Abstract
AIM
To discuss and compare the fundus autofluorescence (FAF) and optical coherence tomography (OCT) in acute or chronic central serous chorioretinopathy (CSCR).
METHODS
Medical records of 100 cases of CSCR were reviewed. Acute and chronic cases were evaluated according to the duration of decreased visual acuity, serous retinal detachment (RD) and focal leakage on fluorescein angiography (FA). Chi-square test was used for statistical analysis.
RESULTS
Forty cases had acute and 60 cases had chronic CSCR. FAF showed focal hypo-autofluorescence in 34 (85%) and iso-autofluorescence in 6 (15%) of acute cases and hypo-autofluorescence in 51 (85%), hyper-autofluorescence in 6 (10%) and iso-autofluorescence in 3 (5%) of chronic cases. OCT showed serous RD with distinct borders correlated with FAF findings (hypo-autofluorescence) in all acute CSCR cases. In chronic CSCR group, OCT showed serous RD with indistinct borders correlated with FAF findings. The differences between the OCT and FAF findings of the two groups were significant (P=0.000).
CONCLUSION
OCT and FAF findings can support the clinical observations in differential diagnosis of acute and chronic CSCR and help clinicians to evaluate retinal pigment epithelium, outer segments of photoreceptors and the components of serous RD.
Keywords: central serous chorioretinopathy, fluorescein angiography, fundus autofluorescence, optical coherence tomography
INTRODUCTION
Central serous chorioretinopathy (CSCR), which was described by von Grafe in 1886, is a frequent retinal disease and characterized by serous sensorial retinal detachment (RD) due to the primary dysfunction at the level of the choroid and one or more focal lesions of retinal pigment epithelium (RPE)[1]-[4]. Although macula is frequently affected and the disease can be recurrent, the visual prognosis is generally good. The disease has several names like CSCR, idiopathic central serous choroidopathy and central serous epitheliopathy because of unclear etiopathogenesis[3],[4]. It can be classified as acute (symptoms and diagnostic findings <6mo), chronic (symptoms and diagnostic findings ≥6mo) and recurrent (more than 1 acute attack). The duration of the disease is the most important factor for the discrimination of acute and chronic terminology.
Fundus autofluorescence (FAF) has been widely used in the diagnosis of retinal diseases for recent years. It is an in-vivo imaging method for metabolic mapping of naturally or pathologically occurring fluorophore of the ocular fundus[5],[6]. Lipofuscin precipitate is the main source of autofluorescence. The main component of lipofuscin is A2E and its formers are A2PE-H2, A2PE and A2-rodopsine which are all autofluorescent and found in outer segments of photoreceptors before phagocytosis[7]. In normal conditions FAF image of optic disc reveal no autofluorescence because of the absence of RPE and FAF of macula reveals hypo-autofluorescence because of the absorbance of blue light by lutein and zeaxanthin. The increased metabolic activity of RPE is generally associated with hyper-autofluorescence and the opposite is also correct. Except these conditions, the uniform appearance caused by physiological level and distribution of lipofuscin in RPE is known as isoautofluorescence[5]-[7].
Although indocyanine green angiography (ICG) is the golden standard, FAF and optical coherence tomography (OCT) are also important for the diagnosis of the disease. The FAF of CSCR cases was thought to arise not only from the RPE, but also from the back surface of the retina with a diffuse and a punctuate characteristic of autofluorescence[8]. OCT shows hyper intense granules caused by the occurrence of brush border appearance in the area which fits with the outer segments of photoreceptors and outer segments which were widely scattered in subretinal space and over RPE due to the prolonged serous detachment especially after 6wk[9]-[11].
In our study, our aim was to discuss and compare the fundus FAF and OCT findings of acute or chronic CSCR cases.
SUBJECTS AND METHODS
Medical records of 40 acute and 60 chronic CSCR cases that had been diagnosed between June 2010 and October 2011 in Retina Department of Ulucanlar Eye Research Hospital, were reviewed. CSCR had been diagnosed on the basis of clinical findings, color fundus photography, fluorescein angiography (FA) leakage pattern and OCT. All study procedures were conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from all the participants. Our presentation was approved by Ethics Committee of Ankara University, school of medicine.
