Abstract
AIM
To report the long-term vision-threatening complications in patients who underwent phakic intraocular lens (pIOLs) implantation for high myopia.
METHODS
This study was designed from a consecutive series of phakic intraocular lens complication and corrective surgeries. Sixteen eyes of 13 patients had implantation of phakic intraocular lens for correction high myopia and developed serious complications have been included in this study. The mean age of patients was 38.6±6.35y (range 32-50y) and the mean time of history of pIOL implantation for high myopia was 6±2y (range 2-10y). Before corrective surgery, best spectacle-corrective visual acuity (BSCVA) ranged from perception to 20/200 in the eyes in which severe complications occurred.
RESULTS
Corneal decompensation occurred in 12 eyes of 9 high myopic patients after anterior chamber pIOL implantation. Rhegmatogenous retinal detachment (RRD) occurred in 4 eyes of 4 high myopic patients following anterior chamber and posterior chamber pIOL implantation. Patients with corneal decompensation, had combined procedures consisting of pIOL removal and penetrating keratoplasty (PKP). Removals of pIOL, phacoemulsification and pars plana vitrectomy (PPV) with silicone oil tamponade were performed in patients with RRD. After corrective surgeries, all patients but one (P+, patient 2, right eye) achieved moderate BSCVA ranged from 20/200 to 20/50 at the last visit.
CONCLUSION
Phakic IOLs may be effective for the correction of high myopia. Although these IOLs may have severe complications and it affects safety and efficacy of this surgery. As seen here, corneal decompensation and rhegmatogenous retinal detachment are possible postoperative vision-threatening complications of phakic IOLs. Patients must be carefully examined before and after surgery for possible endothelial cell loss and vitreoretinal problems.
Keywords: phakic intraocular lens, high myopia, complications, corneal decompensation, rhegmatogenous retinal detachment
INTRODUCTION
Implantation of phakic intraocular lens (pIOL) is an alternative way to correct high myopia in patients who are dissatisfied with the quality of spectacles and contact lenses or not candidates for corneal laser surgery[1],[2]. PIOLs such as iris-fixated or angle-supported anterior chamber and posterior chamber have been reported to be effective for the correction of high myopia in many studies[3]-[7]. However long-term vision-threatening complications of this pIOLs, affect safety and efficacy of this surgery.
In this study, we reported patients who developed serious vision treating late complications after implantation of pIOL for the correction of high myopia.
SUBJECTS AND METHODS
This retrospective, interventional case series was designed from a consecutive series of phakic intraocular lens complication and corrective surgeries performed in a single center by 1 surgeon. Between 1998 and 2009, 16 eyes of 13 patients had implantation of pIOL to correct high myopia in our (7 eyes of 5 patients) or another clinic and presented with serious complications between 2008 and 2011 have been included in this study. All patients were adequately informed and signed a consent form. The study adhered to the tenets of the Declaration of Helsinki.
Patients with the mean age of 38.6±6.35y (range 32-50y), whom had history of pIOL implantation for high myopia the mean 6±2y (range 2-10y), were referred to our clinic. Eyes with a previous history of refractive, corneal or intraocular surgery, glaucoma, cataract, corneal degeneration, recurrent or chronic uveitis, retinopathy, shallow anterior chamber depth (ACD) (from the epithelium) of less than 3.2 mm in Artisan and I Care and 2.8 mm in implantable collamer lens (ICL) group, endothelial cell count (ECC) of less than 2000 /mm2 were not performed initial refractive surgery. All patients were older than 18y and had stable myopia for at least 2y.
Before initial refractive surgery, all patients underwent a complete ophthalmic examination. It included uncorrected visual acuity (UCVA), best spectacle-corrected visual acuity (BSCVA), cycloplegic and manifest refraction, applanation tonometry for intraocular pressure (IOP), anterior chamber depth (ACD) (IOLmaster, Carl Zeiss Meditec AG), endothelial cell density (ECD) (Topcon-SP, Tokyo, Japan), corneal topography, slit lamp evaluation, biometry (IOLmaster, Carl Zeiss Meditec AG) measurement and dilated fundus evaluation. White-to-white (WTW) diameter by surgical calipers and corneal pachymetry were also measured in ICL groups.
