Abstract
Celiac disease is a multisystemic dietary, gluten-induced autoimmune disorder characterized by the presence of transglutaminase (TG) 2 serum autoantibodies. Distinct autoantibodies targeting members of the TG family (TG2, TG3 and TG6) are found deposited in small-bowel mucosa and in extraintestinal tissues affected by the disease. Serum autoantibodies against other self-antigens also emerge in untreated celiac disease patients. Although villous atrophy and crypt hyperplasia in small-bowel biopsy samples are still the gold standards in diagnostics, celiac disease-specific antibodies are widely used as diagnostic aids. Gluten-induced small-bowel mucosal T-cell response is the cornerstone in the pathogenesis of the disorder, but humoral immunity may also play a central role. This review article is focused on the autoantibodies that occur in the context of celiac disease. The article summarizes the diagnostic utility of different celiac-related antibodies and discusses their roles in the pathogenesis of the disease.
Keywords: antibody, celiac disease, diagnostics, pathogenesis
An overview of celiac disease: the players in the game
Celiac disease is an autoimmune disorder that occurs in genetically susceptible individuals in response to dietary gluten in wheat, rye and barley. In clinical and pathological terms, our understanding of the disease has considerably improved in recent decades. Traditionally, celiac disease has been considered a fairly uncommon gastrointestinal disorder affecting mainly children, but according to current knowledge, the disease has evolved to become a common systemic condition affecting individuals of all age groups. In many Western countries, the disease affects approximately 1% of the population, but it has recently been shown that the true prevalence of celiac disease is increasing over time.1 The prevalence also increases by age within a country; for example, in Finland, the prevalence is 1.5% in children,2 2% in adults1and 2.7% in the elderly.3 The symptoms and signs of celiac disease vary from mild to severe, and some with celiac disease can be asymptomatic for years or decades. The classical symptoms include the following: malabsorption, chronic diarrhea, iron deficiency anemia and weight loss. In children, short stature is also a symptom. In addition to gastrointestinal symptoms, the disease has extraintestinal manifestations, such as osteoporosis,4 dermatitis herpetiformis,5 neurological problems,6 liver disorders,7 arthritis8 and obstetric problems.9 Celiac disease is also associated with other autoimmune disorders, such as type one diabetes mellitus and autoimmune thyroid diseases.10
Celiac disease is associated with major histocompatibility complex class II genes and the alleles encoding the human leukocyte antigen molecules (HLA)-DQ2 and HLA-DQ8. Almost all patients with celiac disease carry these HLA alleles.11 However, 30–40% of healthy individuals also carry the DQ2 and DQ8 alleles. The majority of these individuals never develop the disorder. To explain this discrepancy, genome-wide linkage and association studies have been conducted, and a set of chromosomal regions that may harbor gene variants or polymorphisms conferring additional risk for developing celiac disease has been identified.12, 13
Although other environmental factors (‘second hits') in addition to gluten may be involved in triggering celiac disease, the disease goes into remission when gluten is removed from the diet. This suggests that gluten is a major player in the pathogenesis of the disease. Gluten-containing cereal prolamins, such as gliadin in wheat, secalin in rye and hordein in barley, have a high number of repetitive glutamine- and proline-rich sequences, making them highly resistant to proteolytic degradation by human gastric, pancreatic and intestinal brush-border enzymes, even in healthy individuals.14, 15 Such proteolytic resistance results in the persistence of relatively large peptides, which are thought to activate the small-bowel mucosal immune system, thereby leading to the development of celiac disease. Under normal physiological conditions, intestinal epithelium is fairly impermeable to long peptides, such as wheat-derived gliadin peptides. However, in untreated celiac disease, the epithelial barrier function is compromised, and gliadin peptides gain access across the epithelial layer.16 Studies performed with small-bowel biopsy organ cultures and different in vitro cell cultures support the idea that gluten can activate the innate immunity mechanisms. This activation is thought to be mediated by toxic gluten-derived gliadin peptides (the α-gliadin peptide 31–43), which eventually results in intestinal epithelial cell damage.17, 18, 19, 20
However, a different set of gliadin peptides, the so-called immunogenic peptides (peptides within the α-gliadin 33-mer peptide 56–89), activate the adaptive immune response. First, these peptides are post-translationally modified by a ubiquitously expressed multifunctional enzyme, transglutaminase (TG) 2, which catalyzes the deamidation of distinct glutamine amino acids to glutamic acid residues.21, 22 Such deamidation greatly enhances the ability of the peptides to bind to HLA-DQ2, which thereby potentiates celiac patient T-cell stimulation.21, 23 As a result, proinflammatory cytokines are secreted during small-bowel mucosal tissue remodeling and damage, which is characterized by villous atrophy, crypt hyperplasia and inflammation. During this process, B cells start to secrete antibodies against the trigger, gliadin and various self-antigens.24 This review is focused on the importance of gluten-induced disease-pathognomonic antibodies as a diagnostic tool and discusses their roles in celiac disease pathogenesis in the intestinal and extraintestinal environment.
Celiac disease antibodies: different targets, different clinical utility
Serum antibodies
The gold standard in diagnosing celiac disease is the presence of histological changes in small-bowel mucosal biopsies. In other words, villous atrophy, crypt hyperplasia and profound inflammation characterize celiac disease. However, because of the multifaceted nature of the disease, clinicians have long used various serum-based antibody tests in case finding (Table 1) before proceeding to diagnostic upper gastrointestinal endoscopies with multiple small-bowel mucosal biopsies. Among the first serum-based antibody tests applied in celiac disease, diagnostics are the antigliadin antibody (AGA) assays. Currently, these tests are no longer used as diagnostic aids because their sensitivities and specificities are fairly poor.25 In addition, individuals suffering from gastrointestinal conditions other than celiac disease and healthy individuals without celiac-type genetics have been reported to have elevated AGA levels.29
Table 1. Sensitivities and specificities of IgA-class serological tests in untreated celiac disease.
| Antibodies | Sensitivity | Specificity | References |
|---|---|---|---|
| AGA | 75–95% | 80–95% | 25 |
| ARA | 78–97% | 98–100% | 26 and 27 |
| EMA | 80–97% | 97–100% | 25 |
| Anti-TG2 | 85–98% | 95–99% | 25 |
| Anti-DGP | 74–98% | 90–99% | 28 |
Abbreviations: AGA, anti-gliadin antibodies; anti-DGP, deamidated gliadin peptide antibodies; anti-TG2, transglutaminase 2 antibodies; ARA, R1-type reticulin antibodies; EMA, endomysial antibodies.
