Abstract
AIM
To report the visual outcome based on various patterns of optical coherence tomography (OCT) morphology in diabetic macular edema (DME), following treatment with anti-VEGF intravitreal bevacizumab (IVB) injection.
METHODS
Sixty-seven consecutive subjects with centre involving DME underwent intravitreal injection of Bevacizumab (1.25 mg/0.05 mL) in this retrospective, comparative, non randomized study. The DME was classified into one of four categories: focal, diffuse, focal cystoid and neurosensory detachment based on OCT. Best corrected visual acuity (BCVA), macular appearance, and OCT findings were used to decide whether the subject should have a repeat injection of intravitreal bevacizumab. Outcome measures were a change in mean BCVA (Snellen converted to logMAR) and central macular thickness (CMT) in each group during the six month follow-up period.
RESULTS
The mean BCVA improved to logMAR 0.23 at final follow-up from a baseline of 0.32 logMAR (P=0.040) in the focal group, logMAR 0.80 at final follow-up from a baseline of 0.82 logMAR (P=0.838) in the diffuse group, worsened to logMAR 0.53 at final follow-up from a baseline of 0.43 logMAR (P=0.276) in the focal cystoid group, and improved to logMAR 0.79 at final follow-up from a baseline of 0.93 logMAR (P=0.490) in the neurosensory detachment group. The mean CMT before treatment were 298.8±25.03 µm in the focal group, 310.8±40.6 µm in the diffuse group, 397.15±31.05 µm in the focal cystoid group and 401.03±75.1 µm in the neurosensory detachment group. A mean of 2.05 (range: 1-5) injections in the focal group, 1.32 (range: 1-2) in the diffuse group, 2.6 (range: 1-6) in the focal cystoid group and 2.6 (range: 1-6) in the neurosensory detachment group were performed during the six month follow-up period. Following intravitreal bevacizumab treatment, vision improved, remained unchanged or worsened in 11, 7 and 2 subjects in focal group; 11, 9 and 8 in diffuse group; 0, 2 and 4 in focal cystoid group and 5, 5 and 3 subjects respectively in neurosensory detachment group.
CONCLUSION
OCT morpholgy patterns in DME may predict the effects of intravitreal bevacizumab treatment, and patients with focal DME are most likely to benefit from the improvent of visual acuity from this treatment.
Keywords: optical coherence tomography, diabetic macular edema, intravitreal injection, bevacizumab
INTRODUCTION
Diabetic retinopathy (DR) is one of the leading causes of vision loss in the world[1],[2]. The main cause of vision impairment in diabetic patients is diabetic macular edema (DME). A large epidemiological study indicated that macular edema was present in 15% of the study patients with DR [3].
Vascular endothelial growth factor (VEGF) is a potent endothelial cell angiogenic factor, and a powerful mediator of vascular permeability. It leads to the breakdown of the blood-retinal barrier in DR, causing leakage of intravascular fluid from abnormal retinal capillaries, resulting in DME [4]. Therefore, at present, treatment with anti-VEGF agents is one of the most promising approaches for the treatment of vision loss due to DME[5],[6]. Various studies have established the safety and efficacy of anti-VEGF agents, including ranibizumab and bevacizumab, in the treatment of DME[7]-[9].
Various patterns of DME have been recognized on optical coherence tomography (OCT). However, there are few published studies reporting visual outcomes for various OCT patterns in DME treated with anti-VEGF therapy, and none from the Arabian Peninsula where diabetes is common and the vast majority of patients suffer from visual disability due to DME[10]. The aim of this study is to report the visual outcome based on various patterns of OCT morphology in DME, following treatment with anti-VEGF intravitreal bevacizumab (IVB) injection in Saudi patients.
SUBJECTS AND METHODS
Subjects
We conducted a retrospective, comparative, non-randomized study on a consecutive series of patients who underwent intravitreal injection of bevacizumab for DME. Sixty-seven consecutive subjects with centre involving DME were included in the study. The study adhered to the tenets of the Declaration of Helsinki, was approved by the Institutional Review Board of the hospital and informed consent was obtained. The subjects were recruited from the retina clinic at Dhahran Eye Specialist Hospital, a tertiary referral ophthalmic hospital in the Eastern province of Saudi Arabia. Patients in the study were identified by injection room log, and were followed up for six months after the initiation of the intravitreal injection treatment. The diagnosis of DME was established in each subject by clinical examination and OCT.