After initial refractive surgery, UCVA, BSCVA, manifest refraction, IOP measurement, ECD, slit-lamp biomicroscopy and dilated fundus examination were repeated at 1d, 1wk, 1, 3, 6 and 12 mo.
There were no pathological findings such as high intraocular pressure or retinal tears and retinal degenerations in 5 patients who had initial refractive surgery in our clinic, before and after 1-year initial refractive surgery.
Patients' data and type of pIOL are shown in the Table 1.
Table 1. All patients data and type of Piol.
| No. | 1Age/eye (a) | Piol | SE(D) | 2Age (a) | ACD (mm) | ECD (mm2) |
| 1 | 36/R | I Care | NF | 46 | NF | NF |
| 2 | 36/L | I Care | NF | 42 | NF | NF |
| /R | I Care | NF | 39 | NF | NF | |
| 3 | 42/L | I Care | NF | 48 | NF | NF |
| 4 | 45/R | I Care | -14.25 | 50 | 3.20 | 2 200 |
| /L | I Care | -13.75 | 49 | 3.20 | 2 240 | |
| 5 | 29/R | I Care | NF | 35 | NF | NF |
| 6 | 32/R | I Care | NF | 38 | NF | NF |
| 7 | 32/R | I Care | 13.25 | 36 | 3.30 | 2 150 |
| 8 | 30/R | I Care | NF | 32 | NF | NF |
| 9 | 30/R | Artisan | -12.75 | 35 | 3.30 | 2 100 |
| /L | Artisan | -10.75 | 34 | 3.20 | 2 250 | |
| 10 | 26/R | Artisan | NF | 34 | NF | NF |
| 11 | 22/L | Artisan | NF | 32 | NF | NF |
| 12 | 31/L | Artisan | -12.25 | 36 | 3.30 | 2 210 |
| 13 | 30/L | ICL | -8.75 | 32 | 3.00 | 2 280 |
1Age at the time of the initial refractive surgery; 2Age at the time of complication development; R: Right eye; L: Left eye; pIOL: Type of phakic intraocular lens; SE: Spherical Equivalent; ACD: Anterior chamber depth; ECD: Endothelial cell density; NF: Not found.
All patients were examined by same authors. Slitlamp examination was significant with corneal decompensation and bullous keratopathy in 12 eyes (8 eyes with angle-supported and 4 eyes with iris-fixated anterior chamber pIOL). In 1 eye out of 12, corneal perforation had occurred due to severe corneal decompensation, spontaneously (Patient 2). In patients who developed corneal decompensation following anterior pIOL implantation, the mean endothelial cell density was 560.64±103.31 /mm2 (range 400-770 /mm2) and the visual acuity ranged from light perception to 20/200. Fundus examination was significant with rhegmatogenous retinal detachment (RRD) in 4 eyes (2 eyes with angle-supported and 1 eye with iris-fixated anterior chamber, 1 eye with posterior chamber pIOL). In this group the visual acuity was hand movement in 3 eyes and light perception in 1 eye. Patient's data at the time of initial examination is shown in the Table 2.
Table 2. Patient's data at the time of initial examination after complication occurred.
| No. | 1Complication time (a) | Complications | Visual acuity after complication | ECD |
| 1 | 10 | Corneal decompensation | P+ | 400 |
| 2 | 6 | Corneal decompensation | 20/200 | 770 |
| 3 | Corneal decompensation and corneal perforation | P+ | NM | |
| 3 | 6 | Corneal decompensation | CF from 1m | 540 |
| 4 | 5 | Corneal decompensation | CF from 1m | 510 |
| 4 | Corneal decompensation | 20/200 | 512 | |
| 5 | 6 | Corneal decompensation | 20/200 | 495 |
| 6 | 6 | Corneal decompensation | 20/400 | 500 |
| 7 | 4 | Retinal detachment | P+ | NM |
| 8 | 2 | Retinal detachment | HM | NM |
| 9 | 5 | Corneal decompensation | CF from 1m | 620 |
| 4 | Corneal decompensation | 20/400 | 700 | |
| 10 | 8 | Corneal decompensation | 20/400 | 540 |
| 11 | 10 | Corneal decompensation | 20/400 | 580 |
| 12 | 5 | Retinal detachment | HM | NM |
| 13 | 2 | Retinal detachment | HM | NM |
1Complication time: Time of complication development after pIOL implantation; ECD: Corneal endothelial cell density; NM: Not measured; P+: Perception; HM: Hand movement; CF: Counting finger.