The problems with the AGA tests were overcome by the advent of the gluten-dependent IgA-class R1-type reticulin (ARA) and endomysial autoantibody (EMA) tests.27, 30, 31, 32, 33 These tests are based on indirect immunofluorescence using rodent (ARA) or primate (EMA) tissues as antigens. In most studies, their sensitivities and specificities are both reported to be above 90% (Table 1), though these tissue-based autoantibody tests are often subjective and laboratory dependent. It has been suggested that symptomatic patients, both children and adults, could be diagnosed based on a positive serum EMA finding.34, 35
In 1997, Dieterich and co-workers identified TG2 as the autoantigen of celiac disease.36 As various TG2-based enzyme-linked immunosorbent assays (ELISA) became available, a new era in celiac disease case finding by serology began.27, 37 Thereafter, it was shown that TG2 was also the specific protein antigen in the ARA and EMA tests,38 indicating that the abovementioned three tests in fact do measure the same autoantibodies. Currently, TG2 ELISA tests are widely used in diagnostic workup of celiac disease.25 However, it is important to bear in mind that the performance of the commercial ELISA TG2-antibody assays may vary depending on the quality of the TG2 antigen and thus, may yield false-positive and false-negative results.25, 39, 40 Therefore, the EMA test appears to hold its place as the gold standard celiac disease-specific antibody test. The superiority of the EMA test in celiac disease diagnostics is also supported by a high concordance between EMA positivity and the presence of the celiac-type HLA-DQ2 or -DQ8, which is not always seen with TG2 ELISA seropositivity.2, 32, 34 Furthermore, the compromised specificity of the TG2 ELISA and the high specificity and sensitivity of the EMA tests suggest that the epitope in the EMA test is somehow specific for celiac disease autoantibodies.
An indication of the constant development of serological tests for celiac disease is the introduction of an ELISA test using deamidated gliadin peptides (DGPs) as antigens. The rationale behind the test is based on the finding that TG2 is known to deamidate gliadin peptides during the pathogenesis of celiac disease.21 It has been shown that selective deamidation specifically increases circulating antibody recognition of gliadin peptides in celiac patients, and such serum DGP antibodies have been proven to be highly accurate indicators of untreated celiac disease (Table 1).
Celiac disease patients with selective IgA deficiency are by definition negative for serum antibodies in the IgA class. Therefore, IgG-based EMA, TG2 and DGP antibody test are esed in such class26, 41, 42. To avoid using total serum IgA measurements, the IgA-class antibody tests have been combined with the IgG-class tests in a single screen assay.43
A common feature for the above listed celiac disease antibodies is that they are gluten-dependent and their levels decrease and become negative within 1 year of being on a strict gluten-free diet. However, it is important to keep in mind that seronegativity in patients consuming gluten does not rule out the possibility of celiac disease.
Antibodies found in the serum of celiac disease patients are not only restricted to the antigen triggering the disease (gliadin, DGP) or the major celiac-specific autoantigen, TG2. Patients have also been reported to have circulating antibodies against other self-antigens, exemplifying a further breakdown of immune tolerance. However, such autoantibodies are generally independent of endomysial staining.24, 38 For instance, celiac patients have gluten-dependent IgA-class autoantibodies against cytoskeletal actin. The presence of these autoantibodies correlates with the severity of the small-bowel villous atrophy.44 Other identified targets for celiac autoantibodies include the following: calreticulin, zonulin and desmin. However, studies on their applicabilities in celiac disease diagnostics are scarce.24
Intestinal autoantibodies
The first evidence of antibody deposits in the intestinal environment was produced in the 1970s by Shiner and Ballard, who reported an increase in extracellular deposits of immunoglobulins, especially IgA, in jejunal mucosa of celiac children after a gluten challenge.45 Immediately thereafter, similar IgA deposits in the basement membrane area were reported in the small-bowel mucosa of untreated celiac patients in conjunction with increases in the number of immunoglobulin-containing cells.46, 47, 48, 49 Immunoelectron microscopic studies revealed heavy deposits of IgA in the basement membrane of surface epithelial cells, in crypt epithelium, around the subepithelial fibroblasts and in the walls of blood vessels in the intestinal mucosa of celiac patients. Such findings differed considerably from those in healthy individuals.46, 47, 48, 49, 50, 51 It was also found that during a gluten-free diet, the antibody deposits disappeared, but when gluten was reintroduced in the diet of celiac disease patients, the antibody deposition rapidly reappeared.49, 52, 53
After the identification of TG2 as the celiac disease autoantigen,36 it was demonstrated that the patient autoantibodies also target the autoantigen TG2 in tissues.54, 55 Furthermore, the IgA deposits in celiac disease patient small-bowel biopsies had the ability to bind external TG2 when added to the tissues.56 Interestingly, it has been shown by phage-display technology that celiac disease autoantibodies are produced locally in the small-bowel mucosa.57
Staining of IgA deposits, which are small-bowel mucosal TG2-targeted autoantibodies, has extensively been used in the diagnostic workups of celiac disease. There are several studies showing that all untreated celiac disease patients, even seronegative ones, characteristically have these deposits in their small-bowel mucosa.55, 56, 58 These deposits appear early on during disease development when the mucosa is still morphologically normal. These deposits appear in the mucosa even before they are detectable in the periphery.55, 59, 60 In IgA-deficient celiac disease patients, these mucosal autoantibody deposits appear in the IgM-class instead.61
Autoantibodies associated with extraintestinal manifestations of celiac disease
Interestingly, in some cases, autoantibodies against distinct self-molecules in addition to TG2 have been reported to coincide with specific clinical manifestations of celiac disease. Even more intriguing is the fact that these autoantigens belong to the same TG protein family as TG2, which is the major celiac disease autoantigen.