Intravitreal Bevacizumab Injection and Follow-up
All subjects received 1.25 mg/0.05 mL intravitreal injection of anti-VEGF agent bevacizumab (Avastin®, Genentech Inc., San Francisco, CA, USA). The lids and conjunctiva were cleansed with 10% and 5% povidone and iodine, respectively, followed by an injection of 2% lidocaine to anesthetise the conjunctiva. Bevacizumab (1.25 mg) was injected with a 30 g needle through the pars plana (3.5 mm from limbus) into the vitreous. An eye pad was placed and 0.3% gatifloxacin topical drops were prescribed to be instilled four times daily for 4d.
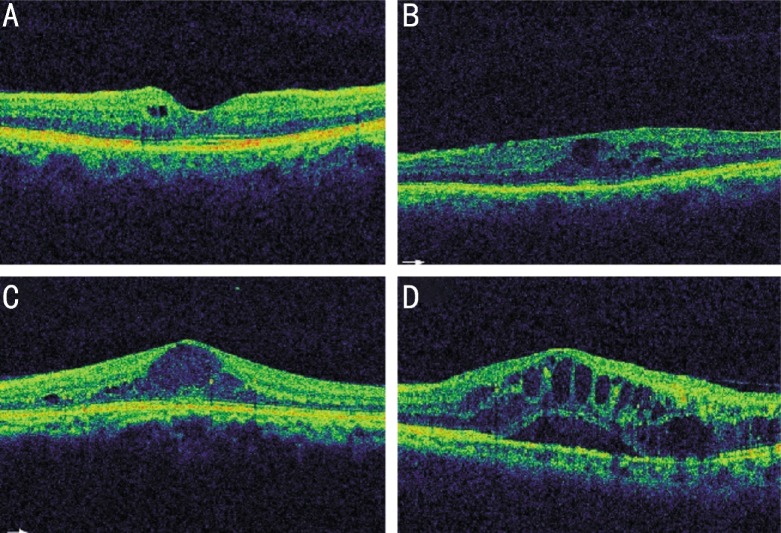
An OCT appearance of the macular image taken at the initiation of treatment was graded in a masked fashion by 2 retina specialists. The DME was classified into one of four categories. Patients with focal DME had limited retinal thickening with cyst formation and preservation of the macular contours (Figure 1A), diffuse DME patients had widespread retinal thickening with a sponge like appearance of the macula (Figure 1B), patients with focal cystoid DME had a mound like appearance of the fovea due to focal collection of fluid at the fovea (Figure 1C), and patients with neurosensory detachment DME had an associated subretinal collection of fluid under the fovea (Figure 1D). Images with mixed features were categorised based on predominant features. Patients who had an OCT appearance of vitreomacular traction and foveal plaque were excluded from the study.
Figure 1. DME classification based on OCT appearance.
A: Patients with focal DME had limited retinal thickening with cyst formation and preservation of the macular contours; B: Diffuse DME patients had widespread retinal thickening with a sponge like appearance of the macula; C: Patients with focal cystoid DME had a mound like appearance of the fovea due to focal collection of fluid at the fovea; D: Patients with neurosensory detachment DME had an associated subretinal collection of fluid under the fovea.
At each follow-up visit, a full ophthalmic assessment including an evaluation of retinal morphology with OCT was performed. Evaluation of the macula with OCT was performed with commercially available equipment (Topcon Medical Systems, Inc) to image any intraretinal edema, subretinal fluid or other macular abnormalities. Retinal thickness was measured in a circle of 3.5 mm in diameter corresponding to five zones (central, nasal, temporal, superior and inferior) centered on the fixation point. Mean thickness on the 1 mm circle centered on the fovea was measured, and thickness of all five zones was used to calculate average central macular thickness (CMT).
Best corrected visual acuity (BCVA), macular appearance, and OCT findings were used to decide whether the subject should have a repeat injection of IVB. However, it was left at the discretion of the treating physician to follow a specific regimen or treat patients on PRN (Pro re nata, as required) basis.
Statistical Analysis
Outcome measures were a change in mean BCVA (Snellen converted to logMAR) and CMT in each group during the six month follow-up period. Statistical analysis, t-test for parametric data, and one way ANOVA was performed with SPSS statistical software (version 17.0.1, IBM Corp., Somers, NY, USA). P<0.05 was considered statistically significant.
