Abstract
Huperzine A, an active Lycopodium alkaloid extracted from traditional Chinese herb, is a potent, selective and reversible acetylcholinesterase (AChE) inhibitor and has been widely used in China for the treatment of Alzheimer's disease (AD). Accordingly, some new mechanisms of action for huperzine A have been discovered over the past decades. In addition to its AChE inhibitory effect, potent multifaceted neuroprotective effect through activating cholinergic system and directly acting on mitochondria have been explored. Moreover, in order to maximize the efficacy and safety of huperzine A therapy, great efforts have been made to optimize drug delivery system. In the present article, an attempt is made to discuss the current progress and future perspective for huperzine A therapy in AD.
Keywords: Alzheimer's disease, huperzine A, acetylcholinesterase, mitochondrion, controlled release
Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by gradually loss of memory and other cognitive functions. Understanding the complexity of AD and its underlying pathophysiological mechanisms of AD provides a potential new paradigm that may help to develop new AD treatment1. Among the limited therapeutic approaches, cholinergic replacement therapy especially acetylcholinesterase inhibitors (AChEIs), has been at the forefront of efforts to pharmacologically ameliorate the symptom of AD patients. So far, four AChEIs, tacrine, donepezil, rivastigmine, and galantamine, have been approved by the United States Food and Drug Administration for the treatment of mild or moderate AD.
Huperzine A is a novel Lycopodium alkaloid isolated from Chinese herb Huperzia serrata (Thunb) Trev (Qian Ceng Ta), which was traditionally used to relieve pain, antidote the poison, as well as alleviate swelling. Huperzine A is widely proved to be a potent, selective and well-tolerated inhibitor of acetylcholinesterase (AChE) (reviewed by2). This effect was accidentally discovered in the 1970s while herbal extraction of Huperzine serrata was used to treat schizophrenia and found with significant cholinergic side effects. Huperzine A was approved by State Food and Drug Administration of China for AD therapy in 1994. Since then, large amount of clinical studies have shown that huperzine A administration can significantly improve the memory, cognitive skills, and daily life abilities of AD patients with no severe side effects. Besides being indicated for AD, huperzine A is also used in treating memory impairment in vascular dementia (VaD) patients, schizophrenia patients and sleep disorder in insomniacs (reviewed by2).
Suggestions on the multifaceted neuroprotective effects of huperzine A came from its superior preclinical and clinical benefits as compared with other clinically used AChEIs, and also from large numbers of laboratory studies evaluating the mechanisms of this compound (reviewed by3). This perspective summarizes the evidences of novel insights of huperzine A to reduce AD risk, the molecular mechanisms involved in the anti-AD effects of huperzine A aside from its traditional AChE inhibition, and the novel delivery system which modified release patterns of this drug.
Multifaceted pharmacological effects of huperzine A: cholinergic-dependent and -independent mechanism
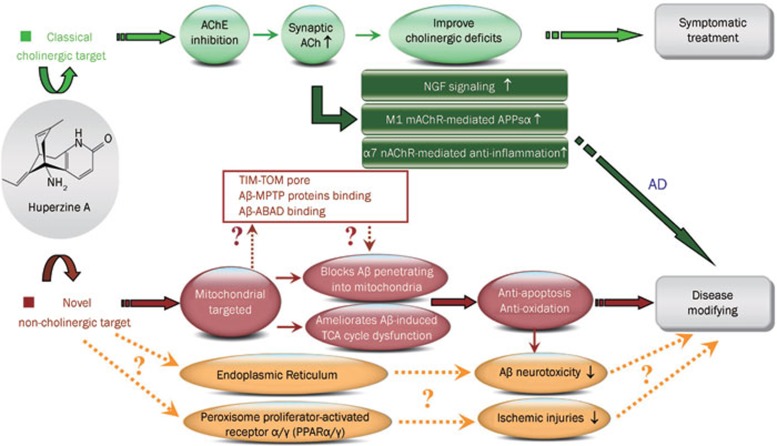
Although we are still at the stage of symptomatic treatments for AD since AChEIs remains the most widely used drugs, we are moving into an age in which disease-modifying agents, particularly, drugs with potent neuroprotective effect are considered to play a significant role in delaying AD development. Interestingly, recent studies reveal that huperzine A, might be of disease-modifying properties. The classical cholinergic effect and novel potential non-cholinergic actions of huperzine A are discussed as following paragraphs and summarized as Figure 1.
