Abstract
AIM
To evaluate the rates of retinopathy without diabetes and diabetic retinopathy (DR), associated with some markers of oxidative stress, antioxidants and cardiometabolic risk factors.
METHODS
We determined the prevalence of DR in 150 type 2 diabetes mellitus (T2DM) patients, that of retinopathy in 50 non diabetics, the levels of body mass index (BMI), waist circumference (WC), blood pressure, lipids, 8-isoprostane, 8-hydroxydeoxyguanosine (8-OHdG), gamma-glutamyl transferase GT (GGT), oxidized low-density lipoprotein (OxLDL), thiobarbituric acid reacting substances (TBARS), reduced glutathione (GSH), superoxide dismutase (SOD), uric acid, creatinine, albumin, total antioxidant status (TAOS), zinc, selenium, magnesium, vitamin C, vitamin D, vitamin E, glucose, apolipoprotein B (ApoB).
RESULTS
The prevalences of DR at 53y and Rtp at 62y were 44% (n=66) and 10% (n=5), respectively. The highest levels of 8-isoprostane, 8-OHdG, TBARS, SOD, and OxLDL were in DR. The lowest levels of vitamin D, vitamin C, TAOS, and vitamin E were in DR. In the case-control study discriminant analysis, the levels of vitamin C, vitamin D, ApoB, 8-OHdG, creatinine, Zn, vitamin E, and WC distinguished significantly non-diabetics without DR (controls), T2DM patients without DR and T2DM patients with DR.
CONCLUSION
Anticipation of DR onset is significantly associated with the exageration of oxidative stress biomarkers or decrease of antioxidants in African type 2 diabetics. Prevention of oxidative stress and abdominal obesity is needed. Supplementation in vitamin C, D, and E should be recommended as complement therapies of T2DM.
Keywords: anticipation, apolipoprotein B, diabetic retinopathy, oxidative stress, discriminant analysis, Africa
INTRODUCTION
Several reports suggest that increased oxidative stress and impaired antioxidant defense are some contributory factors for initiation and progression of microvascular complications in diabetes mellitus (DM)[1]-[13]. Experimental and clinical studies suggest that oxidative stress including aging is increased in diabetic retinopathy (DR) presence because of processes that can damage proteins, lipids, polysaccharides and nucleic acids[1],[3]-[10]. In rats with experimentally induced diabetes, the retinas display more extensive membrane lipid peroxidation and oxidative DNA damage which are the consequences of reactive oxygen species (ROS)-induced injury[11]-[13].
Moreover, “metabolic memory”, phenomenon referred to the persistence of DR after glucose control has been reestablished[14]. The DR is a key marker of atherosclerosis, uncontrolled hypertension, metabolic syndrome (MS) including dyslipidemia, and insulin resistance (IR)[15]-[19].
However, Yamagishi et al[1] showed a significant association between increased oxidative stress, lower antioxidant system, control of diabetes, creatinine, triglycerides, aging and DM whereas the later factors were not associated with DR.
In Democratic Republic of Congo (DRC), Central Africa, DR is one of the leading causes of visual disability (VD) among young adults with shorter DM duration (median 3-5y), demographic, epidemiologic and nutrition transitions as well as increasing risk of cardiometabolic diseases and ocular diabetic complications[14]-[16].
As reported by Ojaimi et al[20] and Pang et al[21], retinopathy signs in people without DM and prediabetics are often observed among black Bantu people from the Western region of DRC (Mvitu Muaka, Unpublished data). The Los Angelos Latino Eye Study (LALES) published only one report of nondiabetic retinopathy in adult Latinos with a higher prevalence of nondiabetic retinopathy in comparison to other population-based studies[22]. This study reported also a lack of association between hypertension and retinopathy in its population[21].
On clinical impression, a new retinopathy Bantu phenotype may point to the dynamics and diversity of oxidative stress and antioxidant defense in different compartments of the body.
We hypothesize that isolated actions of genetics (apolipoprotein B=ApoB, African descent) and environment (Tobacco, obesity, insulin resistance), interactions of genetical and environmental factors, diabetic retinal hemodynamics, aging-related accelerating atherosclerosis and almost of markers of disturbance of balance between oxidative process and antioxidant defenses can distinguish infraclinical retinopathy in non-diabetics, type 2 diabetics without DR, and type 2 diabetics with DR presence[23].
Therefore, the present findings might be important therapeutically in altering the course of DR by supplements of antioxidants such as vitamins, and preventing the risk factors and consequences of DR and oxidative stress[24].
The objectives of this study were to evaluate rates of retinopathy without diabetes, levels of some markers of oxidative stress, antioxidants, cardiometabolic risk of retinopathy in type 2 diabetes mellitus (T2DM) patients without DR, and T2DM patients with DR. We also investigated the discriminant function to separate the study groups.
SUBJECTS AND METHODS
This was a case-control study conducted in Kinshasa town, DRC, from July to September 2010. The cases of 150 T2DM patients were matched for age, sex, residence, and socioeconomic status (SES) to 50 apparently healthy and non diabetic people recruited from public places of each Kinshasa district (party lounges). Interviews, clinical examination and eye examination were performed at Saint Joseph Hospital Division of Ophthalmology in Kinshasa Limete, DRC.
T2DM was known by clinics and defined as a fasting serum glucose ≥126 mg/dL (≥7.0 mmol/L), and hemoglobin A1c (HbA1c) ≥6.5% according to the American Diabetes Association[25]. Sickle Cell disease, chloroquine side effects, children, young adults <20y, fever, cancer, chronic infections, antioxidants supplements, lipid lowering treatment and refusal to participate in the study were excluded. The inclusion criteria for 45 healthy non-diabetic controls were neither been diagnosed by a physician as having retinopathy, DM or use of hypoglycemic medication nor treated by lipid lowering molecules or use of antioxidant supplements or any known disease. Five asymptomatic participants (2 non-diabetics and 3 pre-diabetics) had retinopathy. Patients with complete medical records agreed to enroll according to the Helsinki Declaration. The study was approved by the Ethical Committee of LOMO Medical Center, Kinshasa, Limete, DRC.