Patients with corneal decompensation, had combined procedures consisting of pIOL removal and penetrating keratoplasty (PKP). All transplantations were performed by same surgeon under retrobulbar anesthesia. Removals of pIOL, phacoemulsification and pars plana vitrectomy (PPV) with silicone oil tamponade were performed in patients with RRD. No complications developed during the surgical procedures. All patients were followed up for at least 1y after PKP and retinal reattachment surgery. Surgical procedures and last visual acuity are shown in Table 3.
Table 3. Surgical procedures and last visual acuity after surgery.
| No. | Surgery | Visual acuity after surgery |
| 1 | pIOL removal and PKP | 20/100 |
| 2 | pIOL removal, lens aspiration, IOL implantation, PKP | 20/100 |
| pIOL removal, lensectomy, anterior vitrectomy, PKP | P+ | |
| 3 | pIOL removal and PKP | 20/50 |
| 4 | pIOL removal and PKP | 20/200 |
| pIOL removal and PKP | 20/100 | |
| 5 | pIOL removal and PKP | 20/100 |
| 6 | pIOL removal andPKP | 20/200 |
| 7 | PPV with removal of pIOL and phocoemuisification | 20/100 |
| 8 | PPV with removal of pIOL and phocoemuilsification | 20/200 |
| 9 | pIOL removal and PKP | 20/100 |
| pIOL removal and PKP | 20/200 | |
| 10 | pIOL removal and PKP | 20/100 |
| 11 | pIOL removal and PKP | 20/100 |
| 12 | PPV with removal of pIOL and phocoemuilsification | 20/200 |
| 13 | PPV with removal of pIOL and phocoemuilsification | 20/200 |
PKP: Penetrating keratoplasty; PPV: Pars plana vitrectomy; Visual acuity after surgery: Visual acuity at last examination.
RESULTS
Corneal decompensation occurred in 12 eyes of 9 high myopic patients after angle-supported (8 eyes of 6 patients) and iris-fixated (4 eyes of 3 patients) anterior chamber pIOL implantation. Patient age at the time of the initial refractive procedure ranged from 22 to 45y (mean 34.1y). The mean ECD was 2 197.5±68.5 /mm2 and ACD was 3.23±0.05 mm in 2 patients of 4 eyes who had the initial refractive procedure in our clinic. Time interval between initial refractive surgery and presentation with complication was ranged from 3 to 10y. At Presentation, BSCVA ranged from perception to 20/200 in eyes with corneal decompensation. The ECD had decreased from 2240 /mm2 to 512 /mm2 in one (patient 4, left eye) (Figure 1). After corrective surgery, all eyes (Figure 2) but one (patient 2, right eye)(Figure 3) achieved moderate BSCVA ranged 20/200 to 20/50 at the last visit.
Figure 1. Patient 4, left eye.
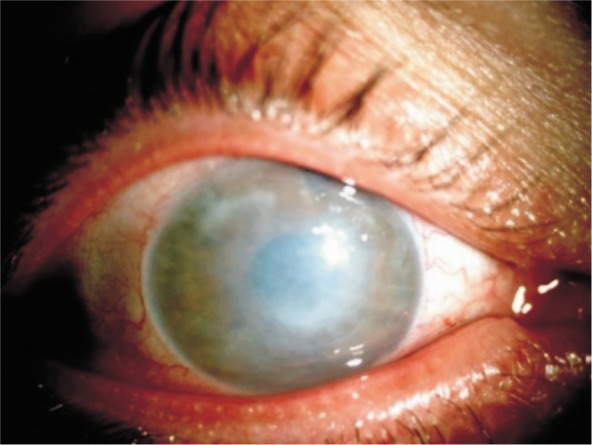
Figure 2. Patient 9, after corrective surgery.