Figure 1.
Summary of classical cholinergic and potential non-cholinergic pharmacological targets of huperzine A.
Multiple lines of evidences proved that huperzine A is a mixed-competitive and reversible AChE inhibitor, which shows higher potency and selectivity of AChE inhibition both in vitro and in vivo as compared with galanthamine, donepezil, tacrine, and rivastigmine (reviewed by2). The potent inhibitory effect on AChE could in turn markedly enhance the synaptic ACh release and consequently cholinergic neurotransmission. Beside aforementioned classical effects, more and more beneficial characters of huperzine A are continuously discovered by employing various AD models, mainly from two aspects: expanded effects of cholinergic system in neuroprotection, and novel pharmacological target independent of its AChE inhibitory effect.
Cholinergic system is well established as an important part of the neuronal circuitry that modulates cognition, while, muscarinic and nicotinic ACh receptor antagonists are well known to produce or exacerbate cognitive impairments, respectively4,5,6,7. Although ACh is generally considered to be a neurotransmitter, it can also function as a cytokine and might participate in various neuroprotective pathways: close association was found between ACh and the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor in the rat hippocampus8; activating M1 muscarinic ACh receptor could activate the non-amyloidogenic APP pathway9,10; α7 nicotinic ACh receptor is increasing believed to be a critical link between inflammation and neurodegeneration in AD11. In line with this observation, huperzine A administration was found to enhance the expression and secretion of NGF, as well as increase p75NTR mRNA in primary astrocytes12, enhance the non-amyloidogenic pathway by increasing the levels of sAPPα13 possibly associated with M1 muscarinic ACh receptor mediated pathway14,15,16, and reduce the hypoxia ischemia- triggered inflammatory response through α7 nicotinic ACh receptor17,18,19. Since previous study has proven that huperzine A had no direct effect on the amplitude or kinetics of nAChRs activation20, above cholinergic system-associated beneficial effects of huperzine A administration may mainly act through the enhancement of synaptic ACh level. These neuroprotective effects are probably potential common performance of cholinergic activation, since similar results are found from other AChEIs21,22,23.
As shown in Figure 1, effect of huperzine A on the cholinergic system may simultaneously contribute to symptomatic and disease modifying efficacy in AD. Meanwhile, huperzine A was recently found to exhibit additional benefits that appear to be independent of AChE inhibition, and differentiate the drug from other AChEIs. It is well known that mitochondria are the powerhouse of the cell which participates in a number of physiological functions24, and the mitochondrial dysfunction is considered as one of the key intracellular lesions associated with the pathogenesis of AD25. We recently discovered that huperzine A was able to effectively ameliorate brain mitochondrial malfunction under Aβ26,27,28 or ischemia insult29. We further elucidated that the ameliorative effects of huperzine A on Aβ-induced mitochondrial dysfunction are associated with the reduced production in reactive oxygen species (ROS) and the increases in the activities of some key components of the respiratory chain and key enzymes in tricarboxylic acid (TCA) cycle26,27,28. These findings clearly implicate that mitochondrion appears to be an important target of huperzine A, which is further supported by the findings that huperzine A inhibited the penetration of Aβ into mitochondria and ameliorated Aβ-induced TCA cycle dysfunction in isolated brain cortical mitochondria28. The mitochondria-targeted effects of huperzine A are clearly independent of cholinergic system since there is no evidence indicated the existence of cholinergic system in isolated brain mitochondria. As mitochondria participate in a number of physiological functions that include calcium homeostasis, signal transduction, oxidative stress and apoptosis. This potential independent pharmacological target of huperzine A on mitochondria may further interpret the anti-oxidative stress30,31,32,33,34,35,36 and anti-apoptotic effects30,31,37,38,39 shown by huperzine A administration in various in vivo and in vitro models (Figure 1). Interestingly, several lines of evidence suggest that Aβ directly interacts with several proteins on and inside mitochondria40: Aβ could import into mitochondria through a pore formed by the outer membrane (TOM40) and the inner membrane (TIM22)41; Aβ could also interact with mitochondrial proteins from the membrane permeability transition pore (MPTP)42, which may in turn affect the mitochondrial membrane potential; Aβ was also reported to bind with β-amyloid binding alcohol dehydrogenase (ABAD) upon entering into mitochondria and leading to mitochondrial dysfunction43,44. Although the precise molecular target of huperzine A on Aβ-induced mitochondrial dysfunction remains to be clarified, whether and how huperzine A affects above mentioned Aβ-mitochondrion interactions could be a very promising future project.
