS
After 14d treatment, refractive errors for FDM group and LIM group were -3.05±0.71D and -2.12±1.29D, respectively, which were significantly more myopic than that of normal controls and fellow control eyes (P<0.01). As for axial length, it was 7.93±0.03 mm for FDM group and 7.89±0.06 mm for LIM group, which were significantly longer than both normal and fellow controls (P<0.01). With respect to both refractory error and axial length, the differences between FDM group and LIM group were not significant (P>0.05). Under light microscope, both FDM group and LIM group showed thinned sclera, disarrangement of fibrosis and enlarged disassociation between fibers. Consistently, ultrastructural examination showed degenerated fibroblasts and thinned fibers in posterior sclera.
CONCLUSION
Following two weeks of myopia induction in guinea pigs, with regard to the degree of myopia, axial length and pathological alterations, there was no significant difference between FDM and LIM models. Therefore, FDM and LIM are equally effective and useful as a model of experimental myopia and guinea pigs are ideal animals for induction of experimental myopia because their high sensitivity to both form-deprivation and lens-induction.
Keywords: form-deprived myopia, lens-induced myopia, pathology
INTRODUCTION
Worldwide, the incidence of myopia is increasing gradually and 30% of the whole population is suffering from myopia. Certain serious myopia syndromes can cause blindness. The establishment of experimental myopia provides a fundamental model for the investigation of myopia etiology. Experimental myopia is only induced in the laboratory, which is now widely used to study human myopia because anatomical structure and characteristics of refraction of some animals are similar to human spontaneous myopia. A variety of animals, including chick, primate, tree shrew, mouse, cat and guinea pig, have been used to investigate the mechanisms of axial myopia[1]-[4]. Both form deprivation and lens induction could elongate axial length of eyes and thus caused myopic refraction of various animals[5]-[7]. With respect to the underlying cellular mechanisms, form-deprived myopia (FDM) and lens-induced myopia (LIM) are two different types of experimental myopia. FDM is induced by forbidding animals to see whereas LIM is induced through wearing concave lens before animals' eyes to disturbing image formation behind the retina, which induces excessive accommodation and extension of axial length, thereby leading to axial myopia[8],[9].
FDM differs from LIM in many ways[10]-[12]. Previous studies have shown that FDM caused local alterations in retina and usually was not associated with the central nerve system. However, LIM could be inhibited by breaking the circadian rhythm, which was not the case for FDM. As for their association with circadian rhythm, the differences between FDM and LIM were confirmed by the recovery of myopia through holding the circadian rhythm. The identical results in FDM and LIM were refractive error, extending axial length and abnormal growth of the sclera[13]. However, to our best knowledge, there are few reports about the differences between these two types of myopia models under the same experimental conditions, such as experimental duration and environment. The current study compares FDM and LIM of guinea pigs under the same experimental conditions by assessing the degree of refractive error, axial length and morphological alterations of the sclera.
MATERIALS AND METHODS
Materials
The animal research protocol used in this study was approved by Experimental Animal Center and Ethnics Committee in China Medical University. All experimental procedures were under Helsinki declaration. Twenty-eight pigmented guinea pigs, approximately 4 weeks old, were obtained from the Experimental Animal Center, China Medical University. All animals underwent biometric measurement for refraction and axial length prior to the experiment. To evaluate the association between myopic development and treatment duration, all animals in the experimental groups underwent biometric measurement after 14 d treatment following removal of the facemasks and lenses. As for the normal control group, all animals underwent biometric measurement at the same time point.
Methods
Guinea pigs were randomly divided into three groups: FDM (n=11), LIM (n=11) and normal control (n=6). LIM group included LIM refraction eyes (one eye wearing contact lens, 11 eyes) and LIM fellow control eyes (the other eye without contact lens, 11 eyes) while FDM group included FDM refraction eyes (one eye wearing opaque eyeshade, 11 eyes) and FDM fellow control eyes (the other eye without any intervention, 11 eyes). Normal controls were not intervened except for eyes related with examination. The animals were raised for 14 d and then measured, and specimens were processed.