Data Collection
Survey methods included interviewer-administered structured and standardized questionnaires, laboratory measurements, physical examination, dietary assessment, and eye examination using photographs. The questionnaire was structured, standardized and pre-coded for including information about demographics (sex, age, residence, socioeconomic status), medical history, DM duration, family history (not defined), medication, lifestyle factors (smoking history, moderate or excessive alcohol intake), and westernization/acculturation (rural-urban migration, postmigration duration in town).
Clinical examination included measurements of current body weight, waist circumference (WC), height and blood pressure (BP). Weight in light clothes was measured to the nearest 100 g using a Soehnle scale (Soehnle-Waagen GmGh Co, Murrhardt, Germany). Height was measured to the nearest 0.5 cm using a portable locally manufactured stadiometer. Body mass index (BMI) was calculated as weight divided by height in meter squared (kg/m2). Three consecutive diastolic blood pressure (DBP) and systolic blood pressure (SBP) measurements were recorded on the right arm using a standard mercury sphygmomanometer with appropriate cuff sizes at intervals longer than 2min after 15min of rest in seated participants. The average of the second and third readings was used in the present analyses. WC was measured after a gentle expiration between the lower rib margins and the iliac crest to the nearest millimeter using flexible tapes, with participants standing with their heels together. Anthropometric measures were performed using standardized protocols[26],[27].
For quality control, blood samples were collected and assessed by the coefficients of variation (CV, intra and interassay).
Enzyme activities for serum gamma-glutamyl transferase (GGT) were determined according to standard laboratory procedures. Serum total cholesterol (TC) for CHOD-PAP Method, triglycerides (TG) for GPO-PAP method, high-density lipoprotein-cholesterol (HDL-C) for IRC method, uric acid, and low-density lipoprotein cholesterol (LDL-C) were measured using enzymatic colorimetric kits (Biomerieux, Marcy l'Etoile, France).
Antibodies against Oxidized LDL-cholesterol (OxLDL) were measured using solid phase two-site immunoassay based on the direct sandwich technique; there are two genetic determinants on the oxidized apoliprotein B molecules (Mercodia AB, Sylveniusgaton 8A, SE 75 450, Uppsala, Sweden). The laboratory tests in LOMO Medical Laboratories were assayed using an automatic analyzer (Hospitex Diagnostics, Florence, Italy). Glycated hemoglobin fractions were measured in fresh anticoagulated blood samples with both migration set-up using a semi-automated multi-parameter instrument (HYDRASYS system, Serbia, Evry, France) and densitometric scanning of unstained gels 9HYDRGEL 7/15 hBa1C) performed on HYRYS Densitometer and gel carrier O and specific HbA1c (NGSP) software (HYRYS densitometer, Serbia, Evry, France). After including a control blood sample into each run of samples, relative concentrations (%) of three fractions in each hemolyzate were yielded by the Densitometer scanning as follows: the most cathodic corresponding to the minor glycated hemoglobin A1C (HbA1c), and the most anodic being the main fraction containing Ao and A2 hemoglobins.
The majority of markers of oxidative stress, total antioxidant status (TAOS), micronutrients (Zinc, Selenium, Magnesium), vitamins (C, D, E), superoxide dismutase (SOD), 8-isoprostane, 8-hydroxydeoxyguanosine (8-OHdG), erythrocyte glutathione peroxidase (GPx), reduced glutathione (GSH), and thiobarbituric acid reacting substances (TBARS) were measured at the Analytical Chemistry Laboratory of the Walter Sisulu University, Mthatha, South Africa. Commercial Cayman's Kits (Cayman Chemical Company, Ann Arbor, MI, USA) were used to measure TAOS, SOD, and 8-isoprostane in plasma. Serum 8-OHdG levels were measured by utilizing a competitive enzyme linked immunosorbent assay method on Biomerieux Reader version and commercial kits supplied by Northwest Laboratories (Northwest Life Science Specialities, LLC, Vancouver, WA 98662, Canada).
Levels of TBARS were measured from serum samples and expressed as mmol/L[28]. For that, samples were run simultaneously with quality control samples of pooled serum nonstudy normal healthy people. If analysis of the quality control TBARS level varied from ±2SD of a mean determined by a total of 60 runs, and between-week runs, the analysis was deemed unacceptable and was repeated. GPx activity was measured from the washed packed cell fraction based on a kinetic method and units of activity normalized per milligram of hemoglobin[28].
The serum Se, Mg, and Zn concentrations were determined by direct electrothermal atomic-absorption spectrometry on AAS Unicam (Serum mineralized in microwave system, Milestone, Italy).
Serum α-tocopherol (vitamin E), ascorbic acid (vitamin C), and 1.25 (OH) 2D (1,25-dihydroxy-vitamin D) were analyzed using reverse phase high performance liquid chromatography (HPLC) with multiwavelength.
The very low density lipoprotein cholesterol (VLDL-C) was calculated based on the Friedewald equation and excluding participants with TG ≥400 mg/dL: VLDL=TG/5[29].
The relative homeostasis model-based insulin resistance (HOMA-IR) was calculated using the formula of Mathews: HOMA-IR=(fasting insulin×fasting plasma glucose)/22.5 in case of glucose concentrations in mmol/L and by 405 in case of glucose concentrations in mg/dL[30].
Fasting plasma glucose (FPG), fasting plasma insulin and serum creatinine (µmol/L) were measured with commercially prepared kits (Biomerieux, Marcy l'Etoile, France). The coefficient of variation levels were as follows: 3.5% for GGT, 2% for TC, 3% for HDL-C, 2.5% for TG, 3% for OxLDL, 4% for LDL-C, 2% for HbA1c, 2.2% for ApoB, 2.3% (2.6% interassay) for 8-OHdG, 2.9% (3.2% interassay) for 8-isoprostane, 3% (2.5% interassay) for GSH, 4.5% (5.4% interassay) for TBARS, 3.5% for TAOS, 6% for SOD,8% for GPx, 5.4% for Zn, 2.8% for vitamin C, 5.3% for vitamin E, 3.6% for vitamin D, 1.5% for FPG, and 2% for insulin.