Emerging evidence implies that a ligand-activated nuclear transcription factor, peroxisome proliferator-activated receptor (PPAR), could be a potential pharmacological target against inflammation and brain damage after ischemic injury45,46,47, and potent dual PPARα/γ agonist exerted anti-inflammatory and neuroprotective effects48. Moreover, similar as mitochondrion, endoplasmic reticulum (ER) is a multifunctional organelle that plays a central role in various malignant events in AD (reviewed by49). Considerable studies have proved that huperzine A could effectively ameliorate ischemic injuries in various in vivo and in vitro models15,17,18,19,29, and our preliminary data also showed that huperzine A could affect Aβ-associated abnormal ER function (unpublished data). In order to further understand the above beneficial effects, it is also worthy to explore whether huperzine A could target on PPAR or ER.
Controlled delivery of huperzine A as a better drug administrtion strategy
Based on multifaceted beneficial profiles and well confirmed memory improvement effects, huperzine A has been proved to be one of the most promising agents for palliative therapy of cognitive deficits in patients with AD. The recommended dose of huperzine A for the clinical practice in China is 150–250 μg b.i.d, and the most-recently published phase II clinical trial conducted in the United States showed that mild to moderate AD patients treated with huperzine A (400 μg b.i.d.) manifested significant improvement in cognitive function as compared to placebo50. Meanwhile, it is also important to assess the side effects and tolerability of huperzine A therapy at the clinically used doses. As a specific AChE inhibitor, it will be easy to predict that adverse effects of huperzine A should relate to the well-known cholinergic activity. In fact, two US Phase I studies have shown that, although rated as mild scale, adverse symptoms included tachycardia, low energy levels, and hypertension at multiple dose ranges; bradycardia, headache, and intense dreams at a dose of 400 μg b.i.d.; muscle cramps at 400 μg b.i.d.; arthralgia at 300–400 μg b.i.d.
Taken together, the efficacy and safety data from clinical trails of huperzine A treatment indicated that larger doses of huperzine A were needed for better clinical effects in AD patients, however, the increasing of dosage may also increase the possibility of emergency of more severe side effects51. It will be therefore very interesting to unravel the rational behind above phenomenon and search for proper solution. The pharmacokinetic data indicated that huperzine A had a rapid and nearly complete oral absorption and was extensively distributed into tissues after drug administration in dogs52. The concentration of huperzine A in plasma quickly reached its peak at 1.25 h with a steep ratio after oral administration, the oral bioavailability is about 94%52, and approximately 20% of the huperzine A level in plasma reached the cerebrospinal fluid after both intranasal and intravenous administration53. In combination of clinical, pharmacological and pharmacokinetic data, it is suggested that the increased side effect caused by high dosage of huperzine A may attribute to the promptly reached peak plasma drug level after oral administration, while the better efficacy shown by high dosage of huperzine A may due to higher level and longer duration of huperzine A in the plasma at this situation, besides, the high blood-brain-barrier penetration rate allows more huperzine A entering into the brain and maintain at a higher level, which could also enhance the efficacy of huperzine A.