Dermatitis herpetiformis is regarded as a dermal manifestation of celiac disease.5 Dermatitis herpetiformis is an autoimmune, blistering, pruritic papulovesicular gluten-dependent skin rash typically located on the elbows, forearms, buttocks, knees and scalp. Moreover, dermatitis herpetiformis and celiac disease have identical genetic backgrounds that include the presence of HLA-DQ2 or HLA-DQ8.62 The diagnosis of dermatitis herpetiformis is established by the demonstration of granular IgA deposits in the dermal papillae of uninvolved skin. In 2002, Sardy and colleagues identified the epidermal TG, TG3, as the autoantigen of dermatitis herpetiformis.63 Moreover, serum TG3-targeted autoantibodies are found in patients with dermatitis herpetiformis, and they seem to be gluten dependent.64, 65
Celiac disease may also present with neurological complications, such as dementia, brain atrophy, cerebellar ataxia, peripheral neuropathy and epilepsy with occipital calcifications.6 Gluten ataxia is a sporadic cerebellar ataxia associated with the presence of AGA without other apparent etiologies for the ataxia. It can manifest with or without enteropathy.6 It has been shown that gluten ataxia patients have celiac-specific TG2-targeted IgA deposits in the duodenal mucosa as well as in brain blood vessels.66 Interestingly, it was recently shown that serum autoantibodies in gluten ataxia patients also recognize a novel neuronal TG, TG6.67 However, contradictory results exist as to its suitability in clinical practice.67, 68
the role of Celiac disease antibodies in the pathogenesis
Antibodies in the intestinal environment
Although the applicability of celiac disease-specific antibodies in the diagnostics of the disease is well established, the question remains as to how the antibodies are linked with the pathogenesis of the disease (Figure 1), especially considering that gliadin peptides or TG2, which are the targets of the antibodies, have important roles in disease progression. Table 2 lists known biological effects of celiac-type antibodies.
Figure 1.
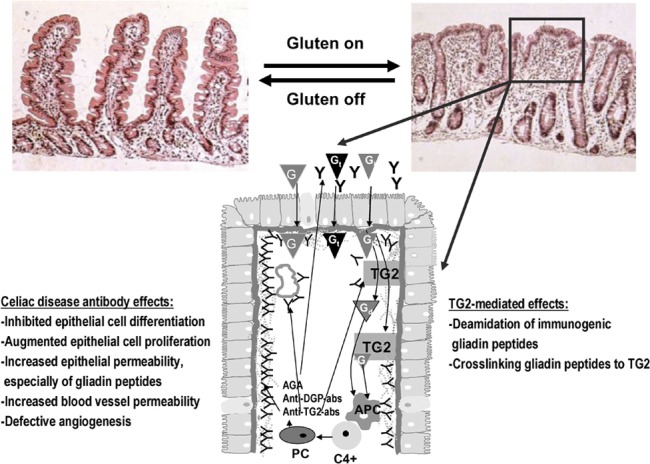
The pathogenesis of celiac disease. In celiac disease patients, the gluten-induced small-bowel mucosal deterioration occurs gradually from normal morphology (shown in the left) to villous atrophy with crypt hyperplasia and inflammation (shown in the right). The schematic presentation (below) shows what happens in the small-bowel mucosa at the cellular level in untreated celiac patients. Gt evoke an innate immunity response, which eventually lead to epithelial cell death and increased epithelial permeability. This enables the Gi to enter the lamina propria where TG2 either deamidates or crosslinks the peptides to themselves. Gd or gliadin-TG2 complexes are taken up by APCs. This is followed by DQ2-/DQ8-dependent activation of CD4+ T cells and the subsequent secretion of antibodies by mucosal PCs. TG2 autoantibodies are found to be deposited in the small-intestinal mucosa below the epithelial basement membrane and around capillaries, but antibodies are also present in the lumen (indicated by Y in the figure). In these distinct locations, the antibodies, which are also targeted against other molecules than TG2, could take part in the disease pathogenesis, and their effects are listed in the figure. When the exogenous trigger gluten is removed, the mucosal damage recovers. AGA, anti-gliadin antibody; anti-DGP-abs, deamidated gliadin peptide antibodies; anti-TG2-abs, TG2 autoantibodies; APC, antigen-presenting cell; G, gliadin; Gd, deamidated gliadin peptide; Gi, immunogenic gliadin peptide; Gt, toxic gliadin peptide; PC, plasma cell; TG2, transglutaminase 2.
Table 2. Biological effects of celiac disease autoantibodies.