Eye examination of each participant included visual acuity (VA) measurement, ocular alignment and motility, pupil reactivity and function, visual fields, intraocular pressure measurement (mm Hg), slit lamp examination of the cornea, iris, lens and vitreous, and dilated funduscopic evaluation. This fundus examination was detailed and performed at the best possible mydriasis, after dilating the pupils with tropicamide (1%) and phenylephrine (10%), by indirect ophthalmoscopy at the slit lamp (Haag Streit 900) with 90D lens. Participants were refracted with the use of standard subjective refraction techniques.
VA was measured under similar lighting conditions by an ophthalmologist. VA was measured separately for each eye and was defined according to the lowest line on the chart for which the majority of letters were read correctly, with the full required distance correction as determined by the decimal optometric scale. For the classification of retinopathy, the modified Airlie House classification as introduced by the Early Treatment Diabetic Retinopathy Study (ETDRS) was used as follows: non-proliferative (NPDR), proliferative (PDR) and maculopathy.
Definitions
Retinopathy was defined by the presence of one of the following lesions in either eye: retinal microaneurysms, blot hemorrhages, cotton-wool spots, hard exsudates, intraretinal microvascular abnormalities, venous beading, new retinal vessels, preretinal or vitreous hemorrhages, or macula edema. DR was defined by nonproliferative and/or proliferative lesions. DR was considered non proliferative retinopathy as microaneurysms, dot or blot hemorrhages, venous abnormalities, cotton-wool spots, and hard exsudats. DR was condirered proliferative retinopathy as growth of new retinal blood vessels (neovascularization), retinal or vitreous hemorrhages, and vitreoretinal traction.
The study groups were constituted of non diabetics without retinopathy, non diabetics with retinopathy, T2DM patients without DR and T2DM patients with DR.
Longer DM duration was ≥5y (the median value). Cardiometabolic risk was defined by the variations of the mean values of age (aging), BMI, TC, 10-year absolute cardiovascular risk (10-year ACVR) according to Framingham equation, TC/HDL ratio, Non-HDL (TC-HDL-C), TG/HDL ratio, LDL/HDL ratio, VLDL, HDL-C, LDL-C. TG, WC, SBP, DBP, and HOMA-IR.
The categorical case-control was constituted of apparently healthy non-diabetics controls without retinopathy, T2DM patients without DR and T2DM patients with DR.
The imbalance of oxidant/antioxidant status was defined by increased levels of lipid peroxidation markers (8-isoprostane, TBARS, OxLDL), DNA oxidation product (8-OHdG), and extracellular catabolism of GSH, SOD, GPx), and non-enzymatic antioxidants (albumin, uric acid, TAOS, vitamin C, vitamin D,vitamin E, Zn, selenium).
Metabolic syndrome (MS) was defined according to IDF criteria with European cutoffs[31], including the following components: a male with a WC ≥94 cm or a female with a WC ≥80 cm MS if they also have two or more of the following: Triglycerides ≥150 mg/dL (1.7 mmol/L) or specific treatment for this lipid abnormality, reduced concentration of HDL-C <40 mg/dL (1.03 mmol/L) in males and <50 mg/dL (1.29 mmol/L) in femalesor specific treatment for this lipid abnormality, SBP≥30 mm Hg or DBP ≥85 mm Hg or treatment of previously diagnosed hypertension, and Fasting Plasma Glucose ≥100 mg/dL (5.6 mmol/L) or previously diagnosed T2DM.
Tolerance glucose in non diabetics was defined using 2 H-post load tests of 75 g of dextrose (PG): normal glucose tolerance with PG<7.8 mmol/L and pre-diabetes with PG=7.8-11 mmol/L.
Statistical Analysis
Data were reported as proportions (%) for categorical variables and mean±standard deviation for normally distributed continuous variables. The Chi-square test was used to compare proportions while comparisons of means between two groups and three groups were performed using the Student's t-test and one Way variance (ANOVA) with Bonferroni Post-Hoc test, respectively.
Kruskal-Wallis H-test, a non parametric test, was used for comparisons of means of asymmetric continuous variables (GGT, HOMA-IR, SOD) across the groups.
We used analysis of the area under the curve (AUC with 95% CI) of receiver operating characteristic curves (ROC) to compare the ability of markers between T2DM patients and non-diabetics without retinopathy. According to the sensitivity and specificity, the best cut-off points elevated for 8-OHdG and decreased for vitamin D, vitamin E, vitamin C, and Zn, were the most accurate biomarkers of imbalance of oxidant/antioxidant status.
Fisher's Stepwise Discriminant function analysis was used to determine which variables discriminate between the groups in the case-control approach and all participants. With this respect canonical analysis, the maximum number of three functions was equal to the number of 4 groups minus one in the case-control study and the maximum number of 4 functions equal to the number of 5 groups minus one in all participants. The larger standardized beta coefficient, the greater was the contribution of the respective variables to the discrimination between groups.
All of the statistical analyses were two-sided and a P<0.05 was considered statistically significant. Data analysis was carried out using the Statistical Package for Social Sciences (SPSS) for Windows version 19 (SPSS Inc, Chicago, IL, USA).
Ethics Statement
The study protocol was approved by the local Ethics Committee (Comité d'Ethique et de Recherche) of LOMO Clinic, Kinshasa, DRC, and was conducted according to the principles of Helsinki Declaration. After informed verbal consent, satisfactory for the local Ethical committee like in all sub-Saharan African countries (the majority being illiterate), patients who completed their comprehensive physical examination were included in the study.