At present, huperzine A is available in the market mainly as tablet or capsule, which has to be given orally 2–3 times per day. The pharmacokinetic parameters of huperzine A determined that it is difficult to optimize both efficacy and safety of the drug with the current two formulations, and it is not convenient for AD patient who suffers memory disorder not to miss scheduled self-medication. Therefore, developing new sustained released drug formulations with long-term efficacy may solve afore-mentioned problems. Currently, several new controlled released formulations are developed, most of them are performed on huperzine A loaded poly (lactic-co-glycolic acid) (PLGA) microspheres using an oil/water emulsion solvent evaporation technique54,55,56, and the period of the sustained release could range from one day to six weeks. While which delivery system exerts best efficacy still remain to be explored, it is reported that ACh has been implicated in cortical synaptic plasticity and memory processes, and suggested the existence of a circadian rhythm in central cholinergic transmission, which modulates memory processes, with high ACh levels during wakefulness and reduced levels during slow-wave sleep57. Therefore, huperzine A sustained release formulation with long lasting over than 12 h may not be a good choice, based on the evidence that low levels of ACh during slow-wave sleep were critical for the consolidation of declarative memory58. Developing novel delivery system with stable and proper plasma concentrations during active cycle (light cycle for human) could be a promising strategy to create better clinical efficacy in AD therapy.
Conclusions and future prospects
AD is a multi-causal progressive neurodegenerative disease with complicated pathogenesis, the major current obstacle of this mysterious syndrome is the lack of effective therapeutic strategy. However, based on the fact that a big gap exists between bench research and clinical application, which is termed as “valley of death”59, large increases in spending on medical research have not produced corresponding increases in new effective therapeutic strategy. Recently, the concept of “translational medicine” is increasingly used to bridge benchside to bedside, in order to broaden and deepen the understanding of the real fact of AD pathogenesis, and also to shorten the period of drug development. One innegligible aspect is to further understand the beneficial profiles and weakness of an existed effective drug when used in clinical practice, trying to unravel the molecular mechanisms and optimize the medicine performance. This article reflects the research progress made in molecular pharmacology and pharmacological therapy for treating AD with huperzine A, showing that the classical cholinergic and potential non-cholinergic target of huperzine A may shed more light on the successes gained from huperzine A in the AD therapy. Accumulative evidence suggests that single target drug always exert limited clinical effects for AD therapy, while combined therapies or drugs with multi-pharmacological activities would be a promising future therapeutic approach to address the varied pathological aspects of the disease. The multiple neuroprotective effects of huperzine A together with the optimized drug delivery system will give this drug new opportunity to prove its merit, however, further studies still need to be performed to validate its efficacy, especially solid evidence from the bedside.
Acknowledgments
This article was supported by the Ministry of Science and Technology of China (No 2011CB510004); the National Natural Science Foundation of China (No 81173034 and 81072646); National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” of China (2012ZX09301001001); the SKLDR/SIMM Projects (No SIMM1105KF-04); Shanghai Science and Technology Development Funds (No 10QA1408100), SA-SIBS Scholarship Program. The author want to thank Ruan ZHI for his effort on graph preparation.
References