| Effect describe | References | ||
|---|---|---|---|
| In vitro | |||
| Induction of epithelial proliferation | 69 and 70 | ||
| Inhibition of epithelial differentiation | 69 | ||
| Increase in epithelial permeability | 71–73 | ||
| Interference with gliadin up-take | 74 | ||
| Inhibition of angiogenesis | 75 and 76 | ||
| Increase in vascular permeability | 77 | ||
| Induction of neuronal apoptosis | 78 | ||
| Binding to placental tissue | 79 | ||
| Induction of trophoblast apoptosis | 80 | ||
| In vivo | |||
| Induction of ataxia | 81 | ||
| No effect | 82–84 | ||
The epithelium in the small-bowel mucosa of untreated celiac disease patients is characterized by an increased number of proliferating cells, a decreased number of differentiated cells, augmented cellular turn over and compromised barrier function. Although proinflammatory cytokines and gliadin are known to induce such features in intestinal epithelial cells,85 there are emerging data suggesting that celiac disease antibodies have similar effects in cell culture conditions. To modulate small-bowel mucosal epithelial biology in vivo in the intestines of celiac disease patients, the celiac-type antibodies are located (deposited) in strategically correct places, such as below the epithelial layer. These antibodies then target extracellular TG2 on subepithelial fibroblasts and on the basement membrane.29, 51, 55 Furthermore, the antibodies are translocated by specific mechanisms to the intestinal lumen.29
Serum IgA in patients with untreated celiac disease and patient-derived anti-TG2 autoantibodies produced by recombinant technology have been shown to induce epithelial cell proliferation.69, 70 Celiac patient IgA is also capable of inhibiting intestinal epithelial cell differentiation.69 In addition, several studies suggest that celiac antibodies also modulate epithelial barrier function.71, 72 In accordance with these results, our recent data demonstrate that IgA derived from untreated celiac disease patients specifically increase the transepithelial passage of gliadin peptides in vitro.73 All of the abovementioned cellular effects are also seen in small-bowel mucosal lesion that is characteristic of untreated celiac disease. Thus, celiac antibodies could collectively promote the development of small-intestinal damage. Yet, based on the aforementioned experiments, it is not completely clear which celiac-type antibody populations are responsible for the effects on epithelial cell biology. It is therefore conceivable that the effects could be exerted by antibodies against DGPs and autoantibodies targeted against self-molecules.
In addition to being deposited below the intestinal epithelium, TG2 autoantibodies also target blood vessel TG2 in the intestinal lamina propria.86 The intestinal vascular network plays important roles in intestinal biology. One of these roles is to provide mechanical support to the villi. Duodenal biopsies derived from untreated celiac patients show disorganization in the vascular network as well as a reduction in vessel maturity.86 Interestingly, the target of the disease-specific autoantibodies, TG2, has been reported to modulate angiogenesis.87 In this context, it is tempting to speculate first that the described defects in the mucosal vasculature of celiac disease patients contribute to the disease pathogenesis and also that the blood vessel TG2-targeted autoantibodies take part in the aberrant organization of the mucosal vasculature. In this respect, our group has shown that celiac patients' autoantibodies that specifically target TG2 have the ability to inhibit angiogenesis in vitro75 by increasing endothelial cell TG2 enzyme activity.76 Moreover, our studies have also demonstrated that blood vessel permeability to macromolecules and lymphocytes in vitro is increased in the presence of patient-derived TG2 autoantibodies.77 Therefore, the celiac disease-specific TG2-targeted autoantibodies deposited around mucosal blood vessels in the patients could contribute to the disorganization of the small-bowel vasculature and their increased permeability.
Although there are data available from in vitro studies that antibodies from celiac disease patients could take part in the remodeling of the small-bowel mucosal architecture and development of villous atrophy and crypt hyperplasia, there are unfortunately no studies demonstrating this in vivo. Neither passive transfer of IgG-class gliadin antibodies83 nor adenovirus vector-mediated expression of celiac patient-derived TG2-specific autoantibodies in mice82 have resulted in any kind of intestinal pathology resembling that seen in human celiac disease. Similarly, experiments to immunize mice with the celiac disease autoantigen TG2 failed to induce any morphological changes in the small bowel.84 Taken together, the participation of celiac disease antibodies in the development of the disease-characteristic small-bowel mucosal lesions remains unclear. It is noteworthy, however, that transgenic mice expressing celiac-type HLA with gluten-specific CD4+ T cells from intestinal biopsies of patients neither developed celiac disease-like conditions.88 As there is currently no animal model for celiac disease that presents with all features of the disorder, this issue certainly warrants further studies.
Antibodies in the extraintestinal environment
As we previously described, dietary gluten leads to the production of a wide range of antibodies in celiac patients once B cells are stimulated (Table 1), and sometimes specific autoantibodies correlate with distinct extraintestinal manifestations (e.g., TG3 with dermatitis herpetiformis and TG6 with gluten ataxia).63, 67 However, patients also have circulating autoantibodies against TG2 in the serum and/or TG2-autoantibody deposits in the small-bowel mucosa, as discussed previously.
Even if TG3 has been identified as the autoantigen in dermatitis herpetiformis and patients produce IgA-class anti-TG3 autoantibodies, neither serum TG3 nor TG2 autoantibodies are able to bind to papillary skin structures.63 This would suggest that the circulating antibodies are not directed against dermal targets and that the IgA/TG3 aggregates in the dermis of patients with dermatitis herpetiformis may represent immune complexes. The prevailing hypothesis for the pathogenesis of dermatitis herpetiformis postulates that the dermal IgA originates in the form of immune complexes from the small intestine, binds to the skin and activates the subsequent inflammatory response.63 Although, to our knowledge, there are no in vivo studies directly proving that dermally deposited IgA is the cause of skin lesions; it is of great interest that a proportion of HLA-DQ8 transgenic mice in a non-obese diabetic background develop skin pathology reminiscent of dermatitis herpetiformis with dermal IgA deposits.89
The other extraintestinal manifestation of celiac disease that results in an additional autoantigen to TG2 is gluten ataxia, as already mentioned. Gluten ataxia is characterized by for instance a substantial loss of Purkinje cells.6 Interestingly, it has been reported that in cell culture, patient anti-TG2 autoantibodies induce neuronal cell apoptosis, which is mediated by mitochondria-dependent mechanisms.78 The authors reported that the apoptotic effect was more pronounced in cultures supplemented with antineuronal antibodies than in those supplemented with combined antigliadin and anti-TG2 antibodies. As this finding was published before TG6 was identified as an autoantigen in gluten ataxia, it would be interesting to know whether the anti-TG6 autoantibodies were responsible for the phenomena. It was later reported that only distinct celiac disease patient-derived anti-TG2 autoantibodies recognize neurons, and interestingly, such autoantibodies crossreact with both TG3 and TG6.81 In the elegant study of Boscolo and co-workers, it was shown that intraventricular injection of both the anti-TG2 or the anti-TG2/3/6 crossreactive autoantibodies provoked transient, but equally intensive, ataxia in mice.81 Although the previous study clearly demonstrates the pathogenetic potential of celiac-specific TG2-targeted autoantibodies to induce gluten ataxia, the precise contribution of TG6 autoantibodies still remains to be elucidated.