RESULTS
In total, 200 participants (45 non-diabetics without retinopathy, and 150 T2DM patients in case-control study +5 non-diabetics with retinopathy) were investigated. Table 1 summarizes the comparisons of levels of sociobiographical and clinical variables in the case-control study. Only higher levels of smoking, BMI, low SES, and WC but lower levels of moderate alcohol intake were significantly associated with T2DM. The levels of smoking, low SES, moderate alcohol intake, HbA1c, BMI and WC varied significantly between non-diabetics without retinopathy, T2DM patients without DR (the highest levels) and T2DM patients with DR and the lowest proportion of moderate alcohol intake.
Table 1. Comparisons of levels of sociobiographical and clinical variables in the case-control.
| Parameters | Non diabetics without retinopathy | All type 2 diabetics | T2DM without DR | T2DM with DR | P |
| Sex | 0.638 | ||||
| M | 21 (46.7) | 65 (43.3) | 39 (46.4) | 26 (39.4) | |
| F | 24 (53.3) | 85 (56.7) | 45 (53.6) | 40 (66.6) | |
| Age (a) | 50.7±13 | 55.2±13 | 56.6±12.4 | 53.4±13.6 | 0.046 |
| Smoking | 2 (4.4) | 2 (4.4) | 34 (22.7) | 8 (9.5) | 26 (39.4) |
| Alcohol intake | |||||
| Moderate | 17 (37.8) | 27 (18) | 16 (19) | 11 (16.7) | 0.014 |
| Excessive | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Low SES | 19 (42.2) | 102 (68) | 55 (65.5) | 47 (71.2) | 0.003 |
| DM duration ≥5a | - | 105 (70) | 51 (60.7) | 54 (81.8) | <0.004 |
| VD | 5 (11.1) | 97 (64.7) | 63 (75) | 34 (51.5) | <0.0001 |
| Medications | |||||
| Insulin therapy | 49 (58.3) | 39 (59.1) | |||
| Diabetic antioral | 35 (41.7) | 27 (40.9) | |||
| Non control | |||||
| Glycemia | 118 (78.7) | 66 (71.4) | 52 (78.8) | >0.05 | |
| Hypertension | 54 (36) | 29 (34.5) | 25 (37.9) | >0.05 | |
| HbA1c (%) | 5±0.6 | 9.3±4.1 | 9.8±4.3 | 9.8±4.3 | <0.0001 |
| BMI(kg/m2) | 22.4±2.9 | 25.8±5.0 | 26.3±5.0 | 25.2±5 | <0.0001 |
| WC(cm) | 81.0±15.0 | 94.7±14.2 | 95.4±12.2 | 93.8±16.4 | <0.0001 |
| Migration time(a) | 35.8±14.3 | 36.0±17.1 | 41.9±16 | 0.047 |
x±s, n (%)
Compared with non-diabetics without retinopathy, all T2DM patients had significantly higher levels of TC, LDL-C, TG, non HDL, VLDL, ApoB, Homa-IR, abdominal obesity, MS, IR, SOD, 8-Isoprostane, TBARS, OxLDL, 8-OHdG, uric acid, GGT, but significantly lower levels of HDL-C, Gpx, TAOS, vitamin C, vitamin E, vitamin D, Zn, Se and Mg (Tables 2–4). Except for uric acid and reduced Glutathione, the rest of variables varied significantly across the case-control study groups: the highest level of oxidant markers and the lowest level of antioxidants in T2DM with DR. There was a significant association between highest levels of ages (62.4±5.6y; P<0.0001), ApoB (2.5±2.2 g/L; P<0.0001), and GGT (52.4±10.4 UI/L; P=0.006), but lowest levels of SBP (106±19.5 mm Hg; P=0.011), and BMI (21.8±2.3 kg/m2) with retinopathy without T2DM compared to those of the rest groups in all participants. The level of Homa-IR was higher in non diabetics with retinopathy (6±6; P<0.0001) than that in non diabetics without retinopathy, and the half value in T2DM patients with DR (P<0.0001).
Table 2. Comparisons of levels of cardiometabolic biomarkers in the case-control study.
| Cardiometabolic markers | Non diabetics without retinopathy | All type 2 diabetics | T2DM without DR | T2DM with DR | P |
| TC (mg/dL) | 174.2±141 | 203.2±56.2 | 205.1±54.6 | 200.8±58.6 | 0.006 |
| HDL-C (mg/dL) | 56.3±14.5 | 45.3±27.5 | 46.6±118.8 | 43.5±35.8 | 0.030 |
| LDL-C (mg/dL) | 63.1±27 | 86.7±45.7 | 86.2±42.8 | 47.3±49.5 | 0.005 |
| TG (mg/dL) | 75.6±7.4 | 131.8±45.5 | 125.2±45.6 | 137±45.1 | <0.0001 |
| Non HDL (mg/dL) | 117.9±40 | 150.8±54.6 | 146.4±56.3 | 154.3±53.3 | <0.001 |
| VLDL (mg/dL) | 15.1±1.5 | 26.5±9.2 | 25.5±9.4 | 27.4±9 | <0.0001 |
| ApoB (g/L) | 0.54±0.33 | 1.3±1.5 | 0.79±0.62 | 1.9±1.97 | <0.0001 |
| Homa-IR (n) | 3.2±3.3 | 10.7±9.9 | 8.7±10.1 | 13.4±9.1 | <0.0001 |
| Abdominal obesity n(%) | 4 (8.9) | 88 (58.7) | 52 (61.9) | 36 (54.5) | <0.0001 |
| MS n (%) | 7 (15.6) | 100 (73.3) | 60 (71.4) | 50 (75.8) | <0.0001 |
| IR n (%) | 5 (11.1) | 76 (51.7) | 30 (36.6) | 46 (71.8) | <0.0001 |
x±s, n (%)
Table 4. Comparisons of levels of antioxidant biomarkers in the case-control study.