All of the cases had a complete ophthalmological examination. Also their color fundus photography, FAF (488 nm) with Heidelberg Retina Angiograph 2 (HRA 2, Heidelberg engineering, Germany), FA (KOVA imaging systems, Japan) and spectral-domain OCT (SD-OCT) (Heidelberg Engineering, Heidelberg, Germany) findings in the initial and follow-up visits were also reviewed.
Cases were divided into acute and chronic-recurrent CSCR according to the duration of decreased visual acuity (VA), clinical findings of CSCR and focal leakage on FA. Acute cases were defined as: duration of decreased VA, clinical findings (ophthalmoscopically visible neurosensory detachment) and FA findings (focal leakage) <6mo (group 1). Chronic-recurrent cases were defined as: duration of decreased VA, residual and recurrent symptoms, FA findings (one or more sites of focal, diffuse or mottled leakage, RPE alterations) ≥6mo (group 2). Chi-square test was used for statistical analysis.
RESULTS
Group 1 consisted of 30 (75%) male and 10 (25%) female cases with the mean age of 38.4±6.9 (27-59 years) and group 2 consisted of 52 (86.7%) male and 8 (13.3%) female cases with the mean age of 46.7±8.1 (35-65 years) (Table 1).
Table 1. Comparison of the demographic characteristics and best-corrected visual acuity values of the cases.
| Parameters | Acute CSCR | Chronic CSCR | P |
| No. of cases (n) | 40 | 60 | |
| Age(a) | 38.4±6.9 | 46.7±8.1 | 10.000 |
| Sex, n (%) | |||
| M | 30 (75) | 52 (86.7) | 20.08 |
| F | 10 (25) | 8 (13.3) | |
| BCVA (logMAR) | 0.1±1 | 0.2±1.3 | 10.01 |
BCVA: Best-corrected visual acuity; CSCR: Central serous chorioretinopathy; 1Statistically significant, t-test; 2Statistically insignificant, Chi-square test.
The mean best-corrected visual acuity (BCVA) (logMAR) at the time of the diagnosis was 0.1±1 in acute CSCR cases and 0.2±1.3 in chronic cases. Acute CSCR cases had higher initial VA levels than chronic cases and the difference was statistically significant (P=0.01) (Table 1).
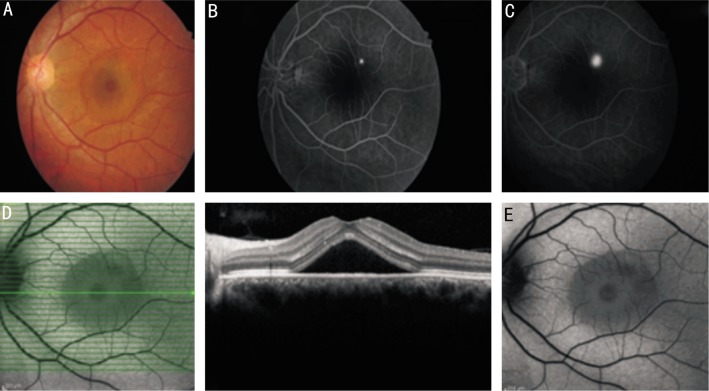
In initial examinations of all acute CSCR cases (Figure 1A), FA showed hyper-fluorescence due to the leakage from the site of the RPE defect in the serous detachment area in late phases (Figure 1B, 1C). OCT of the same shows serous detachment with distinct border but without any changes in the outer segments of photoreceptors (Figure 1D). FAF photography revealed hypo-autofluorescence in 34 (85%) cases (Figure 1E) and iso-autofluorescence in 6 (15%) cases in the areas which fitted with the focal leakage sites in FA. In 36 (90%) cases hypo-autofluorescence and in 4 (10%) cases isoautofluorescence were seen in FAF photography in the areas which fitted with the serous detachment. In group 1, OCT showed pigment epithelial detachment (PED) and serous RD with distinct borders in all cases (Table 2) (Figure 1D) and the mean central macular thickness was 360±140 µm. After the resorbtion of serous detachment, 15 (37.5%) cases in group 1 showed speckled hyper-autofluorescence in FAF photography. Figure 1D shows horizontal section of macular OCT and the leakage point can not be seen. If OCT section crossed the point of leakage which was shown by FA, the leakage site of RPE could be observed by OCT. Figure 1F shows OCT section from the leakage point observed in FA.