RESULTS
Sixty-seven consecutive subjects with DME were included in the study. There were 13 males and 7 females in the focal group, 19 males and 9 females in the diffuse group, 4 males and 2 females in the focal cystoids group and 11 males and 2 females in the neurosensory detachment group. The mean BCVA before treatment was 0.32±0.268 logMAR (range: 0.0 logMAR to 0.9 logMAR) in the focal group, 0.82±0.73 logMAR (range: 0.0 logMAR to 3.0 logMAR) in the diffuse group, 0.43±0.16 logMAR (range: 0.2 logMAR to 0.7 logMAR) in the focal cystoids group and 0.93±0.64 logMAR (range: 0.0 logMAR to 2.0 logMAR) in the neurosensory detachment group. The mean CMT before treatment were 298.8±25.03 µm in the focal group, 310.8±40.6 µm in the diffuse group, 397.15±31.05 µm in the focal cystoids group and 401.03±75.1 µm in the neurosensory detachment group (Table 1).
Table 1. Characteristics of groups of patients in the study.
| Parameters | Focal group | Diffuse group | Focal cystoid group | Neurosensory detachment group |
| No. of patients | 20 | 28 | 6 | 13 |
| Age (range) | 53.2 (30-72) | 60.9 (30-94) | 58.1 (46-64) | 52.15 (38-74) |
| Sex (M/F) | 13/7 | 19/9 | 4/2 | 11/2 |
| Number of IVB: mean (range) | 2.05 (1-5) | 1.32 (1-2) | 2.6 (1-6) | 2.6 (1-6) |
| Pre- IVB visual acuity LogMAR: x±s (range) |
0.32±0.268 (0.0-0.9) |
0.82±0.73 (0.0-3.0) |
0.43±0.16 (0.2-0.7) |
0.93±0.64 (0.0-2.0) |
| Pre IVB OCT thickness Microns: x±s (range) |
298.8±25.03 (264.5-349.6) |
310.8±40.6 (231.9-414.4) |
397.15±31.05 (364.7-445.7) |
401.03±75.1 (309.8-540.7) |
IVB: Intravitreal bevacizumab.
A mean of 2.05 (range: 1-5) injections in the focal group, 1.32 (range: 1-2) in the diffuse group, 2.6 (range: 1-6) in the focal cystoids group and 2.6 (range: 1-6) in the neurosensory detachment group were performed during the six month follow-up period.
The mean BCVA improved to logMAR 0.235 at final follow-up from a baseline of 0.32 logMAR (P=0.040) in the focal group, logMAR 0.80 at final follow-up from a baseline of 0.82 logMAR (P=0.838) in the diffuse group, worsened to logMAR 0.53 at final follow-up from a baseline of 0.43 logMAR (P=0.276) in the focal cystoids group, and improved to logMAR 0.79 at final follow-up from a baseline of 0.93 logMAR (P=0.490) in the neurosensory detachment group (Table 2).
Table 2. Statistical analysis of outcome in different groups.
| Groups | Parameter | Pre-treatment | Post IVB injection | P |
| Focal | Visual acuity LogMAR: x±s | 0.32±0.268 | 0.235±0.27 | 0.040 |
| OCT thickness Microns: x±s | 298.8±25.03 | 284.6±24.4 | 0.004 | |
| Diffuse | Visual acuity LogMAR: x±s | 0.82±0.73 | 0.80±0.76 | 0.838 |
| OCT thickness Microns: x±s | 310.8±40.6 | 291.94±50.1 | 0.021 | |
| Focal cystoid | Visual acuity LogMAR: x±s | 0.43±0.16 | 0.53±0.25 | 0.276 |
| OCT thickness Microns: x±s | 397.15±31.05 | 374.2±50.23 | 0.208 | |
| Neurosensory detachment | Visual acuity LogMAR: x±s | 0.93±0.64 | 0.79±0.62 | 0.490 |
| OCT thickness Microns: x±s | 401.03±75.1 | 365.0±57.6 | 0.052 |
IVB: Intravitreal bevacizumab.
The mean CMT decreased from 298.8±25.03 µm before treatment to 284.6±24.4 µm at the last visit (P=0.004) in the focal group, from 310.8±40.6 µm before treatment to 291.94±50.1 µm at the last visit (P=0.021) in the diffuse group, from 397.15±31.05 µm before treatment to 374.2±50.23 µm at the last visit (P=0.208) in the focal cystoids group and from 401.03±75.1 µm before treatment to 365.0±57.6 µm at the last visit (P=0.052) in the neurosensory detachment group (Table 2).
Following IVB treatment, vision improved, remained unchanged or worsened in 11, 7 and 2 subjects in focal group; 11, 9 and 8 in diffuse group; 0, 2 and 4 in focal cystoid group and 5, 5 and 3 subjects respectively in neurosensory detachment group (Table 3).