| Parameters | Non-diabetics without retinopathy | All type 2 diabetics | T2DM without DR | T2DM with DR | P |
| Non enzymatic markers | |||||
| TAOS (mmol/L) | 1.7±0.8 | 1.1±0.7 | 1.1±0.7 | 1.1±0.7 | <0.0001 |
| Vitamin C (mg/dL) | 4.5±1.5 | 1.7±0.7 | 2.0±0.6 | 1.2±0.7 | <0.0001 |
| Vitamin E (µmol/L) | 28.2±3.6 | 16.8±4.5 | 17.4±5 | 15.9±3.6 | <0.0001 |
| Vitamin D (mmol/L) | 77.3±17 | 32.3±14.4 | 38±11.3 | 25.1±14.8 | <0.0001 |
| Albumin (g/dL) | 4.3±0.4 | 4.6±1.3 | 4.6±0.8 | 4.6±1.8 | >0.05 |
| Uric acid (mg/dL) | 4.7±1.6 | 5.4±1.9 | 5.4±2 | 5.4±1.8 | >0.05 |
| Zn (µg/dL) | 97.1±3.6 | 68.1±19.6 | 70.3±21.3 | 66.5±18.2 | <0.0001 |
| Se (µg/L) | 126.4±12.1 | 101.8±19.8 | 101.2±19.4 | 102.7±20.4 | <0.0001 |
| Mg (mg/dL) | 0.83±0.11 | 0.68±0.25 | 0.70±0.25 | 0.65±0.25 | <0.0001 |
| Enzymatic markers | |||||
| Glutathione peroxidase(GPx:mg/dL packed red blood cell) | 43.2±10.4 | 41.8±10.8 | 41.4±10.8 | 42.3±10.7 | >0.05 |
| SOD (U/mL) | 0.9±0.5 | 2.7±1.6 | 2.4±1.6 | 3.2±1.6 | <0.0001 |
x±s, n (%)
In the case-control study discriminant analysis, the levels of vitamin C, vitamin D, ApoB, 8-OHdG, creatinine, Zn, vitamin E, and WC distinguished significantly non-diabetics without DR (controls), T2DM patients without DR and T2DM patients with DR (Table 5, Figure 1).
Table 5. Classification functions between non-diabetics without retinopathy, T2DM patients without DR and T2DM patients with DR in the case-control study.
| Parameters | Non-diabetics without retinopathy | T2DM without DR | T2DM with DR |
| Vitamin C (mg/dL) | 2.362 | 0.899 | 1.785 |
| Vitamin D (mmol/L) | 0.259 | 0.074 | 0.012 |
| ApoB | 1.478 | 1.380 | 2.128 |
| 8-OHdG (mg/mL) | 0 | 0.056 | 0.088 |
| Creatinine (mg/dL) | 1.016 | 1.339 | 1.378 |
| Zn | 0.278 | 0.175 | 0.193 |
| Vitamin E (mmol/L) | 1.443 | 0.888 | 0.829 |
| WC (cm) | 0.398 | 0.488 | 0.482 |
| Constant | -100.211 | -91.118 | -93.379 |
Excluded from the Fisher's linear discriminant functions: Se, non HDL, OxLDL, TC, 8-isoprostane, SOD, age, HDL-C, TBARS, GGT, Homa-IR, BMI, TAOS and post migration living time in town.
Figure 1. Discriminant functions for retinopathy presence groups in the case-control study.
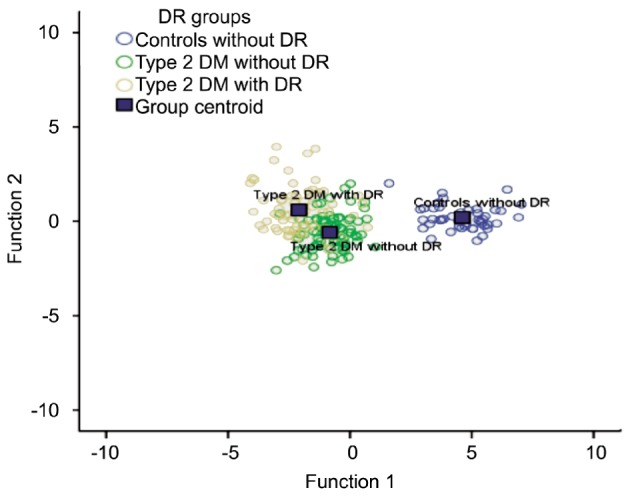
Canonical Discriminate Functions case population: case polulation.
Table 3. Comparisons of levels of oxidant biomarkers in the case-control study.
| Parameters | Non-diabetics without retinopathy | All type 2 diabetics | T2DM without DR | T2DM with DR | P |
| Lipid peroxidation | |||||
| 8-isoprostane (ng/mL) | 39.9±18.6 | 81.9±43.8 | 73.9±48.6 | 92.1±34.7 | <0.0001 |
| TBARS (mmol/L) | 4.4±1.4 | 8.9±4.2 | 9.1±5.2 | 8.7±2.5 | <0.0001 |
| OxLDL (U/L) | 53.8±14.8 | 61.8±22.2 | 60.2±19.5 | 63.9±25.3 | 0.044 |
| DNA oxidation | |||||
| 8-OHdG (mg/mL) | 31.4±15.2 | 62.1±25.2 | 54.5±25.3 | 71.7±21.6 | <0.0001 |
| Catabolism of GSH | |||||
| GGT (UI/L) | 10.6±6.4 | 36.7±50.2 | 33.8±47.2 | 40.3±53.9 | <0.0001 |
x±s, n (%)
The Discriminant Analysis identified the following as the most important parameters to separate all non diabetics (controls without DR thus without retinopathy and controls with alike DR thus with retinopathy) from T2DM without DR and T2DM with DR: 8-OHdG (Wilks' Lambda=0.516), TBARS (Wilks' Lambda=0.462), BMI (Wilks' Lambda=0.449), Fasting Plasma Glucose (Wilks' Lambda=0.435), and TAOS (Wilks' Lambda=0.414) (Figure 2).
Figure 2. Discriminant functions for retinopathy presence groups in all participants.