Figure 1. Images of a case with acute CSCR.
A: Color fundus photography of an acute CSCR 37-year-old male case shows serous detachment; B,C: FA of the same case shows hyper-fluorescence at the leakage site which begins at the 35th second and increases in late phases; D: Horizontal section of OCT of the same case shows serous detachment with distinct border but without any changes in the outer segments of photoreceptors and serous fluid; E: Fundus autofluorescence photography reveals hypo-autofluorescence in the serous detachment area with distinct borders and also hypo-autofluorescence at the leakage site in FA surrounded by iso-autofluorescence area of normal retina.
Table 2. Comparison of the FA, FAF and OCT findings of the cases.
| Parameters | Acute CSCR | Chronic CSCR | P |
| FA findings, | |||
| Hypo-fluorescence | 34 (85) | 0 (0) | 10.000 |
| Hyper-fluorescence | 0 (0) | 60 (100) | |
| Iso-fluorescence | 6 (15) | ||
| FAF at the focal leakage site in FA | |||
| Hypo-autofluorescence | 34(85) | 51 (85) | 0.48 |
| Hyper-autofluorescence | 0 (0) | 6 (10) | |
| Iso-autofluorescence | 6 (15) | 3 (5) | |
| FAF of serous RD | |||
| Hypo-autofluorescence | 36 (90) | 0 (0) | 10.000 |
| Hyper-autofluorescence | 0 (0) | 54 (90) | |
| Iso-autofluorescence | 4 (10) | 6(10) | |
| PED+ | 3 (7.5) | 3 (5) | 0.44 |
| PED− | 37 (92.5) | 57 (95) | |
| OCT | |||
| Distinct borders | 40 (100) | 0 (0) | 10.000 |
| Indistinct borders | 0 (0) | 60 (100) |
FA: Fluorescein angiography; CSCR: Central serous chorioretinopathy; FAF: Fundus autofluorescence; RD: Retinal detachment; OCT: Optical coherence tomography; PED: Pigment epithelial detachment; 1Statistically significant.
n (%)
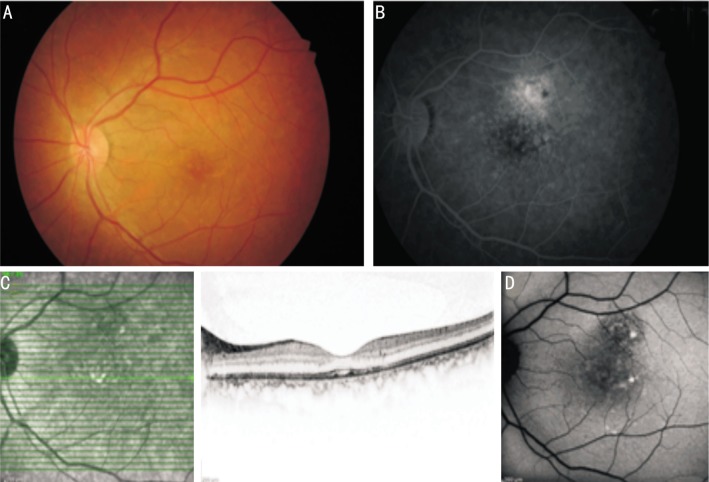
After the resorption of acute attack (Figure 2A), FA shows window defect due to RPE atrophy at the leakage site (Figure 2B). OCT shows probably resorbed serous detachment and some irregularities associated with the outer segments of photoreceptors (Figure 2C) and related mottled hyper-autofluorescence in FAF (Figure 2D). Figure 2C shows horizontal section of macular OCT and it is difficult to say that it shows resorbed serous detachment definitely. But a shallow serous detachment under fovea and some irregularities of RPE and atrophic regions can be seen but not so clearly. Shallow serous detachment associated with CSCR may remain for a long time and cause changes and irregularities of RPE and IS/OS band.