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–22. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin. 2006;27:1–26. doi: 10.1111/j.1745-7254.2006.00255.x. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Tang XC. Neuroprotective effects of huperzine A: new therapeutic targets for neurodegenerative disease. Trends Pharmacol Sci. 2006;27:619–25. doi: 10.1016/j.tips.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Domer FR, Schueler FW. Investigations of the amnesic properties of scopolamine and related compounds. Arch Int Pharmacodyn Ther. 1960;127:449–58. [PubMed] [Google Scholar]
- Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–21. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Tariot PN, Weingartner H, Murphy DL, Newhouse PA, Mueller EA, et al. Pharmacologic modelling of Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:599–610. doi: 10.1016/0278-5846(86)90030-8. [DOI] [PubMed] [Google Scholar]
- Knipper M, da Penha Berzaghi M, Blochl A, Breer H, Thoenen H, Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994;6:668–71. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Fisher A, Michaelson DM, Brandeis R, Haring R, Chapman S, Pittel Z. M1 muscarinic agonists as potential disease-modifying agents in Alzheimer's disease. Rationale and perspectives. Ann N Y Acad Sci. 2000;920:315–20. doi: 10.1111/j.1749-6632.2000.tb06941.x. [DOI] [PubMed] [Google Scholar]
- Rossner S, Ueberham U, Schliebs R, Perez-Polo JR, Bigl V. The regulation of amyloid precursor protein metabolism by cholinergic mechanisms and neurotrophin receptor signaling. Prog Neurobiol. 1998;56:541–69. doi: 10.1016/s0301-0082(98)00044-6. [DOI] [PubMed] [Google Scholar]
- Conejero-Goldberg C, Davies P, Ulloa L. Alpha7 nicotinic acetylcholine receptor: a link between inflammation and neurodegeneration. Neurosci Biobehav Rev. 2008;32:693–706. doi: 10.1016/j.neubiorev.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LL, Wang R, Tang XC. Effects of huperzine A on secretion of nerve growth factor in cultured rat cortical astrocytes and neurite outgrowth in rat PC12 cells. Acta Pharmacol Sin. 2005;26:673–8. doi: 10.1111/j.1745-7254.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Yan H, Tang XC. Huperzine A enhances the level of secretory amyloid precursor protein and protein kinase C-alpha in intracerebroventricular beta-amyloid-(1–40) infused rats and human embryonic kidney 293 Swedish mutant cells. Neurosci Lett. 2004;360:21–4. doi: 10.1016/j.neulet.2004.01.055. [DOI] [PubMed] [Google Scholar]
- Tang LL, Wang R, Tang XC. Huperzine A protects SHSY5Y neuroblastoma cells against oxidative stress damage via nerve growth factor production. Eur J Pharmacol. 2005;519:9–15. doi: 10.1016/j.ejphar.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Tang LL, Yan H, Wang YJ, Tang XC. Effects of huperzine A on memory deficits and neurotrophic factors production after transient cerebral ischemia and reperfusion in mice. Pharmacol Biochem Behav. 2006;83:603–11. doi: 10.1016/j.pbb.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Yan H, Zhang HY, Tang XC. Involvement of M1-muscarinic acetylcholine receptors, protein kinase C and mitogen-activated protein kinase in the effect of huperzine A on secretory amyloid precursor protein-alpha. Neuroreport. 2007;18:689–92. doi: 10.1097/WNR.0b013e3280c1e28c. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Tang XC. Huperzine A protects C6 rat glioma cells against oxygen-glucose deprivation-induced injury. FEBS Lett. 2007;581:596–602. doi: 10.1016/j.febslet.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Wang J, Zhang HY, Tang XC. Huperzine A exhibits anti-inflammatory and neuroprotective effects in a rat model of transient focal cerebral ischemia. J Neurochem. 2008;106:1594–603. doi: 10.1111/j.1471-4159.2008.05504.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang HY, Tang XC. Huperzine A improves chronic inflammation and cognitive decline in rats with cerebral hypoperfusion. J Neurosci Res. 2010;88:807–15. doi: 10.1002/jnr.22237. [DOI] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol Pharmacol. 2004;66:658–66. doi: 10.1124/mol.104.000042. [DOI] [PubMed] [Google Scholar]
- Shigeta K, Ootaki K, Tatemoto H, Nakanishi T, Inada A, Muto N. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by a Coptidis Rhizoma extract and protoberberine alkaloids. Biosci Biotechnol Biochem. 2002;66:2491–4. doi: 10.1271/bbb.66.2491. [DOI] [PubMed] [Google Scholar]