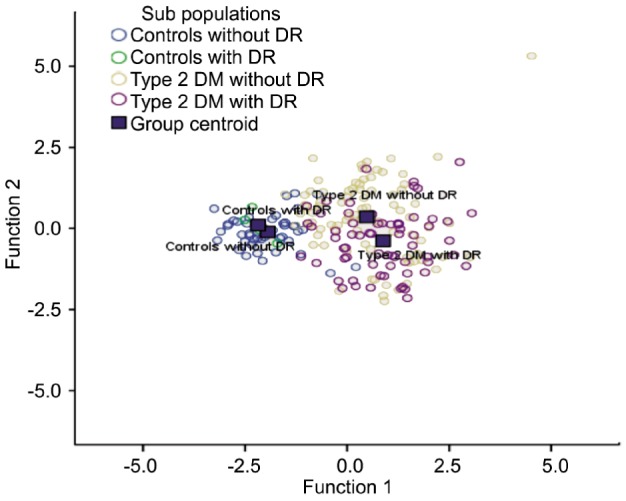
Canonical discriminate functions.
DISCUSSION
This study provided the first regionally representative burden of DR in diabetic black Central Africans. The potential mechanisms of DR development were investigated.
Among non diabetic controls, 10% had the typical combination and distribution of features characteristics of DM without veno-occlusive diseases, retinal vasculopathies, hyperviscosity syndrome, or other conditions. This finding confirmed the literature reports of retinopathy among pre-diabetic probably latent diabetic people[8],[9],[20],[21].
The present prevalence of 44% of DR among T2DM patients was within rates of DR estimated in 7-63% of sub-Saharan Africans[5]-[7]. Within the expected increased prevalence and incidence of T2DM in Kinshasa population[3],[4], due in part to urbanization, poverty, westernization, globalization, metabolic syndrome, and decreasing physical activity, the burden of DR might be expected to increase in non diabetics and diabetics as well. The World Health Organization and the American Diabetes Association criteria for diagnosing DM assume the presence of a glycemic threshold with high sensitivity for identifying retinopathy[31]. Interestingly, the present study suggests that oxidative stress contributes to onset of DR not only in the context of biological definition of DM, but also in people with normal glucose tolerance and pre-diabetes[31],[32]. This phenomenon is named “metabolic memory”[14],[33].
In characterizing the oxidative stress, the antioxidant capacities and the cardiometabolic risk of blood and biophysical markers of obesity, the present study provided evidence for the involvement of oxidant/antioxidant imbalance in the pathogenesis of DR. The oxidant/antioxidant imbalance was characterized by an increase in lipid peroxidation, DNA oxidation damage products, and enzymes of liver disease. At the same time, adequate levels of the enzymatic and non enzymatic antioxidants, responsible for scavenging free radicals, were not efficient.
The older age, and high proportion of metabolic syndrome observed in controls with retinopathy, already indicators of oxidative stress, may explain the higher risk of retinopathy in this group in comparison with controls without retinopathy[34]. These controls with retinopathy had also the highest level of GGT (another marker of oxidative stress) among all participants. The highest levels of 8-OHdG, 8-isoprostane, TBARS, and OxLDL, but the lowest values of TAOS, vitamin C, vitamin D, and vitamin E among the present T2DM group with DR confirmed the significant impact of the imbalance of oxidant/antioxidant status in the pathophysiology of DR from several studies[10]-[17].
However, SOD had the highest values among T2DM patients with DR. This finding takes place among conflicting data about SOD, GSH, uric acid, albumin and oxidative-induced conditions. Kumawat et al[35] reported recently a positive correlation of SOD with GSH in type 2 diabetes patients with nephropathy, one of the leading vascular complications in diabetes. Many other studies have also reported a decrease in SOD level in blood[35]. The SOD activity becomes low in diabetic patients because about 50% of SOD in erythrocytes is glycated[36],[37]. Results from animal and human studies reported both increase and decrease in SOD activity in erythrocytes[38]. The effects of diabetes on activity of antioxidant enzymes including SOD are contradictory in various tissues[38],[39]. Increased activity of SOD in our study may be a compensatory response to oxidative stress.
T2DM in general and T2DM with DR in particular, were associated with increased frequency of metabolic syndrome and hypertension which lead to increased free radical activity and atherosclerotic diseases[34]. Obesity, physical inactivity, and insulin resistance related to metabolic syndrome and hypertension induce oxidative stress through low grade inflammation status and endothelial dysfunction.
TBARS, BMI, and 8-OHdG alongside with FPG and TAOS were the most important and powerful parameters able to discriminate controls without retinopathy, controls with retinopathy, T2DM patients without DR, and T2DM patients with DR. These findings suggest that primordial and primary preventions on DR are better than cure in terms of clinical implications.
Clinical Implications and Perspectives for Public Health
Our findings will impact on the management (etiology, diagnosis, and prevention)[15]. Primordial prevention of DR in non diabetics and primary prevention in T2DM patients are still feasible to prevent DR, whereas antioxidant supplements in T2DM may be late, questionable with conflicting results. Measures of controlling obesity are needed in controls without DR to reduce the levels of 8-OHdG. The hydroxyl radicals may attack DNA strands causing the addition to DNA bases, which lead to the generation of variety of oxidation products such as 8-OHdG. Evidence suggests that 8-OHdG is the most commonly detected by-product of DNA damage and considered as a biomarker of oxidative stress, strongly associated with DR[4],[40].
As many Africans with T2DM present malnutrition, decrease in BMI may worse oxidative stress defined by increased levels of TBARS, a product of lipid peroxidation[41]. The process of lipid peroxidation occurs in response to elevated levels of ROS. Lipid peroxidation of cellular sctructures, a free-radical-induced activity, may play a crucial role in ageing, atherosclerosis and diabetic microvascular complications.
Nutrition education is needed for these Central Africans as the background of decreased levels of TAOS, vitamin C, vitamin D, and vitamin E in T2DM patients with DR should be an appropriate diet rich in antioxidants[34].