The various extraintestinal manifestations of celiac disease are not always characterized by specific autoantibodies other than those targeting TG2. TG2-targeted deposits have been reported to exist, for instance, in the liver of untreated celiac disease patients,55 and interestingly, liver problems are reported as one extraintestinal manifestation of celiac disease. To date, there are no studies addressing the effects of disease-specific TG2 autoantibodies on hepatocytes or liver function.
Celiac disease is sometimes associated with poor pregnancy outcomes, including increased incidence of miscarriages and intrauterine growth restriction.9 Although IgA, the characteristic class of celiac disease-specific autoantibodies is not able to pass from the maternal to the fetal site of the placenta, it has been show that celiac patient IgA-class autoantibodies in vitro bind to placental TG2.79 However, the IgG-class TG2 autoantibodies in an undiagnosed pregnant woman do cross the placental barrier and may target the autoantigen, causing ill health to the fetus and newborn. Recently, Di Simone and colleagues showed that IgG-class anti-TG2 antibodies, both commercial and celiac disease patient derived, bind to cultured human primary trophoblasts and cause increased time- and dose-dependent apoptosis of these cells.80 In pregnancy, the umbilical cord and placental vasculature are vitally important as they assure efficient delivery of nutrients and other essential compounds to the growing embryo. Therefore, it can be envisaged that the effects of the celiac-specific autoantibodies on vascular biology75, 76, 77 also contribute to adverse pregnancy outcomes.
Following the same logic, the celiac patient TG2 autoantibodies have been suggested to be involved in the development of other celiac disease-related extraintestinal manifestations. For instance, celiac disease-specific autoantibodies recognize TG2 in bone,90 thyroidal gland91 and heart muscle tissues,92 and furthermore, celiac disease has been associated with osteoporotic fractures and with thyroidal and cardiac autoimmunity and dysfunction.90, 91, 92 Currently, we can only speculate that the disease-specific autoantibodies may have an active role in the pathophysiology of these conditions; although this suggestion is attractive, its validity awaits verification.
Conclusions
A hallmark of celiac disease is the presence of distinct autoantibody populations both in the patient's serum and in different tissues. Some of these autoantibodies (EMA and anti-TG2) are highly disease-specific and are used in clinics for presumptive diagnosis. Today, evidence is emerging in favor of their pathogenic potential, as discussed in the present review. However, it remains to be determined which antibody populations contribute to the disease progression at the small-bowel mucosal and extraintestinal levels and the mechanism involved.
Box 1. Role of B cells in autoimmunity, return to the past.
Over the past few years, concepts in autoimmunity and immunology have changed so that some researchers are starting to think that the role of B cells in the regulation of immunology is different than was previously thought. In fact, some researchers in the areas of rheumatology or endocrinology have concluded that B cells have a central role in autoimmunity. This concept is based on Burnett's forbidden clone hypothesis, which states that an indolent lymphoproliferative disorder of B cells underlines the development of autoimmune disease. This event could be a consequence of a mutation in the regulatory genes of B-cell precursors. Thus, the precursors could have relevance in autoimmunity through their B-cell receptors, which could match self-antigens. Such cells would escape the homeostatic mechanisms and then proliferate, differentiate to plasma cells and produce relevant quantities of antibodies. The antibodies could modulate the physiology of the target tissue either by themselves or together with T cells and other inflammatory cells to produce the final damage. Because B-cell receptors have different specificities, their effects would be different. This intriguing hypothesis is supported by experimental evidence. In many autoimmune diseases, such as rheumatoid arthritis, Sjögren's syndrome, myositis, Grave's disease or even insulin-dependent diabetes mellitus, B cells have been recognized as key players that are essential for disease progression.93 The most convincing data speaking in favor of this hypothesis are those demonstrating B-cell depletion to be an effective from of intervention in many autoimmune conditions.93
Acknowledgments
Celiac Disease Study Group has been financially supported by the Academy of Finland, the Sigrid Juselius Fundation, the Pediatric Research Foundation, the Competitive Research Funding of Tampere University Hospital and the European Commission IAPP frant TRANSCOM (contract number PIA-GA-2010-251506).