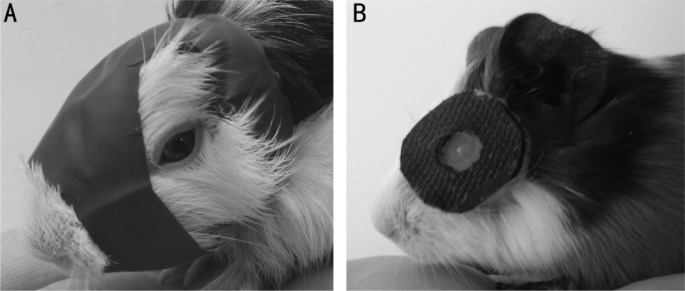
FDM was induced by applying monocularly deprived facemask as previously described[6]. LIM was induced by cutting Velcro belt into a ring with 2 cm in diameter, and then creating a hole with 0.8 cm in diameter in the middle of the ring and inserting PMMA contact lens (Lens diameter 13.5 mm, optical diameter 10.5 mm, base curve 9.6 mm, diopter -7D) into the hole with convex plane facing outwards. PMMA contact lens was placed in front of a random eye by gluing Velcro belt on the animal with celloidin (Figure 1). The animals were specially cared by animal care personnel in the room with 14/10 hour light cycle at 20-25°C. The animals were checked once every two hours in the daytime to clean the dirty lens with swab or to refix lens in front of the eye if needed. Lenses and facemasks were demounted 14d after treatment and animals were processed for further experiment.
Figure 1. FDM (A) and LIM (B) in guinea pigs.
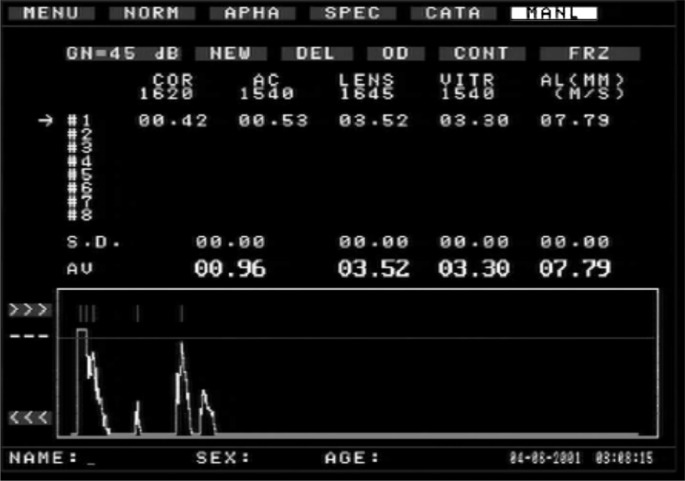
Refraction examination and eye axial length measurement were performed before and after myopia induction, guinea pigs were administered with 10 g/L tropicamide-phenylephrine ophthalmic solution on both eyes' conjunctival sac four times with 5 min interval, and then eyes were examined with streak retinoscopy in the darkroom. Meanwhile, the axial length of both eyes were also manually measured 10 times by A-scan ultrasonography (Maida Corporation, China) with accuracy at 0.01 mm and then mean values were calculated for analysis (Figure 2). All procedures were performed following anaesthetizing guinea pigs with 0.4% oxybuprocatine (1/5min, 2-3 times).
Figure 2. Results of the A-scan of the axial length of guinea pig's eyes.
Specimens processing
Eyeballs were extracted under aseptic condition after guinea pigs were sacrificed. Front part of eye tissue and vitreous body were removed by splitting eyeball along ora serrata under surgery microscope (Topcon, Japan). The sclera tissue was stained by Hematoxylin & Eosin and observed under Olympus BH-2 microphotoscope to examine histopathological changes. Hitachi H-600 electric microscope was used to study the ultrastructural changes of sclera fiber.
Statistical Analysis
The refractive status and axial length were compared among the different groups with one-way ANOVA after Bonferroni correction (SPSS Version 16.0, IBM SPSS, USA). Absolute values of biometric results were also compared among the different groups using the same statistical analysis. The difference was defined as significant at P<0.05 and highly significant at P<0.01.