Figure 2. Images of a case with chronic CSCR.
A: Color fundus photography of a 45-year-old chronic CSCR case shows yellowish-white precipitates in macular area and RPE atrophy; B: The late phases of FA of the same case shows patchy hyper-fluorescence due to diffuse leakage just above fovea and a window defect due to RPE atrophy at the leakage site; C: Horizontal section of OCT shows resorbed serous detachment and precipitations over RPE; D: FAF photography shows scattered hyper-autofluorescence in the area of resorbed serous detachment.
In chronic cases, FA showed hyper-fluorescence at the original leakage sites in late phases in all cases (Figure 2B). FAF revealed hypo-autofluorescence in 51 (85%) cases, hyper-autofluorescence in 6 (10%) cases and iso-autofluorescence in 3 (5%) cases at the original leakage sites which fitted with the hyper-fluorescence in FA. In 54 (90%) cases hyper-autofluorescence and in 6 (10%) cases iso-autofluorescence were seen in FAF photography in the areas which fitted with the serous detachment (Table 2). OCT showed shallow serous RD in all chronic CSCR cases and the mean central macular thickness was 250±101 µm. 45 (75%) cases in group 2 showed mottled hyper-autofluorescence in FAF photography. In chronic cases with shallow or prominent serous detachment, OCT showed hyper-intense granules caused by scattered outer segments of photoreceptors and brush border appearance. FAF photography of these sites revealed hyper-autofluorescence and was accordant with OCT findings. Color fundus photography of none of the chronic CSCR cases show drusen, edema, exuda or choroidal neovascular membrane related with age-related macular degeneration (ARMD) and because of this ARMD was not thought in the differential diagnosis.
DISCUSSION
The current study examines FA, FAF and OCT findings in CSCR and compares these findings in acute and chronic cases. In spite of its unclear etiology, the liquid is inversely pumped into the subretinal space and cause neurosensory detachment in CSCR by the dysfunctions of mainly RPE but also choroid[1]-[4]. Guyer et al[12] investigated the indocyanine green angiography (ICG) of CSCR cases and postulated that serous RD was caused by defects in RPE structure and its metabolic activity induced by the pressure on RPE due to the increase in choroidal permeability. Recurrent epinephrine injection was reported to cause similar clinical findings like in CSCR in animal models and histopathological examination showed focal RPE degeneration and the endothelial cell defects in choriocapillaris[13].
Emotional stress, corticosteroid use, some systemic diseases, familial and racial factors and pregnancy have been reported to be risk factors of the disease[1]-[4],[14]-[16]. Although spontaneous resorbtion generally occurs in 3-4mo, 4% of chronic cases developed RPE insufficiency named as sick RPE syndrome[1]. The disease is more frequent in young men and it reaches its peak at the age of 45. Older and female cases are known to have chronic CSCR more than male and younger cases[1]-[4]. Also in our study, chronic cases were found to be significantly older than acute CSCR cases but both of the groups had male predominance.