References
- Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- Mäki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, et al. Prevalence of celiac disease among children in Finland. N Engl J Med. 2003;348:2517–2524. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- Vilppula A, Kaukinen K, Luostarinen L, Krekelä I, Patrikainen H, Valve R, et al. Increasing prevalence and high incidence of celiac disease in elderly people: a population-based study. BMC Gastroenterol. 2009;9:49. doi: 10.1186/1471-230X-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustalahti K, Collin P, Sievänen H, Salmi J, Mäki M. Osteopenia in patients with clinically silent coeliac disease warrants screening. Lancet. 1999;354:744–745. doi: 10.1016/S0140-6736(99)01990-X. [DOI] [PubMed] [Google Scholar]
- Fry L. Dermatitis herpetiformis. Baillieres Clin Gastroenterol. 1995;9:371–393. doi: 10.1016/0950-3528(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Hadjivassiliou M, Sanders DS, Grunewald RA, Woodroofe N, Boscolo S, Aeschlimann D. Gluten sensitivity: from gut to brain. Lancet Neurol. 2010;9:318–330. doi: 10.1016/S1474-4422(09)70290-X. [DOI] [PubMed] [Google Scholar]
- Rubio-Tapia A, Murray JA. The liver in celiac disease. Hepatology. 2007;46:1650–1658. doi: 10.1002/hep.21949. [DOI] [PubMed] [Google Scholar]
- Chakravarty K, Scott DG. Oligoarthritis—a presenting feature of occult coeliac disease. Br J Rheumatol. 1992;31:349–350. doi: 10.1093/rheumatology/31.5.349. [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterology. 2005;129:454–463. doi: 10.1016/j.gastro.2005.05.065. [DOI] [PubMed] [Google Scholar]
- Collin P, Kaukinen K, Välimäki M, Salmi J. Endocrinological disorders and celiac disease. Endocr Rev. 2002;23:464–483. doi: 10.1210/er.2001-0035. [DOI] [PubMed] [Google Scholar]
- Karell K, Louka AS, Moodie SJ, Ascher H, Clot F, Greco L, et al. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol. 2003;64:469–477. doi: 10.1016/s0198-8859(03)00027-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Juo SH, Holopainen P, Terwilliger J, Tong X, Grunn A, et al. Genomewide linkage analysis of celiac disease in Finnish families. Am J Hum Genet. 2002;70:51–59. doi: 10.1086/338453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois PC, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A, et al. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet. 2010;42:295–302. doi: 10.1038/ng.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol. 2002;283:G996–G1003. doi: 10.1152/ajpgi.00136.2002. [DOI] [PubMed] [Google Scholar]
- Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray GM, et al. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–2279. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- Smecuol E, Bai JC, Vazquez H, Kogan Z, Cabanne A, Niveloni S, et al. Gastrointestinal permeability in celiac disease. Gastroenterology. 1997;112:1129–1136. doi: 10.1016/s0016-5085(97)70123-9. [DOI] [PubMed] [Google Scholar]
- Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Raia V, Auricchio S, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–37. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- Hue S, Mention JJ, Monteiro RC, Zhang S, Cellier C, Schmitz J, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–377. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Cinova J, Palova-Jelinkova L, Smythies LE, Cerna M, Pecharova B, Dvorak M, et al. Gliadin peptides activate blood monocytes from patients with celiac disease. J Clin Immunol. 2007;27:201–209. doi: 10.1007/s10875-006-9061-z. [DOI] [PubMed] [Google Scholar]
- Nikulina M, Habich C, Flohe SB, Scott FW, Kolb H. Wheat gluten causes dendritic cell maturation and chemokine secretion. J Immunol. 2004;173:1925–1933. doi: 10.4049/jimmunol.173.3.1925. [DOI] [PubMed] [Google Scholar]
- Molberg O, McAdam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- Vader LW, de Ru A, van der Wal Y, Kooy YM, Benckhuijsen W, Mearin ML, et al. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J Exp Med. 2002;195:643–649. doi: 10.1084/jem.20012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wal Y, Kooy Y, van Veelen P, Pena S, Mearin L, Papadopoulos G, et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol. 1998;161:1585–1588. [PubMed] [Google Scholar]
- Alaedini A, Green PH. Autoantibodies in celiac disease. Autoimmunity. 2008;41:19–26. doi: 10.1080/08916930701619219. [DOI] [PubMed] [Google Scholar]
- Rostom A, Dubé C, Cranney A, Saloojee N, Sy R, Garritty C, et al. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology. 2005;128:S38–S46. doi: 10.1053/j.gastro.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Mäki M, Hällström O, Vesikari T, Visakorpi JK. Evaluation of a serum IgA-class reticulin antibody test for the detection of childhood celiac disease. J Pediatr. 1984;105:901–905. doi: 10.1016/s0022-3476(84)80074-8. [DOI] [PubMed] [Google Scholar]
- Sulkanen S, Halttunen T, Laurila K, Kolho KL, Korponay-Szabo IR, Sarnesto A, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998;115:1322–1328. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- Lewis NR, Scott BB. Meta-analysis: deamidated gliadin peptide antibody and tissue transglutaminase antibody compared as screening tests for coeliac disease. Aliment Pharmacol Ther. 2010;31:73–81. doi: 10.1111/j.1365-2036.2009.04110.x. [DOI] [PubMed] [Google Scholar]
- Mäki M.The humoral immune system in coeliac diseaseIn: Howdle PD (ed.) Coeliac disease. Baillière's Clinical Gastroenterology 19959231–249. [DOI] [PubMed] [Google Scholar]
- Seah PP, Fry L, Hoffbrand AV, Holborow EJ. Tissue antibodies in dermatitis herpetiformis and adult coeliac disease. Lancet. 1971;1:834–836. doi: 10.1016/s0140-6736(71)91499-1. [DOI] [PubMed] [Google Scholar]
- Chorzelski TP, Sulej J, Tchorzewska H, Jablonska S, Beutner EH, Kumar V. IgA class endomysium antibodies in dermatitis herpetiformis and coeliac disease. Ann NY Acad Sci. 1983;420:325–334. doi: 10.1111/j.1749-6632.1983.tb22220.x. [DOI] [PubMed] [Google Scholar]