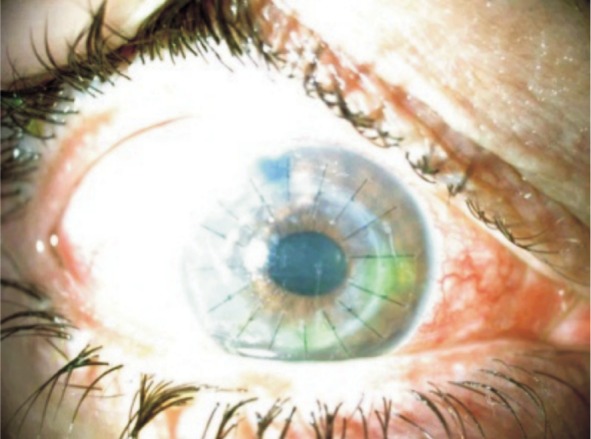
Figure 3. Patient 2, right eye.
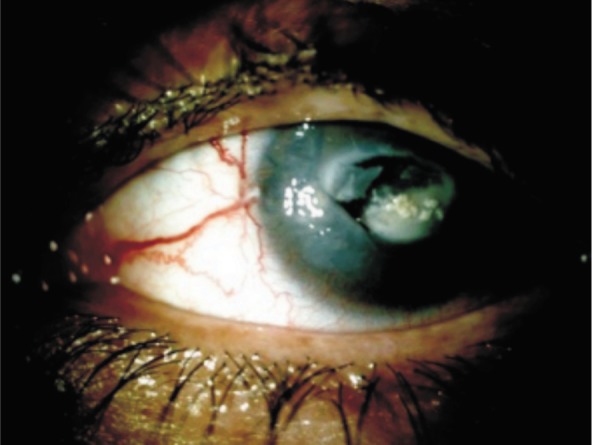
Patient 2 is described in detail as representative example of severe complication.
A 39-year old male patient referred our clinic for spontaneous corneal perforation in the right eye. The patient had a history of phakic angle-supported anterior chamber IOL implantation in both eyes for correction of high myopia 3y. One-year after implantation, he had loss of vision in the right. On presentation his visual acuity was only perception in the right eye and BCVA 20/40 in the left eye. Slitlamp examination revealed phakic angle-supported anterior chamber IOL in the left eye and an edematous cornea with central corneal perforation in which pIOL was embedded to central cornea was observed in the right eye (Figure 3). The endothelial cell density was 1150 /mm2 and there was no sign of corneal decompensation in the left eye. Visual acuity of the right eye with corneal perforation remained at light perception following PKP, pIOL removal, lensectomy and anterior vitrectomy.
After 3y later (42 years old) his visual acuity was perception in the right eye and 20/200 in the left eye. Slitlamp examination was significant for corneal decompensation and the endothelial cell density was 770 /mm2 in the left eye. The right eye was preftisic with opaque and vascularized graft. The patient was scheduled for anterior chamber phakic IOL removal and penetrating keratoplasty. He refused surgery until his visual acuity decreased to the level of finger count. Surgery was performed under retrobulber anesthesia, pIOL was removed, cataratous lens was aspirated without need for phacoemulsification, foldable hydrophilic lens was implanted and PKP was performed without any complication. At last follow-up BCVA was perception in the right eye and 20/100 in the left eye.
Rhegmatogenous retinal detachment occurred in 4 eyes of 4 high myopic patients after angle-supported (2 eyes of 2 patients) and iris-fixated (1 eye of 1 patient) anterior chamber, and posterior chamber (1 eye of 1 patient) pIOL implantation. Patients age at the time of the initial refractive procedure was ranged from 30 to 32y (mean 30.85y) and in fundus examination there were no retinal tears or retinal degenerations that may lead to postoperative rhegmatogenous detachment. The time to the complications following initial pIOL implantation was ranged from 2 to 5y (mean 3.25y). All eyes had 1 causative break and macula-off RRD but there was no ocular USG or retinal image of RRD. It is one of the limitations of this study. Preoperative BCVA in the eyes which RRD was occurred ranged from perception to hand movements. Visual acuity after reattachment was maintained in all patients. Median final BSCVA was 20/175 (range, 20/200 to 20/100) at final visit.