RESULTS
As for refractive error and eye axial length, there were no statistical significance among LIM, FDM and normal control groups before intervention and both eyes of guinea pigs did not show significant difference (P>0.05 for all, Table 1). All eyes were under hypermetropia status prior to the experiment. Fourteen days after treatment, refractive errors for FDM group and LIM group were -3.05±0.71D and -2.12±1.29D respectively, which were significantly more myopic than that of normal control and fellow control eyes (P<0.01 for all). There was no significant difference between FDM and LIM groups (P>0.05 for both, Table 2, Figure 3). With respect to axial length, it was 7.93±0.03 mm for FDM group and 7.89±0.06 mm for LIM group, which were significantly longer than that of normal control group and fellow control eyes (P<0.01 for all, Table 3, Figure 4). As for both refractive error and axial length, there were no significant differences between FDM and LIM groups (P>0.05).
Table 1. Refraction and axial length of the experimental animals before experiment.
| Groups | n | Refraction (Diopter) | Axial length (mm) |
| Experimental | 28 | 2.71±1.29 | 7.63±0.04 |
| Fellow | 28 | 2.57±1.31 | 7.64±0.04 |
x±SD
Table 2. Refraction of three groups post-experiment.
| Groups | n | Refraction (Diopter) |
|
| Experimental | Fellow | ||
| NOR | 6 | 3.15±0.94 | 3.25±1.31 |
| FDM | 11 | -3.05±0.71b | 2.29±1.01 |
| LIM | 11 | -2.12±1.29b | 1.92±1.05 |
bP<0.01 vs normal control group.
x±SD
Figure 3. Comparison of refraction post experiment.
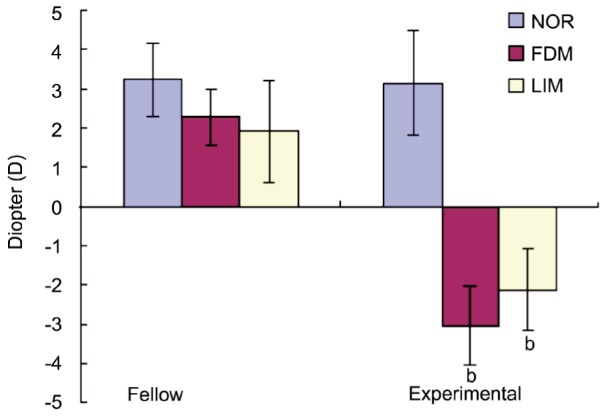
bP<0.01.
Table 3. Axial Length of three groups post-experiment.
| Groups | n | Axial length (mm) |
|
| Experimental | Fellow | ||
| NOR | 6 | 7.75±0.03 | 7.75±0.04 |
| FDM | 11 | 7.93±0.03b | 7.75±0.02 |
| LIM | 11 | 7.89±0.06b | 7.75±0.03 |
bP<0.01 vs normal control group.
x±SD
Figure 4. Comparison of axial length post experiment.
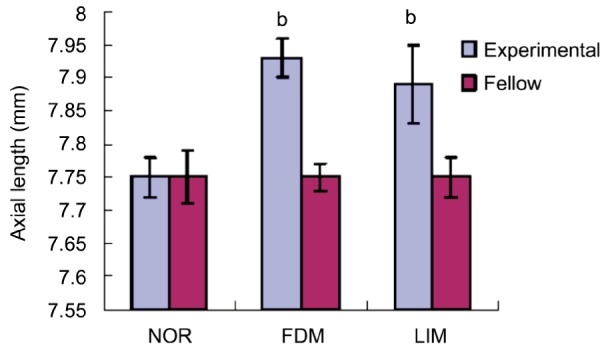
bP<0.01.
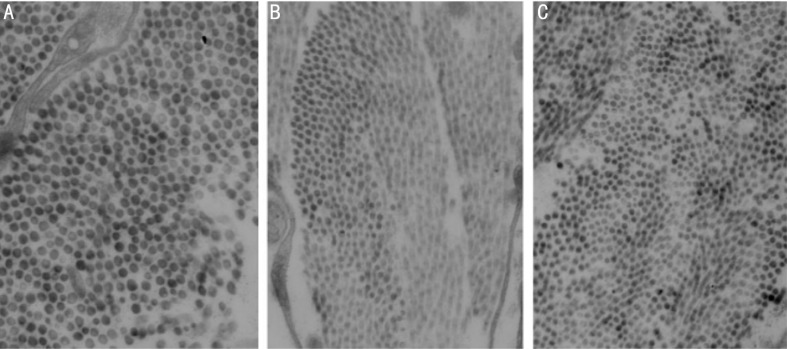
Under light microscope, both FDM and LIM groups showed thinned sclera, disarrangement of fibrosis and enlarged disassociation between fibers (Figure 5). Furthermore, thinner diameter of the posterior sclera collagen fiber and vacuolar degeneration in fibroblasts was observed using electronic microscope (Figure 6).