- Mäki M, Holm K, Lipsanen V, Hällström O, Viander M, Collin P, et al. Serological markers and HLA genes among healthy first-degree relatives of patients with coeliac disease. Lancet. 1991;338:1350–1353. doi: 10.1016/0140-6736(91)92234-s. [DOI] [PubMed] [Google Scholar]
- Volta U, Molinaro N, de Franceschi L, Fratangelo D, Bianch FB. IgA antiendomysial antibody test. A step forward in celiac disease screening. Dig Dis Sci. 1991;36:752–756. doi: 10.1007/BF01311232. [DOI] [PubMed] [Google Scholar]
- Kurppa K, Collin P, Viljamaa M, Haimila K, Saavalainen P, Partanen J, et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136:816–823. doi: 10.1053/j.gastro.2008.11.040. [DOI] [PubMed] [Google Scholar]
- Kurppa K, Ashorn M, Iltanen S, Koskinen LL, Saavalainen P, Koskinen O, et al. Celiac disease without villous atrophy in children: a prospective study. J Pediatr. 2010;157:373–380. doi: 10.1016/j.jpeds.2010.02.070. [DOI] [PubMed] [Google Scholar]
- Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, et al. Identification of tissue transglutaminase as the autoantigen of coeliac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- Dieterich W, Laag E, Schopper H, Volta U, Ferguson A, Gillett H, et al. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317–1321. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- Korponay-Szabo IR, Laurila K, Szondy Z, Halttunen T, Szalai Z, Dahlbom I, et al. Missing endomysial and reticulin binding of coeliac antibodies in transglutaminase 2 knockout tissues. Gut. 2003;52:199–204. doi: 10.1136/gut.52.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock RJ, Stevens S, Pitcher MC, Unsworth DJ. Is immunoglobulin A anti-tissue transglutaminase antibody a reliable serological marker of coeliac disease. Eur J Gastgroenterol Hepatol. 2004;16:467–470. doi: 10.1097/00042737-200405000-00005. [DOI] [PubMed] [Google Scholar]
- Hopper AD, Cross SS, Hurlstone DP, McAlindon ME, Lobo AJ, Hadjivassiliou M, et al. Pre-endoscopy serological testing for coeliac disease: evaluation of a clinical decision tool. BMJ. 2007;334:729. doi: 10.1136/bmj.39133.668681.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay-Szabo IR, Halttunen T, Szalai Z, Laurila K, Kiraly R, Kovacs JB, et al. Evaluation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease. Gut. 2003;52:1567–1571. doi: 10.1136/gut.52.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin P, Mäki M, Keyriläinen O, Hällström O, Reunala T, Pasternack A. Selective IgA deficiency and coeliac disease. Scand J Gastroenterol. 1992;27:367–371. doi: 10.3109/00365529209000089. [DOI] [PubMed] [Google Scholar]
- Kurppa K, Lindfors K, Collin P, Saavalainen P, Partanen J, Haimila K, et al. Antibodies against deamidated gliadin peptides in early-stage celiac disease J Clin Gastroenterolin press. Doi:10.1097/MCG.Ob013e3181fbdfa6. [DOI] [PubMed]
- Clemente MG, Musu MP, Frau F, Brusco G, Sole G, Corazza GR, et al. Immune reaction against cytoskeleton in coeliac disease. Gut. 2000;47:520–526. doi: 10.1136/gut.47.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiner M, Ballard J. Antigen-antibody reactions in jejunal mucosa in childhood coeliac disease after gluten challenge. Lancet. 1972;1:1202–1205. doi: 10.1016/s0140-6736(72)90924-5. [DOI] [PubMed] [Google Scholar]
- Savilahti E. Intestinal immunoglobulins in children with coeliac disease. Gut. 1972;13:958–964. doi: 10.1136/gut.13.12.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jos J, Labbe F. Ultrastructural localization of IgA globulins in normal and coeliac intestinal mucosa using immunoenzymatic methods. Biomedicine. 1976;24:425–434. [PubMed] [Google Scholar]
- Lancaster-Smith M, Packer S, Kumar PJ, Harries JT. Immunological phenomena in the jejunum and serum after reintroduction of dietary gluten in children with treated coeliac disease. J Clin Pathol. 1976;29:592–597. doi: 10.1136/jcp.29.7.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jos J, Labbe F, Geny B, Griscelli C. Immunoelectron-microscopic localization of immunoglobulin A and secretory component in jejunal mucosa from children with coeliac disease. Scand J Immunol. 1979;9:441–450. doi: 10.1111/j.1365-3083.1979.tb03066.x. [DOI] [PubMed] [Google Scholar]
- Lancaster-Smith M, Joyce S, Kumar P. Immunoglobulins in the jejunal mucosa in adult coeliac disease and dermatitis herpetiformis after the reintroduction of dietary gluten. Gut. 1977;18:887–891. doi: 10.1136/gut.18.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala I, Mäki M, Laasonen A, Visakorpi JK. Periodate-lysine-paraphormaldehyde as a fixative for the study of duodenal mucosa. Morphologic and immunohistochemical results at light and electron microscopic levels. Acta Pathol Microbiol Scand A. 1985;93:165–173. doi: 10.1111/j.1699-0463.1985.tb03936.x. [DOI] [PubMed] [Google Scholar]
- Karpati S, Kosnai I, Török E, Kovacs JB. Immunoglobulin A deposition in jejunal mucosa of children with dermatitis herpetiformis. J Invest Dermatol. 1988;91:336–339. doi: 10.1111/1523-1747.ep12475672. [DOI] [PubMed] [Google Scholar]
- Koskinen O, Villanen M, Korponay-Szabo I, Lindfors K, Mäki M, Kaukinen K. Oats do not induce systemic or mucosal autoantibody response in children with coeliac disease. J Pediatr Gastroenterol Nutr. 2009;8:559–565. doi: 10.1097/MPG.0b013e3181668635. [DOI] [PubMed] [Google Scholar]
- Korponay-Szabo IR, Sulkanen S, Halttunen T, Maurano F, Rossi M, Mazzarella G, et al. Tissue transglutaminase is the target in both rodent and primate tissues for celiac disease-specific autoantibodies. J Pediatr Gastroenterol Nutr. 2000;31:520–527. doi: 10.1097/00005176-200011000-00013. [DOI] [PubMed] [Google Scholar]
- Korponay-Szabo IR, Halttunen T, Szalai Z, Laurila K, Kiraly R, Kovacs JB, et al. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut. 2004;53:641–648. doi: 10.1136/gut.2003.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi TT, Collin P, Korponay-Szabo IR, Laurila K, Partanen J, Huhtala H, et al. Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut. 2006;55:1746–1753. doi: 10.1136/gut.2005.071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, et al. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol. 2001;166:4170–4176. doi: 10.4049/jimmunol.166.6.4170. [DOI] [PubMed] [Google Scholar]