DISCUSSION
Although phakic IOL implantation reported to be an effective procedure for correcting high myopia, severe, vision-threatening complications can occur. Different types of phakic IOLs are in clinical use: anterior chamber IOLs (angle-supported and iris-fixated lenses) and posterior chamber IOL (implantable collomer lens). The main complications of angle-supported anterior chamber pIOLs are glare and halos, pupil ovalization and corneal endothelial cell loss; the main complications of iris-fixated pIOLs are chronic subclinical inflammation, corneal endothelial cell loss, dislocation or pupillary block glaucoma; and the main complications of posterior pIOLs are anterior subcapsular cataract formation, pigment dispersion and pupillary block glaucoma[7]. For all types of pIOLs, there is no established direct relationship between pIOL and RD[7].
Endothelial cell loss following anterior chamber phakic IOLs implantation has been reported in many studies[4],[8],[9]. The mean corneal endothelial cell loss after implantation of an I-Care pIOL was 6.1% and after implantation of Artisan/Verisyse pIOL was 6.8 % after 1y, as reported by Gierek-Ciaciura et al[10]. Moshirfar et al[11] report a 6.2 % decrease in corneal endothelial cells 2y after Artisan/ Verisyse implantation. Damage to the corneal endothelium may be due to direct contact between the pIOLs and the inner surface of the cornea during implantation or from postoperative changes in pIOLs position[7]. Moreover, subclinical inflammation may cause direct toxicity to the endothelium[7]. In addition of this natural loss of corneal endothelial cells is also about 0.6% per year, as reported by Bourne et al[12].
In our study group, 12 eyes of 9 patients with corneal decompensation and decreased visual acuity following anterior chamber phakic IOL implantation necessitated corneal transplantation. Endothelial cell density was measured in all patients except one (patient 2, right eye) before complication corrective surgeries. Patients operated at our clinic had very low endothelial cell density at presentation with complication. The mean endothelial cell density was 56.64±103.31/mm2 (range 400-770/mm2) in 11 eyes. Endothelia cell loss rate could not be calculated for all patients due to unavailability of data's for all. But it is presumed to be significantly high. The surgical procedure and phakic IOL implantation most likely proposed factors for the corneal decompensation. Visual acuity after PKP was maintained in all cases except one (patient 2, right eye).
As for all intraocular surgeries, implantation of a phakic IOL to correct high myopia has a potential risk for vitreoretinal complications. Although no vitreoretinal complications have been shown to be causally related to phakic IOL implantation[7]. Budo et al[13] reported 2 cases of RD in the European multicenter study of Artisan IOL over 8y. In the Güell et al[14] study of Artisan/ Verisyse phakic IOL, RD occurred in 1 case. Martinez-Castillo et al[15] published the largest series of RD after ICL posterior phakic IOL surgery and they reported 16 eyes with RD after ICL implantation. All this studies showed that RD was part of the natural history of RD in high myopia and RD rates similar in the highly myopic population that did not have refractive surgery[7].
In our study we reported 4 eyes with RD after phakic IOL surgery. The initial refractive surgery performed all patients except one (patient 8) in our clinic. The mean axial length of the 3 eyes was 32±0.17 mm and there was no risk factor for RD except high myopia. As discussed previously, in our study high myopia and long axial length are predisposing factors for rhegmatogenous retinal detachment and there is no showed direct relationship between phakic IOL and retinal detachment.
It is our observation that patients with high myopia are highly satisfied with pIOL implantation. Even corneal decompensation develops in one eye and fellow eye with decreasing ECD, they refuse explantation of pIOL until their visual acuity decrease to ambulatory levels.
In summary the cases we report suggest that phakic IOLs may have severe complications and it affects safety and efficacy of this surgery. As seen here, corneal decompensation and rhegmatogenous retinal detachment are potential postoperative complications of phakic IOLs. Patients must be carefully examined before and after surgery for possible endothelial cell loss and vitreoretinal problems.
Acknowledgments
Conflicts of Interest: Sayman Muslubas IB, None; Kandemir B, None; Aydın Oral AY, None; Kugu S, None; Dastan M, None.