Table 3. Visual status of different groups following IVB injection.
| OCT morphology (groups) | Improved | No change | Worse | Total |
| Focal | 11 | 7 | 2 | 20 |
| Diffuse | 11 | 9 | 8 | 28 |
| Focal cystoid | 0 | 2 | 4 | 6 |
| Neurosensory detachment | 5 | 5 | 3 | 13 |
A one-way between subjects ANOVA was conducted to compare the effect of OCT morphology on final visual acuity following the injection of IVB. There was a significant effect on visual acuity at the P<0.05 for the focal OCT morphology [F (3.63)=4.14, P=0.010]. Post hoc comparisons using the Tukey HSD test indicated that the mean score for the focal group (mean=0.23; SD=0.27) was significantly different than other OCT morphology groups. Taken together, these results suggest that focal appearance of macular edema on OCT was associated with improved visual acuity following IVB injection.
DISCUSSION
DME is a major cause of vision loss in patients with diabetic retinopathy[11]. It results from the disruption of the blood retinal barrier with a consequent intraretinal accumulation of fluid, and when the pumping ability of the retinal pigment epithelium is overwhelmed, subretinal fluid accumulation can also occur. Although a stereoscopic clinical assessment of the macula with a fundus lens at slit lamp is the gold standard for diagnosing DME, the use of technology such as OCT, offers the opportunity to assess the macula in histologic cross sections, and make objective and quantitative evalauations of DME[12].
Various morphologic subtypes of DME have been recognised on OCT imaging[13]. It is likely that each morphologic subtype of DME may have distinctive aspects that could be responsible for different responses to treatment with the currently favoured anti-VEGF therapy. In our study, DME was classified into four categories; Focal, diffuse, focal cystoid and neurosensory detachment. However, there is not yet a commonly accepted classification for DME morphology on OCT, and other studies have mainly used 3 subtypes: diffuse, cystoid and neurosensory detachment. We opted to create an additional category of ‘focal’, as we felt that it has distinct morphologic OCT features, and may respond differently following IVB treatment.
Our study found that focal DME group was associated with most improvement of visual acuity following IVB, where mean BCVA improved to logMAR 0.23 at final follow-up from a baseline of 0.32 logMAR (P=0.040). There was marginal improvent in the diffuse DME group, which was not statistically significant. The mean OCT macular thickness after IVB treatment decreased in all groups. However, this was most pronounced in the focal DME group (P=0.004). The results in focal DME group also showed a higher number of subjects (11/20, 55%) achieving visual improvement following IVB treatment as compared to other groups.
Several implications can be inferred from significant improvement of BCVA in the focal DME group in our study. Patients with focal DME have limited retinal thickening with cyst formation, and preservation of the macular contours. As the retinal architecture is relatively preserved, they have better visual acuity at presentation, and consequent better response to anti-VEGF therapy. Other investigators have found variable results for visual improvement in different groups after IVB treatment. This may be related to a different classification of DME or patient selection criterion for their study groups. Kim et al[14] found that intravitreal injection of bevacizumab was more effective in the diffuse retinal thickening than in the focal cystoid or neurosensory detachment types of DME. On the other hand Wu et al[15] found that patients with cystoid change gained greater improvement in visual acuity and macular thickness and volume after IVB injection. Koytak et al[16] found that there was no statistically significant variation between focal, cystoid and neurosensory detachment groups regarding the change in BCVA. Most investigators, like our study, noted a reduction in the macular thickness following IVB treatment. However, this did not necessarily translate into the improvement of visual acuity except in focal or cystoid groups.
Some recent studies have reported that the appearance of the photoreceptor layer, and the integrity of the inner segment-outer segment junction (IS/OS) line visualized by SD OCT can be used to predict the functional outcomes after macular surgery[17],[18]. A similar association in patients undergoing DME treatment was found, and that visual acuity improvement was closely associated with IS/OS integrity[19]. In our study, we did not look at these parameters, and only evaluated the various patterns of OCT morphology in DME. Our study is retrospective, and has limited number of patients. However, the results point to improved visual acuity following IVB injection in patients with focal appearance of macular edema on OCT.
In conclusion, OCT morpholgy patterns in DME may predict the effects of IVB treatment, and patients with focal DME are most likely to benefit from the improvent of visual acuity from this treatment. However, prospective randomized studies, and with a uniform classification system for OCT morphology in DME are needed to confirm the results of our study.
Acknowledgments
Conflicts of Interest: Cheema HR, None; Al-Habash A, None; Al-Askar E, None.