An intensive therapy with insulin, oral antidiabetics and diet rich in fibers may reduce the risk of DR. Control of blood glucose may also prevent DR among non diabetics. Indeed, in the discriminant Analysis, a significant distance related to FPG separated controls without DR and controls with DR.
Control of blood pressure, laser photocoagulation for DR and regular dilated indirect ophthalmoscopy, and use of aspirin will complete the preventive measures for DR.
Control of dyslipidemia (metabolic syndrome, increase in Apo B) is needed.
Study Limitations
The present study could be limited to some degree because of its cross-sectional design and small size. DR must be interpreted with caution out of longitudinal and prospective studies capable to demonstrate definitive causal association between DR and the imbalance of Oxidant/Antioxidant status in these Central Africans.
These findings demonstrate that DR is a real public health problem among T2DM patients and non diabetics. There is a significant association between increased oxidative stress, antioxidant deficiencies, metabolic syndrome and DR prevalence in diabetes.
Supplements in vitamin C, D, and E are recommended as complement therapies in the management of T2DM.
Acknowledgments
We thank Mr. Simon Stimela Mathabatha in Memoriam, Analytical Chemistry Laboratory from Walter Sisulu University, Mthatha, South Africa and the staff of LOMO Medical Laboratory, Kinshasa, Limete, DRC.
Conflicts of Interest: Longo-Mbenza B, None; Mvitu Muaka M, None; Masamba W, None; Muizila Kini L, None; Longo Phemba I, None; Kibokela Ndembe D, None; Tulomba Mona D, None.
REFERENCES
- 1.Yamagishi S, Ueda S, Matsui T, Nakamura K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in diabetic retinopathy. Curr Pharm Des. 2008;14(10):962–968. doi: 10.2174/138161208784139729. [DOI] [PubMed] [Google Scholar]
- 2.Chilelli NC, Burlina S, Lapolla A. AGEs, rather than hyperglycemia, are responsible for microvascular complications in diabetes: a “glycoxidation-centric” point of view. Nutr Metab Cardiovasc Dis. 2013;23(10):913–919. doi: 10.1016/j.numecd.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Mancino R, Di Pierro D, Varesi C, Cerulli A, Feraco A, Cedrone C, Pinazo-Duran MD, Coletta M, Nucci C. Lipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samples from patients with diabetic retinopathy. Mol Vis. 2011;17:1298–1304. [PMC free article] [PubMed] [Google Scholar]
- 4.Aldebasi Y, Mohieldein A, Almansour Y, Almoteri B. Imbalance of oxidant/antioxidant status and risk factors for Saudi type 2 diabetic patients with retinopathy. BJMMR. 2011;1(4):371–384. [Google Scholar]
- 5.El-Mesallamy HO, Rizk KA, Hashad IM. Role of oxidative stress, inflammation and endothelial dysfunction in the pathogenesis of diabetic retinopathy. IIOABJ. 2011;2(6):91–97. [Google Scholar]
- 6.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabet. 2008;26(2):77–82. [Google Scholar]
- 7.Spijkerman AM, Gall MA, Tarnow L, Twisk JWR, Lauritzen E, Lund-Andersen H, meis J, Parving HH, tehouwe CDA. Endothelial dysfunction and low-grade inflammation and the progression of retinopathy in type 2 diabetes. Diabet Med. 2007;24(9):969–976. doi: 10.1111/j.1464-5491.2007.02217.x. [DOI] [PubMed] [Google Scholar]
- 8.Desai ViDya, RaVi sheKhaR, siVa PRaboDh, NVs ChoWDaRy, MC Das M JoJi ReDDy. Oxidative stress in diabetic retinopathy. J Clin Diagn Res. 2011;5(5):994–997. [Google Scholar]
- 9.Soliman GZ. Blood lipid per oxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med J. 2008;49(2):129–136. [PubMed] [Google Scholar]
- 10.Renu A. Kowluru, Bindu Menon, Dennis L. Gierhart. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Ophthalmol Vis Sci. 2008;49(4):1645–1651. doi: 10.1167/iovs.07-0764. [DOI] [PubMed] [Google Scholar]
- 11.Kumari S, Panda S, Mangaraj M, Mandal MK, Mahapatra PC. Plasma MDA and antioxidant vitamins in diabetic retinopathy. Indian J Clin Biochem. 2008;23(2):158–162. doi: 10.1007/s12291-008-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamta Kanwar, Pooi-See Chan, Timothy S Kern, Renu A. Kowluru. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48(8):3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 13.Zhong Q, Kowluru RA. Epigenetic modification of Sod2 in the development of diabetic retinopathy and in the metabolic memory: role of histone methylation. Invest Ophthalmol Vis Sci. 2013;54(1):244–250. doi: 10.1167/iovs.12-10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longo-Mbenza B, Nkondi Mbadi A Sungu J, Mbungu Fuele S. Higher pulse pressure, systolic arterial hypertension, duration and family history of diabetes in Central Africans. Int J Diabetes & Metab. 2008;16:17–23. [Google Scholar]
- 15.Longo-Mbenza B, Muaka MM, Mbenza G, Mbungu-Fuele S, Mabwa-Mbalanda L, Nzuzi Babeki V, Mbadi-A-Sungu J. Risk factors of poor control of HbA1c and diabetic retinopathy: Paradox with insulin therapy and high values of HDL in African diabetic patients. Int J Diabetes & Metab. 2008;16:69–78. [Google Scholar]
- 16.Nkondi Mbadi AN, Longo-Mbenza B, Mvitu Muaka M, Mbungu FS, Lemogoum D. Relationship between pulse pressure, visual impairment and severity of diabetic retinopathy in sub-Saharan Africa. Mali Med. 2009;24(3):17–21. [PubMed] [Google Scholar]
- 17.Bosy-Westphal A, Booke CA, Blöcker T, Kossel E, Goele K, Later W, Hitze B, Heller M, Glüer CC, Müller MJ. Measurement site for waist circumference affects its accuracy as an index of visceral and abdominal subcutaneous fat in a Caucasian population. J Nutr. 2010;140(5):5954–5961. doi: 10.3945/jn.109.118737. [DOI] [PubMed] [Google Scholar]