Figure 5. Representative H.E pictures of posterior scleral fibers of normal control (A), FDM (B) and LIM (C), ×200.
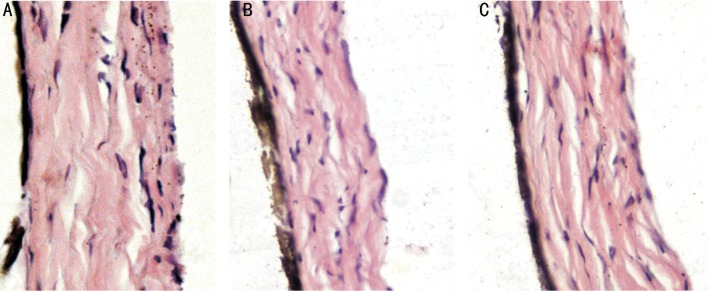
Figure 6. Representative ultrastructural pictures of posterior scleral fibers from normal control (A), FDM (B) and LIM (C), ×6000.
DISCUSSION
To the best of our knowledge, the current study for the first time, under the same experimental conditions, compared the differences of axial myopia between two models of experimental myopia of guinea pigs by investigating the degree of myopia, axial length and pathological changes. Our study showed that both form deprivation and lens induction could elongate eye axial length and cause myopic refraction effectively.
With the development of molecular biology, the studies of myopia etiology have been widely carried out and been well-developed especially after the introduction of the form-deprived myopia animal model by Wiesel and Raviola in 1977[7]. Axial elongation caused by myopia could be induced in developing animals by visual form deprivation or by raising the animal with negative lenses in place[6],[14]-[16]. The mechanisms of myopia development were further investigated. However, previous studies showed controversial results about the animal models. The usual applying models were avian (chick) and mammalian (tree shrews or primate). Newly-hatched chickens were most susceptible to myopic development as myopia ranging from -4 to -6 diopters could be induced within 1 wk of form deprivation[17],[18]. The experimental -8.06D myopia was induced by semitransparent goggles for four days and -10.58D myopia was induced by -15D lens for 11d[19],[20]. In the chick eyes, the sclera consisted of an inner cartilaginous layer and an outer fibrous layer, and eye elongation involved the increase in total protein accumulation by proteoglycan synthesis and proteoglycan accumulation and growth of the inner cartilaginous layer[21]-[23]. However, in the mammalian eyes, in which the sclera comprises a single fibrous layer, ocular expansion did not involve net growth rather than a remodeling of the sclera with decreased proteoglycan synthesis and a net loss of scleral tissue[24]. In addition, the accommodating muscle of chick was not smooth muscle but striated muscle and the receptor not nicotine but hydroxycholine. The mechanics of accommodation and the innervation were different compared to mammalian eyes. Therefore, chick is not an ideal model for the study of human myopia due to the differences between human and chicken regarding the visual axis, ocular anatomy and accommodation mechanisms. Primates are the most ideal models for human myopia as they are most similar to human in terms of ocular anatomy and physiology[25],[26]. However, availability of primates is very limited and the cost is higher than other animals. Moreover, the myopia induced in primates generally requires a longer duration than other animals. As an alternative to the primate model, tree shrews and guinea pigs could develop myopia ranging from -3 to -6 diopters within 1 wk of form deprivation[27]. As for mice, they were hyperopic and their eyes were shorter at the end of the lid-suture period, therefore they developed myopia only when the lid-suture was discontinued and form vision was restored. Furthermore, mice were unlikely to have accommodation since they lacked ciliary muscles in their eyes[28]. Previous study also showed that form deprivation in cats did not consistently induce axial myopia[29]. Therefore, guinea pigs appear to be a suitable animal model for the study of human myopia.