REFERENCES
- 1.Romero-Aroca P. Current status in diabetic macular edema treatments. World J Diabetes. 2013;4(5):165–169. doi: 10.4239/wjd.v4.i5.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall HN, Chinn DJ, Sinclair A, Styles CJ. Epidemiology of blindness attributable to diabetes in Scotland: change over 20 years in a defined population. Diabet Med. 2013;30(11):1349–1354. doi: 10.1111/dme.12223. [DOI] [PubMed] [Google Scholar]
- 3.Petrella RJ, Blouin J, Davies B, Barbeau M. Prevalence, demographics, and treatment characteristics of visual impairment due to diabetic macular edema in a representative canadian cohort. J Ophthalmol. 2012;2012:159167. doi: 10.1155/2012/159167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciulla TA, Harris A, Latkany P, Piper HC, Arend O, Garzozi H, Martin B. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand. 2002;80(5):468–477. doi: 10.1034/j.1600-0420.2002.800503.x. [DOI] [PubMed] [Google Scholar]
- 5.Thomas BJ, Shienbaum G, Boyer DS, Flynn HW., Jr Evolving strategies in the management of diabetic macular edema: clinical trials and current management. Can J Ophthalmol. 2013;48(1):22–30. doi: 10.1016/j.jcjo.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Stewart MW. Anti-vascular endothelial growth factor drug treatment of diabetic macular edema: the evolution continues. Curr Diabetes Rev. 2012;8(4):237–246. doi: 10.2174/157339912800840488. [DOI] [PubMed] [Google Scholar]
- 7.Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, Weichselberger A, Wolf S. Ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetic Retinopathy Clinical Research Network. Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, Friedman SM, Greven CM, Maturi RK, Pieramici DJ, Shami M, Singerman LJ, Stockdale CR. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arevalo JF, Sanchez JG, Wu L, Maia M, Alezzandrini AA, Brito M, Bonafonte S, Lujan S, Diaz-Llopis M, Restrepo N, Rodríguez FJ, Udaondo-Mirete P, Pan-American Collaborative Retina Study Group Primary intravitreal bevacizumab for diffuse diabetic macular edema: the Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology. 2009;116(8):1488–1497. doi: 10.1016/j.ophtha.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Al-Shaaln FF, Bakrman MA, Ibrahim AM, Aljoudi AS. Prevalence and causes of visual impairment among Saudi adults attending primary health care centers in northern Saudi Arabia. Ann Saudi Med. 2011;31(5):473–480. doi: 10.4103/0256-4947.84624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glover SJ, Burgess PI, Cohen DB, Harding SP, Hofland HW, Zijlstra EE, Allain TJ. Prevalence of diabetic retinopathy, cataract and visual impairment in patients with diabetes in sub-Saharan Africa. Br J Ophthalmol. 2012;96(2):156–161. doi: 10.1136/bjo.2010.196071. [DOI] [PubMed] [Google Scholar]
- 12.Pelosini L, Hull CC, Boyce JF, McHugh D, Stanford MR, Marshall J. Optical coherence tomography may be used to predict visual acuity in patients with macular edema. Invest Ophthalmol Vis Sci. 2011;52(5):2741–2748. doi: 10.1167/iovs.09-4493. [DOI] [PubMed] [Google Scholar]
- 13.Kim BY, Smith SD, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol. 2006;142(3):405–412. doi: 10.1016/j.ajo.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Lee P, Kim Y, Yu SY, Kwak HW. Effect of intravitreal bevacizumab based on optical coherence tomography patterns of diabetic macular edema. Ophthalmologica. 2011;226(3):138–144. doi: 10.1159/000330045. [DOI] [PubMed] [Google Scholar]
- 15.Wu PC, Lai CH, Chen CL, Kuo CN. Optical coherence tomographic patterns in diabetic macula edema can predict the effects of intravitreal bevacizumab injection as primary treatment. J Ocul Pharmacol Ther. 2012;28(1):59–64. doi: 10.1089/jop.2011.0070. [DOI] [PubMed] [Google Scholar]
- 16.Koytak A, Altinisik M, Sogutlu Sari E, Artunay O, Umurhan Akkan JC, Tuncer K. Effect of a single intravitreal bevacizumab injection on different optical coherence tomographic patterns of diabetic macular oedema. Eye (Lond) 2013;27(6):716–721. doi: 10.1038/eye.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh MH, Seo JM, Park KH, Yu HG. Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol. 2009;147(3):473–480. doi: 10.1016/j.ajo.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Sano M, Shimoda Y, Hashimoto H, Kishi S. Restored photoreceptor outer segment and visual recovery after macular hole closure. Am J Ophthalmol. 2010;149(2):284–290. doi: 10.1016/j.ajo.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Shin HJ, Lee SH, Chung H, Kim HC. Association between photoreceptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):61–70. doi: 10.1007/s00417-011-1774-x. [DOI] [PubMed] [Google Scholar]