- 18.Raman R, Gupta A, Kulothungan V, Shama T. Prevalence and risk factors of diabetic retinopathy in subjects with suboptimal glycemic, blood pressure and lipid control. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, Report 33) Curr Eye Res. 2012;37(6):513–523. doi: 10.3109/02713683.2012.669005. [DOI] [PubMed] [Google Scholar]
- 19.Sasongko MB, Wong TY, Nguyen TT, Kawasaki R, Jenkins AJ, Shaw J, Robinson C, Wang JJ. Serum apolipoproteins are associated with systematic and retinal microvascular function in persons with diabetes. Diabetes. 2012;61(7):1785–1792. doi: 10.2337/db11-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ojaimi E, Nguyen TT, Klein R, Islam FM, Cotch MF, Klein BE, Wang JJ, Wong TY. Retinopathy signs in people without diabetes: the multi-ethnic study of atherosclerosis. Ophthalmology. 2011;118(4):656–662. doi: 10.1016/j.ophtha.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang C, Jia L, Jiang S, Liu W, Hou X, Zuo Y, Gu H, Bao Y, Wu Q, Xiang K, Gao X, Jia W. Determination of diabetic retinopathy prevalence and associated risk factors in Chinese and pre-diabetic subjects: Shanghai diabetic complications study. Diabetes Metab Res Rev. 2012;28(3):276–283. doi: 10.1002/dmrr.1307. [DOI] [PubMed] [Google Scholar]
- 22.Varma R, Paz SH, Azen SP, Klein R, Globe D, Torres M, Shufelt C, Preston-Martin S, Los Angeles Latino Eye Study Group The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111(6):1121–1131. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Hu A, Luo Y, Guo X, Ding X, Zhu X, Wang X, Tang S. Low serum apolipoprotein A1/B ratio is associated with proliferative diabetic retinopathy in type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2012;250(7):957–962. doi: 10.1007/s00417-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 24.Chao JR, Lai MY, Azen SP, Klein R, Varma R. Retinopathy in persons without diabetes: the los angeles latino eye study. Invest Ophthalmol Vis Sci. 2007;48(9):4019–4025. doi: 10.1167/iovs.07-0206. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association Diagnosis and Classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 26.Lohman TG, Roche AF, Martorell R. Anthropometric standardization Reference Manual. Human Kinetics Books, Champaign, Ill. 1998.
- 27.Ernst DJ, Balance LO, Calam RR, Call RM, Smith SS, Szamosi DJ, Warunek DJ. H3-A6 procedures for the collection of diagnostic blood specimens by venipuncture. Approved Standard-Sixth Edition. 2008 [Google Scholar]
- 28.Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol. 1994;366:43–58. doi: 10.1007/978-1-4615-1833-4_4. [DOI] [PubMed] [Google Scholar]
- 29.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 32.Yun YW, Shin MH, Lee YH, Rhee JA, Choi JS. Arterial stiffness is associated with diabetic retinopathy in Korean type 2 diabetic patients. J Prev Med Public Health. 2011;44(6):260–266. doi: 10.3961/jpmph.2011.44.6.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salido EM, de Zavalía N, Schreier L, De Laurentiis A, Rettori V, Chianelli M, Keller Sarmiento MI, Arias P, Rosenstein RE. Retinal changes in an experimental model of early type 2 diabetes in rats characterized by non-fasting hyperglycemia. Exp Neurol. 2012;236(1):151–160. doi: 10.1016/j.expneurol.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Hutcheson R, Rocic P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: the great exploration. Exp Diabetes Res. 2012;2012:271028. doi: 10.1155/2012/271028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumawat M, Sharma TK, Singh I, Singh N, Ghalaut VS, Vardey SK, Vijay Shankar. Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. N Am J Med Sci. 2013;5(3):213–219. doi: 10.4103/1947-2714.109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arai K, Lizuka S, Tada Y, Oikawa K, Taniguelui N. Increase in the glycosylated form of erythrocyte Cu Zn SOD in diabetes and association of non-enzymatic glycosylation with enzyme activity. Biochim Biophys Acta. 1987;924(2):292–296. doi: 10.1016/0304-4165(87)90025-0. [DOI] [PubMed] [Google Scholar]
- 37.Hamden K, Carreau S, Jamoussi K, Miladi S, Lajmi S, Aloulou D, Ayadi F, Elfeki A. 1Alpha, 25 dihydroxyvitamin D3: therapeutic and preventive effects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats. J Nutr Sci Vitaminol. 2009;55(3):215–222. doi: 10.3177/jnsv.55.215. [DOI] [PubMed] [Google Scholar]
- 38.Taheri E, Djalali M, Saedisomeolia A, Malekshahi Moghadam A, Djazayeri A, Qorbani M. The relationship between the activates of antioxidant enzymes in red blood cells and body mass index in Iranian type 2 diabetes and healthy subjects. J Diabetes Metab Disord. 2012;11(1):3. doi: 10.1186/2251-6581-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan HZ, Zhang H, Chang D, Li H, Sui H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br J Ophthalmol. 2008;92(4):548–551. doi: 10.1136/bjo.2007.130542. [DOI] [PubMed] [Google Scholar]
- 40.Dong QY, Cui Y, Chen L, Song J, Sun L. Urinary 8-hydroxydeoxyguanosine levels in diabetic retinopathy patients. Eur J Ophthalmol. 2008;18(1):94–98. doi: 10.1177/112067210801800116. [DOI] [PubMed] [Google Scholar]
- 41.Al-Shabrawey M, Smith S. Prediction of diabetic retinopathy: role of oxidative stress and relevance of apoptotic biomarkers. EPMA J. 2010;1(1):56–72. doi: 10.1007/s13167-010-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