REFERENCES
- 1.Pesudovs K, Garamendi E, Elliott DB. A quality of life comparison of people wearing spectacles or contact lenses or having undergone refractive surgery. J Refract Surg. 2006;22(1):19–27. doi: 10.3928/1081-597X-20060101-07. [DOI] [PubMed] [Google Scholar]
- 2.Huang D, Schallhorn SC, Sugar A, Farjo AA, Majmudar PA, Trattler WB, Tanzer DJ. Phakic intraocular lens implantation for the correction of myopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(11):2244–2258. doi: 10.1016/j.ophtha.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Kamiya K, Shimizu K, Igarashi A, Hikita F, Komatsu M. Four-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Arch Ophthalmol. 2009;127(7):845–850. doi: 10.1001/archophthalmol.2009.67. [DOI] [PubMed] [Google Scholar]
- 4.Pere'z-Santonja J, Alio' JL, Jime'nez-Alfaro I, Zato MA. Surgical correction of severe myopia with an angle-supported intraocular lens. J Cataract Refract Surg. 2000;26(9):1288–1302. doi: 10.1016/s0886-3350(00)00543-5. [DOI] [PubMed] [Google Scholar]
- 5.Dick HB, Budo C, Malecaze F, Güell JL, Marinho AAP, Nuijts RA, Luyten G, Menezo JL, Kohnen T. Foldable artiflex phakic intraocular lens for the correction of myopia two-year follow-up results of a prospective European multicenter study. Ophthalmology. 2009;116(4):671–677. doi: 10.1016/j.ophtha.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 6.Menezo JL, Peris-Martinez C, Cisneros AL, Martinez-Costa R. Phakic intraocular lenses to correct high myopia: Adatomed, Staar, and Artisan. J Cataract Refract Surg. 2004;30(1):33–44. doi: 10.1016/j.jcrs.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Kohnen T, Kook D, Morral M, Güell JL. Phakic intraocular lenses part 2: results and complications. J Refract Surg. 2010;36(12):2168–2194. doi: 10.1016/j.jcrs.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Patel SR, Chu DS, Ayres BD, Hersh PS. Case reports: corneal edema and penetrating keratoplasty after anterior chamber phakic intraocular lens implantation. J Cataract Refract Surg. 2005;31(11):2212–2215. doi: 10.1016/j.jcrs.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Kim JK, Lee HK. Corneal endothelial decompensation after iris-claw phakic intraocular lens implantation. J Cataract Refract Surg. 2008;34(3):517–519. doi: 10.1016/j.jcrs.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Gierek-Ciaciura S, Gierek-Lapinska A, Ochalik K, Mrukwa-Kominek E. Correction oh high myopia with different phakic anterior chamber intraocular lenses: I CARE angle-supported lens and Verisyse iris-claw lens. Graefes Arch Clin Exp Ophthalmol. 2007;245(1):1–7. doi: 10.1007/s00417-006-0374-7. [DOI] [PubMed] [Google Scholar]
- 11.Moshirfar M, Holz HA, Davis DK. Two-year follow-up of the Artisan/ Verisyse iris-supported phakic intraocular lens for the correction of high myopia. J Cataract Refract Surg. 2007;33(8):1392–1397. doi: 10.1016/j.jcrs.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38(3):779–782. [PubMed] [Google Scholar]
- 13.Budo C, Hessloehl JC, Izak M, Luyten GPM, Menezo JL, Sener BA, Tassignon MJ, Termote H, Worst JGF. Multicenter study of the Artisan phakic intraocular lens. J Cataract Refract Surg. 2000;26(8):1163–1171. doi: 10.1016/s0886-3350(00)00545-9. [DOI] [PubMed] [Google Scholar]
- 14.Güell JL, Morral M, Gris O, Gaytan J, Sisquella M, Manero F. Five-year follow-up of 399 phakic Artisan-Verisyse implantation for myopia, hyperopia, and/or astigmatism. Ophthalmology. 2008;115(6):1002–1012. doi: 10.1016/j.ophtha.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Castillo V, Boixadera A, Verdugo A, Elies D, Coret A, Garcia-Arumi J. Rhegmatogenous retinal detachment in phakic eyes after posterior chamber phakic intraocular lens implantation for severe myopia. Ophthalmology. 2005;112(4):580–585. doi: 10.1016/j.ophtha.2004.09.025. [DOI] [PubMed] [Google Scholar]