- Giacobini E, Mori F, Lai CC. The effect of cholinesterase inhibitors on the secretion of APPS from rat brain cortex. Ann N Y Acad Sci. 1996;777:393–8. doi: 10.1111/j.1749-6632.1996.tb34451.x. [DOI] [PubMed] [Google Scholar]
- Mori F, Lai CC, Fusi F, Giacobini E. Cholinesterase inhibitors increase secretion of APPs in rat brain cortex. Neuroreport. 1995;6:633–6. doi: 10.1097/00001756-199503000-00012. [DOI] [PubMed] [Google Scholar]
- Frey TG, Mannella CA. The internal structure of mitochondria. Trends Biochem Sci. 2000;25:319–24. doi: 10.1016/s0968-0004(00)01609-1. [DOI] [PubMed] [Google Scholar]
- Silva DF, Esteves AR, Oliveira CR, Cardoso SM. Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer's disease. Curr Alzheimer Res. 2011;8:563–72. doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- Gao X, Tang XC. Huperzine A attenuates mitochondrial dysfunction in beta-amyloid-treated PC12 cells by reducing oxygen free radicals accumulation and improving mitochondrial energy metabolism. J Neurosci Res. 2006;83:1048–57. doi: 10.1002/jnr.20791. [DOI] [PubMed] [Google Scholar]
- Gao X, Zheng CY, Yang L, Tang XC, Zhang HY. Huperzine A protects isolated rat brain mitochondria against beta-amyloid peptide. Free Radic Biol Med. 2009;46:1454–62. doi: 10.1016/j.freeradbiomed.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Yang L, Ye CY, Huang XT, Tang XC, Zhang HY. Decreased accumulation of subcellular amyloid-beta with improved mitochondrial function mediates the neuroprotective effect of huperzine A. J Alzheimers Dis. 2012;31:131–42. doi: 10.3233/JAD-2012-120274. [DOI] [PubMed] [Google Scholar]
- Zheng CY, Zhang HY, Tang XC. Huperzine A attenuates mitochondrial dysfunction after middle cerebral artery occlusion in rats. J Neurosci Res. 2008;86:2432–40. doi: 10.1002/jnr.21681. [DOI] [PubMed] [Google Scholar]
- Xiao XQ, Zhang HY, Tang XC. Huperzine A attenuates amyloid beta-peptide fragment 25–35-induced apoptosis in rat cortical neurons via inhibiting reactive oxygen species formation and caspase-3 activation. J Neurosci Res. 2002;67:30–6. doi: 10.1002/jnr.10075. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang HY, Tang XC. Huperzine A attenuates cognitive dysfunction and neuronal degeneration caused by beta-amyloid protein-(1–40) in rat. Eur J Pharmacol. 2001;421:149–56. doi: 10.1016/s0014-2999(01)01030-5. [DOI] [PubMed] [Google Scholar]
- Xiao XQ, Wang R, Han YF, Tang XC. Protective effects of huperzine A on beta-amyloid(25–35) induced oxidative injury in rat pheochromocytoma cells. Neurosci Lett. 2000;286:155–8. doi: 10.1016/s0304-3940(00)01088-0. [DOI] [PubMed] [Google Scholar]
- Xiao XQ, Wang R, Tang XC. Huperzine A and tacrine attenuate beta-amyloid peptide-induced oxidative injury. J Neurosci Res. 2000;61:564–9. doi: 10.1002/1097-4547(20000901)61:5<564::AID-JNR11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Xiao XQ, Yang JW, Tang XC. Huperzine A protects rat pheochromocytoma cells against hydrogen peroxide-induced injury. Neurosci Lett. 1999;275:73–6. doi: 10.1016/s0304-3940(99)00695-3. [DOI] [PubMed] [Google Scholar]
- Wang LM, Han YF, Tang XC. Huperzine A improves cognitive deficits caused by chronic cerebral hypoperfusion in rats. Eur J Pharmacol. 2000;398:65–72. doi: 10.1016/s0014-2999(00)00291-0. [DOI] [PubMed] [Google Scholar]
- Shang YZ, Ye JW, Tang XC. Improving effects of huperzine A on abnormal lipid peroxidation and superoxide dismutase in aged rats. Zhongguo Yao Li Xue Bao. 1999;20:824–8. [PubMed] [Google Scholar]
- Zhang HY, Tang XC. Huperzine A attenuates the neurotoxic effect of staurosporine in primary rat cortical neurons. Neurosci Lett. 2003;340:91–4. doi: 10.1016/s0304-3940(03)00023-5. [DOI] [PubMed] [Google Scholar]
- Zhou J, Tang XC. Huperzine A attenuates apoptosis and mitochondria-dependent caspase-3 in rat cortical neurons. FEBS Lett. 2002;526:21–5. doi: 10.1016/s0014-5793(02)03107-1. [DOI] [PubMed] [Google Scholar]
- Wang R, Xiao XQ, Tang XC. Huperzine A attenuates hydrogen peroxide-induced apoptosis by regulating expression of apoptosis-related genes in rat PC12 cells. Neuroreport. 2001;12:2629–34. doi: 10.1097/00001756-200108280-00009. [DOI] [PubMed] [Google Scholar]