FAF is one of the most important diagnostic methods of retinal diseases for recent years. Macula is hypo-autofluorescent in iso-autofluorescent retina in healthy people because of lutein and zeaxanthin pigments which protect it from oxidative stress. They can filter blue light and reduce lipofuscin formation[5]-[7]. In spite of the different pathogenesis of serous detachment in different retinal disorders; the main source of autofluorescence of the macula is the precipitates of lipofuscin granules in outer retinal layers and subretinal space, due to the impaired phagocytosis of outer segments of photoreceptors by RPE[5]-[7]. In our study, the FAF images showed hypo-autofluorescence at the focal leakage site in FA in the great majority of acute and chronic CSCR cases. Except chronic recurrent cases the area of serous RD was also hypo-autofluorescent but in recurrent cases, hyper-autofluorescence was detected in the RD area. In many other reports, the FAF images also showed hypo-autofluorescence at the focal leakage site which was thought to be caused by the destruction of RPE and/or blockage by subretinal fluid accumulation[17]-[18]. Dinc et al[17] showed hypo-autofluorescence in 80% of acute CSCR and 88.2% of chronic recurrent CSCR in FAF photography. Also in our study, 85% of acute cases and 85% of chronic cases showed hypo-autofluorescence which was thought to be related with reduced metabolic activity. But von Rückmann et al[18] found an opposite result and postulated that increased autofluorescence might occur at the site of the leakage and in the area of RD in acute CSCR cases probably caused by defective degradation of phagosomal material which would result in an increase of autofluorescence. They thought this could be due to inadequate production of degradative enzymes or acidification of the phagosomes. But increased metabolic activity of the RPE was a confusing cause of hyper autofluorescence in their cases because of the malnutrition of photoreceptors and RPE-cells.
FAF shows different characteristics in acute and chronic CSCR cases as in our study[16]-[19]. In acute cases hypo-autofluorescence is predominant because of the blockage by serous effusion. Autofluorescence increases in time in chronic CSCR and cases with persistent serous RD[18],[20],[21]. The reason of increased autofluorescence in these cases is the accumulation of the outer segments of photoreceptors in subretinal space and the dispersed chromophores in the field of serous RD. So not only RPE atrophy but also serous RD may cause hyper-autofluorescence[5],[8],[16],[20]. Spaide and Klancnik[8] demonstrated that the sources of these subretinal precipitations were the outer segments themselves and macrophage cells those phagosited these outer segments. These precipitations cause mottled hyper-autofluorescence which was observed in 75% of our chronic cases. Hyper-autofluorescence may also be observed after the resorbtion of serous detachment. Dinc et al[17] observed mottled hyper-autofluorescence in 36% of their cases after resorbtion of RD like in 37.5% of our cases (Figure 2D).
OCT is also a useful diagnostic tool in CSCR. Matsumoto et al[22] demonstrated the elongation of outer segments of photoreceptors and narrowing of the outer nuclear layer in OCT. In Kon et al[10] study, OCT showed, yellow hyper reflective subretinal and intraretinal precipitations not only in the posterior of RD but also within the RD. Dinc et al[17] demonstrated hyper reflective foci in OCT which were associated with hyperautofluorescent regions in FAF photography. In our study, we also observed hyper reflective foci in acute and chronic CSCR both in serous detachment area and posterior of RD in OCT which was in accordance with hyperautofluorescent regions in FAF.
This report emphasizes the importance of OCT and FAF findings in addition to clinical ophthalmic examination not only in the diagnosis but also in discrimination of different stages of CSCR. As some changes occur in the leakage point of RPE and serous RD area in time, acute and chronic CSCR cases show different clinical and imaging characteristics. At that point, the results of FA, FAF and OCT support and complement each other and they can help the clinicians not only in diagnosis and follow-up but also in planning their cases treatments perfectly.
Acknowledgments
Conflicts of Interest: Teke MY, None; Elgin U, None; Nalcacioglu-Yuksekkaya P, None; Sen E, None; Ozdal P, None; Ozturk F, None.