Guinea pigs were applied widely as experimental animals in biomedical studies. They are an ideal model because of low cost, mild temper and easy raise. Moreover, the ocular anatomic structure, biologic structure and physiological function of guinea pigs were similar to human and primates. Because guinea pigs were sensitive to form-deprivation and lens-induction, they were applied in myopia study recently[30],[31]. High myopia could be induced in short time and had emmetropia process postnatally. McFadden et al[3] reported form deprivation induced -6.6D myopia in five-day old guinea pigs and prolonged axial length by 0.146 mm. Form deprivation also induced -5.8D myopia and prolonged axial length by 0.170 mm in two-day old guinea pig. Lu et al's[6] study showed that form-deprived 3-wk guinea pig for 14d induced -2.2D myopia. Consistently, our study showed that 14 d of form deprivation on four-week old guinea pigs induced -3.05D myopia and prolonged 0.18 mm of axial length. Furthermore, a -7D concave lens placed over the eye induced -2.12D and prolonged 0.14 mm.
After 14 d treatment, refractive errors for FDM group and LIM group were -3.05±0.71D and -2.12±1.29D, respectively. Significant differences, compared with that of normal control group and fellow control eyes, were observed. In terms of axial length, it was 7.93±0.03 mm for FDM group and 7.89±0.06 mm for LIM group. With respect to both refractive error and axial length, significant differences between FDM group and LIM group were not presented. However, there were no significant differences among three fellow groups which showed axial myopia induced by FD and LI. There were no difference in the diopter and axial length following fourteen days treatment.
Under light microscope, both FDM group and LIM group showed thinned sclera, disarrangement of fibrosis and enlarged disassociation between fibers. Strikingly, posterior sclera demonstrated degenerated fibroblasts and decreased fibers. The sclera is the outer coating of the eyes which, in addition to protecting the retina and allowing the attachment of the extraocular muscles, controls the size of the eyes and the location of the retina relative to the focal plane. Sclera is made up with fibroblasts and the collagen bundle which parallel the eye wall under electric microscope. The diameter of the posterior sclera fibroblasts was thinner and the disarranged fibrosis was enlarged compared with that from normal control group and there was vacuolar degeneration in the fibroblasts. Previous studies showed that animals' eyeballs had subjective emmetropia process after birth[17],[31]. Moreover, Liu et al[32] reported that the chief differences at posterior sclera were diameter and morphology of fibroblasts between normal and myopia eyes, which were parallel with the results of our study. In terms of the degree of myopia after two-week induction, there was no significant difference between FDM and LIM. Although the underlying mechanism of myopia formation was not identical, the induced myopia was similar.
FDM was induced by wearing facemask and LIM was induced by placing Velcro belt. The Velcro belt was glued on the animal fossa orbitalis with celloidin. Both methods had the advantages which don't oppress the eyelid and eyeball, and removal and operation were easy without need of anesthesia. Our results were consistent with previous studies regarding the myopia diopter and induced axial length[6],[30]. Moreover, the eyes from guinea pigs and human being share similar anatomical structure because both of them are mammal. The methods used here are suitable and effective to construct myopia models. Guinea pigs are an ideal model for induction of experimental myopia because they are sensitive to form-deprivation and lens-induction, both of which induce axial myopia. In our study, the models of experimental myopia established are operation-friendly without the necessity of anaesthesia.
Acknowledgments
Conflicts of Interest: Xiao H, None; Fan ZY, None; Tian XD, None; Xu YC, None.