- Tillement L, Lecanu L, Papadopoulos V. Alzheimer's disease: effects of beta-amyloid on mitochondria. Mitochondrion. 2011;11:13–21. doi: 10.1016/j.mito.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–50. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Suman S, Chandna S, Das TK. Possible role of amyloid-beta, adenine nucleotide translocase and cyclophilin-D interaction in mitochondrial dysfunction of Alzheimer's disease. Bioinformation. 2009;3:440–5. doi: 10.6026/97320630003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–52. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Ohsawa I, Kasahara T, Ohta S. Cytoprotective role of mitochondrial amyloid beta peptide-binding alcohol dehydrogenase against a cytotoxic aldehyde. Neurobiol Aging. 2009;30:325–9. doi: 10.1016/j.neurobiolaging.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Culman J, Zhao Y, Gohlke P, Herdegen T. PPAR-gamma: therapeutic target for ischemic stroke. Trends Pharmacol Sci. 2007;28:244–9. doi: 10.1016/j.tips.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN. Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol Neurobiol. 2010;41:180–6. doi: 10.1007/s12035-010-8103-y. [DOI] [PubMed] [Google Scholar]
- Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, et al. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J Neurosci. 2009;29:6186–95. doi: 10.1523/JNEUROSCI.5857-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang YS, Tang XC, Zhang HY. T33, a novel peroxisome proliferator-activated receptor gamma/alpha agonist, exerts neuroprotective action via its anti-inflammatory activities. Acta Pharmacol Sin. 2011;32:1100–8. doi: 10.1038/aps.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreiro E, Baldeiras I, Ferreira IL, Costa RO, Rego AC, Pereira CF, et al. Mitochondrial- and endoplasmic reticulum-associated oxidative stress in Alzheimer's disease: from pathogenesis to biomarkers. Int J Cell Biol. 2012;2012:735206. doi: 10.1155/2012/735206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii MS, Walsh S, Little JT, Behan K, Reynolds B, Ward C, et al. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology. 2011;76:1389–94. doi: 10.1212/WNL.0b013e318216eb7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BS, Wang H, Wei ZH, Song YY, Zhang L, Chen HZ. Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis. J Neural Transm. 2009;116:457–65. doi: 10.1007/s00702-009-0189-x. [DOI] [PubMed] [Google Scholar]
- Chu D, Liu W, Li Y, Li P, Gu J, Liu K. Pharmacokinetics of huperzine A in dogs following single intravenous and oral administrations. Planta Med. 2006;72:552–5. doi: 10.1055/s-2006-931566. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chen G. Pharmacokinetic behavior of huperzine A in plasma and cerebrospinal fluid after intranasal administration in rats. Biopharm Drug Dispos. 2009;30:551–5. doi: 10.1002/bdd.686. [DOI] [PubMed] [Google Scholar]
- Fu X, Ping Q, Gao Y. Effects of formulation factors on encapsulation efficiency and release behaviour in vitro of huperzine A-PLGA microspheres. J Microencapsul. 2005;22:705–14. doi: 10.1080/02652040500162196. [DOI] [PubMed] [Google Scholar]
- Gao P, Ding P, Xu H, Yuan Z, Chen D, Wei J, et al. In vitro and in vivo characterization of huperzine a loaded microspheres made from end-group uncapped poly(d,l-lactide acid) and poly(d,l-lactide-co-glycolide acid) Chem Pharm Bull (Tokyo) 2006;54:89–93. doi: 10.1248/cpb.54.89. [DOI] [PubMed] [Google Scholar]
- Liu WH, Song JL, Liu K, Chu DF, Li YX. Preparation and in vitro and in vivo release studies of Huperzine A loaded microspheres for the treatment of Alzheimer's disease. J Control Release. 2005;107:417–27. doi: 10.1016/j.jconrel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Bentue-Ferrer D, Bordet R, Tsolaki M, Forstl H. Importance of circadian rhythmicity in the cholinergic treatment of Alzheimer's disease: focus on galantamine*. Curr Med Res Opin. 2008;24:3357–67. doi: 10.1185/03007990802522397. [DOI] [PubMed] [Google Scholar]
- Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci U S A. 2004;101:2140–4. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SF, Fischhoff MA, Sakowski SA, Feldman EL. Perspective: Transforming science into medicine: how clinician-scientists can build bridges across research's “valley of death”. Acad Med. 2012;87:266–70. doi: 10.1097/ACM.0b013e3182446fa3. [DOI] [PubMed] [Google Scholar]