REFERENCES
- 1.Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond) 2010;24(12):1743–1756. doi: 10.1038/eye.2010.130. [DOI] [PubMed] [Google Scholar]
- 2.Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–145. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 3.Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011;22(3):166–173. doi: 10.1097/ICU.0b013e3283459826. [DOI] [PubMed] [Google Scholar]
- 4.Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002;47(5):431–448. doi: 10.1016/s0039-6257(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 5.Spaide RF. Autofluorescence from the outer retina and subretinal space. Hypothesis and Review. Retina. 2008;28(1):5–35. doi: 10.1097/IAE.0b013e318158eca4. [DOI] [PubMed] [Google Scholar]
- 6.Choudhry N, Giani A, Miller JW. Fundus autofluorescence in geographic atrophy: a review. Semin Ophthalmol. 2010;25(5–6):206–213. doi: 10.3109/08820538.2010.518121. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow JR. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem. 2000;275(38):29354–29360. doi: 10.1074/jbc.M910191199. [DOI] [PubMed] [Google Scholar]
- 8.Spaide RF, Klancnik JM., Jr Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology. 2005;112(5):825–833. doi: 10.1016/j.ophtha.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Ozmert E, Batioğlu F. Fundus autofluorescence before and after photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmologica. 2009;223(4):263–268. doi: 10.1159/000210386. [DOI] [PubMed] [Google Scholar]
- 10.Kon Y, Iida T, Maruko I, Saito M. The optical coherence tomography-ophthalmoscope for examination of central serous chorioretinopathy with precipitates. Retina. 2008;28(6):864–869. doi: 10.1097/IAE.0b013e3181669795. [DOI] [PubMed] [Google Scholar]
- 11.Gupta V, Gupta A, Dogra MR, Singh I. Reversible retinal changes in the acute stage of sympathetic ophthalmia seen on spectral domain optical coherence tomography. Int Ophthalmol. 2011;31(2):105–110. doi: 10.1007/s10792-011-9432-1. [DOI] [PubMed] [Google Scholar]
- 12.Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112(8):1057–1062. doi: 10.1001/archopht.1994.01090200063023. [DOI] [PubMed] [Google Scholar]
- 13.Yoshioka H, Katsume Y, Akune H. Experimental central serous chorioretinopathy in monkey eyes: fluorescein angiographic findings. Ophthalmologica. 1982;185(3):168–178. doi: 10.1159/000309239. [DOI] [PubMed] [Google Scholar]
- 14.Spahn C, Wiek J, Burger T, Hansen L. Psychosomatic aspects in patients with central serous chorioretinopathy. Br J Ophthalmol. 2003;87(6):704–708. doi: 10.1136/bjo.87.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16(5):308–314. doi: 10.1097/01.icu.0000179803.42218.cc. [DOI] [PubMed] [Google Scholar]
- 16.Eandi CM, Ober M, Iranmanesh R, Peiretti E, Yannuzzi LA. Acute central serous chorioretinopathy and fundus autofluorescence. Retina. 2005;25(8):989–993. doi: 10.1097/00006982-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Dinc UA, Tatlipinar S, Yenerel M, Görgün E, Ciftci F. Fundus autofluorescence in acute and chronic central serous chorioretinopathy. Clin Exp Optom. 2011;94(5):452–457. doi: 10.1111/j.1444-0938.2011.00598.x. [DOI] [PubMed] [Google Scholar]
- 18.von Rückmann A, Fitzke FW, Fan J, Halfyard A, Bird AC. Abnormalities of fundus autofluorescence in central serous retinopathy. Am J Ophthalmol. 2002;133(6):780–786. doi: 10.1016/s0002-9394(02)01428-9. [DOI] [PubMed] [Google Scholar]
- 19.Framme C, Walter A, Gabler B, Roider J, Sachs HG, Gabel VP. Fundus autofluorescence in acute and chronic-recurrent central serous chorioretinopathy. Acta Ophthalmol Scand. 2005;83(2):161–167. doi: 10.1111/j.1600-0420.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 20.Iida T, Spaide RF, Haas A, Yannuzzi LA, Jampol LM, Lesser RL. Leopard-spot pattern of yellowish subretinal deposits in central serous chorioretinopathy. Arch Ophthalmol. 2002;120(1):37–42. doi: 10.1001/archopht.120.1.37. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Sander B, la Cour M, Larsen M. Clinical characteristics of subretinal deposits in central serous chorioretinopathy. Acta Ophthalmol Scand. 2005;83(6):691–696. doi: 10.1111/j.1600-0420.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto H, Kishi S, Sato T, Mukai R. Fundus autofluorescence of elongated photoreceptor outer segments in central serous chorioretinopathy. Am J Ophthalmol. 2011;151(4):617–623. doi: 10.1016/j.ajo.2010.09.031. [DOI] [PubMed] [Google Scholar]