REFERENCES
- 1.Edwards MH. Animal models of myopia: a review. Acta Ophthalmol Scand. 1996;74(3):213–219. doi: 10.1111/j.1600-0420.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 2.Qian YS, Chu RY, Hu M, Hoffman MR. Sonic hedgehog expression and its role in form-deprivation myopia in mice. Curr Eye Res. 2009;34(8):623–635. doi: 10.1080/02713680903003492. [DOI] [PubMed] [Google Scholar]
- 3.McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44(7):643–653. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Norton TT, Amedo AO, Siegwart JT., Jr The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50(6):564–576. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashby RS, Megaw PL, Morgan IG. Changes in retinal alphaB-crystallin (cryab) RNA transcript levels during periods of altered ocular growth in chickens. Exp Eye Res. 2010;90(2):238–243. doi: 10.1016/j.exer.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Lu F, Zhou X, Zhao H, Wang R, Jia D, Jiang L, Xie R, Qu J. Axial myopia induced by a monocularly-deprived facemask in guinea pigs: a non-invasive and effective model. Exp Eye Res. 2006;82(4):628–636. doi: 10.1016/j.exer.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266(5597):66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 8.Sivak JG. The role of the lens in refractive development of the eye: animal models of ametropia. Exp Eye Res. 2008;87(1):3–8. doi: 10.1016/j.exer.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Troilo D, Quinn N, Baker K. Accommodation and induced myopia in marmosets. Vision Res. 2007;47(9):1228–1244. doi: 10.1016/j.visres.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith EL, 3rd, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53(1):421–428. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51(10):5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- 12.Bartmann M, Schaeffel F, Hagel G, Zrenner E. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Vis Neurosci. 1994;11(2):199–208. doi: 10.1017/s0952523800001565. [DOI] [PubMed] [Google Scholar]
- 13.Morgan IG, Ashby RS, Nickla DL. Form deprivation and lens-induced myopia: are they different? Ophthalmic Physiol Opt. 2013;33(3):355–361. doi: 10.1111/opo.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EL, 3rd, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50(11):5057–5069. doi: 10.1167/iovs.08-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49(2):219–227. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41(3):267–273. doi: 10.1016/s0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 17.Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27(7):1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 18.Pickett-Seltner RL, Sivak JG, Pasternak JJ. Experimentally induced myopia in chicks: morphometric and biochemical analysis during the first 14 days after hatching. Vision Res. 1988;28(2):323–328. doi: 10.1016/0042-6989(88)90160-5. [DOI] [PubMed] [Google Scholar]
- 19.Schmid KL, Wildsoet CF. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004;81(2):137–147. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 20.García de la Cera E, Rodríguez G, Marcos S. Longitudinal changes of optical aberrations in normal and form-deprived myopic chick eyes. Vision Res. 2006;46(4):579–589. doi: 10.1016/j.visres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Rada JA, Thoft RA, Hassell JR. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev Biol. 1991;147(2):303–312. doi: 10.1016/0012-1606(91)90288-e. [DOI] [PubMed] [Google Scholar]
- 22.Rada JA, Johnson JM, Achen VR, Rada KG. Inhibition of scleral proteoglycan synthesis blocks deprivation-induced axial elongation in chicks. Exp Eye Res. 2002;74(2):205–215. doi: 10.1006/exer.2001.1113. [DOI] [PubMed] [Google Scholar]
- 23.Gentle A, Truong HT, McBrien NA. Glycosaminoglycan synthesis in the separate layers of the chick sclera during myopic eye growth: comparison with mammals. Curr Eye Res. 2001;23(3):179–184. doi: 10.1076/ceyr.23.3.179.5466. [DOI] [PubMed] [Google Scholar]
- 24.Gao H, Frost MR, Siegwart JT, Jr, Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis. 2011;17:903–919. [PMC free article] [PubMed] [Google Scholar]
- 25.Rada JA, Nickla DL, Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000;41(8):2050–2058. [PubMed] [Google Scholar]
- 26.Troilo D, Nickla DL, Wildsoet CF. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41(6):1249–1258. [PubMed] [Google Scholar]
- 27.Troilo D, Nickla DL. The response to visual form deprivation differs with age in marmosets. Invest Ophthalmol Vis Sci. 2005;6(6):1873–1881. doi: 10.1167/iovs.04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remtulla S, Hallett PE. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 1985;25(1):21–31. doi: 10.1016/0042-6989(85)90076-8. [DOI] [PubMed] [Google Scholar]
- 29.Kirby AW, Sutton L, Weiss H. Elongation of cat eyes following neonatal lid suture. Invest Ophthalmol Vis Sci. 1982;22(2):274–277. [PubMed] [Google Scholar]
- 30.Dong F, Zhi Z, Pan M, Xie R, Qin X, Lu R, Mao X, Chen JF, Willcox MD, Qu J, Zhou X. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011;17:2824–2834. [PMC free article] [PubMed] [Google Scholar]
- 31.Howlett MH, McFadden SA. Emmetropization and schematic eye models in developing pigmented guinea pigs. Vision Res. 2007;47(9):1178–1190. doi: 10.1016/j.visres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Liu KR, Chen MS, Ko LS. Electron microscopic studies of the scleral collagen fiber in excessively high myopia. Taiwan Yixuehui Zazhi. 1986;85(11):1032–1038. [PubMed] [Google Scholar]