- Koskinen O, Collin P, Lindfors K, Laurila K, Mäki M, Kaukinen K. Usefulness of small-bowel mucosal transglutaminase-2 specific autoantibody deposits in the diagnosis and follow-up of celiac disease. J Clin Gastroenterol. 2010;44:483–488. doi: 10.1097/MCG.0b013e3181b64557. [DOI] [PubMed] [Google Scholar]
- Koskinen O, Collin P, Korponay-Szabo I, Salmi T, Iltanen S, Haimila K, et al. Gluten-dependent small bowel mucosal transglutaminase 2-specific IgA deposits in overt and mild enteropathy coeliac disease. J Pediatr Gastroenterol Nutr. 2008;47:436–442. doi: 10.1097/MPG.0b013e31817b6dec. [DOI] [PubMed] [Google Scholar]
- Kaukinen K, Peräaho M, Collin P, Partanen J, Woolley N, Kaartinen T, et al. Small-bowel mucosal transglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized clinical study. Scand J Gastroenterol. 2005;40:564–572. doi: 10.1080/00365520510023422. [DOI] [PubMed] [Google Scholar]
- Borrelli M, Maglio M, Agnese M, Paparo F, Gentile S, Colicchio B, et al. High density of intraepithelial gammadelta lymphocytes and deposits of immunoglobulin (Ig)M anti-tissue transglutaminase antibodies in the jejunum of coeliac patients with IgA deficiency. Clin Exp Immunol. 2010;160:199–206. doi: 10.1111/j.1365-2249.2009.04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurkland A, Ingvarsson G, Falk ES, Knutsen I, Sollid LM, Thorsby E. Dermatitis herpetiformis and celiac disease are both primarily associated with the HLA-DQ (alpha 1*0501, beta 1*02) or the HLA-DQ (alpha 1*03, beta 1*0302) heterodimers. Tissue Antigens. 1997;49:29–34. doi: 10.1111/j.1399-0039.1997.tb02706.x. [DOI] [PubMed] [Google Scholar]
- Sardy M, Karpati S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002;195:747–757. doi: 10.1084/jem.20011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CM, Liddle M, Hansen N, Meyer LJ, Schmidt L, Taylor T, et al. Elevation of IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis. Br J Dermatol. 2008;159:120–124. doi: 10.1111/j.1365-2133.2008.08629.x. [DOI] [PubMed] [Google Scholar]
- Rose C, Armbruster FP, Ruppert J, Igl BW, Zillikens D, Shimanovich I. Autoantibodies against epidermal transglutaminase are a sensitive diagnostic marker in patients with dermatitis herpetiformis on a normal or gluten-free diet. J Am Acad Dermatol. 2009;61:39–43. doi: 10.1016/j.jaad.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Hadjivassiliou M, Mäki M, Sanders DS, Williamson CA, Grunewald RA, Woodroofe NM, et al. Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Neurology. 2006;66:373–377. doi: 10.1212/01.wnl.0000196480.55601.3a. [DOI] [PubMed] [Google Scholar]
- Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D. Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann Neurol. 2008;64:332–343. doi: 10.1002/ana.21450. [DOI] [PubMed] [Google Scholar]
- Lindfors K, Koskinen O, Laurila K, Collin P, Saavalainen P, Haimila K, et al. IgA-class autoantibodies against neuronal transglutaminase, TG6 in celiac disease: no evidence for gluten dependency. Clin Chim Acta. 2010;9:744–749. doi: 10.1016/j.cca.2010.09.042. [DOI] [PubMed] [Google Scholar]
- Halttunen T, Mäki M. Serum immunoglobulin A from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology. 1999;116:566–572. doi: 10.1016/s0016-5085(99)70178-2. [DOI] [PubMed] [Google Scholar]
- Barone MV, Caputo I, Ribecco MT, Maglio M, Marzari R, Sblattero D, et al. Humoral immune response to tissue transglutaminase is related to epithelial cell proliferation in celiac disease. Gastroenterology. 2007;132:1245–1253. doi: 10.1053/j.gastro.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Zanoni G, Navone R, Lunardi C, Tridente G, Bason C, Sivori S, et al. In celiac disease, a subset of autoantibodies against transglutaminase binds Toll-like receptor 4 and induces activation of monocytes. PLoS Med. 2006;3:e358. doi: 10.1371/journal.pmed.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Menard S, Candalh C, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205:143–154. doi: 10.1084/jem.20071204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhavirta T, Kaukinen K, Mäki M, Lindfors K. Coeliac disease-specific IgA enhances the transepithelial passage of gliadin peptides. Clin Exp Immunil. In press.
- Caputo I, Barone MV, Lepretti M, Martucciello S, Nista I, Troncone R, et al. Celiac anti-tissue transglutaminase antibodies interfere with the uptake of alpha gliadin peptide 31–43 but not of peptide 57–68 by epithelial cells. Biochim Biophys Acta. 2010;1802:717–727. doi: 10.1016/j.bbadis.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Myrsky E, Kaukinen K, Syrjänen M, Korponay-Szabo IR, Mäki M, Lindfors K. Coeliac disease-specific autoantibodies targeted against transglutaminase 2 disturb angiogenesis. Clin Exp Immunol. 2008;152:111–119. doi: 10.1111/j.1365-2249.2008.03600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caja S, Myrsky E, Korponay-Szabo IR, Nadalutti C, Sulic AM, Lavric M, et al. Inhibition of transglutaminase 2 enzymatic activity ameliorates the anti-angiogenic effects of coeliac disease autoantibodies. Scand J Gastroenterol. 2010;45:421–427. doi: 10.3109/00365520903540822. [DOI] [PubMed] [Google Scholar]
- Myrsky E, Caja S, Simon-Vecsei Z, Korponay-Szabo IR, Nadalutti C, Collighan R, et al. Celiac disease IgA modulates vascular permeability in vitro through the activity of transglutaminase 2 and RhoA. Cell Mol Life Sci. 2009;66:3375–3385. doi: 10.1007/s00018-009-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervio E, Volta U, Verri M, Boschi F, Pastoris O, Granito A, et al. Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. . Gastroenterology. 2007;133:195–206. doi: 10.1053/j.gastro.2007.04.070. [DOI] [PubMed] [Google Scholar]
- Anjum N, Baker PN, Robinson NJ, Aplin JD. Maternal celiac disease autoantibodies bind directly to syncytiotrophoblast and inhibit placental tissue transglutaminase activity. Reprod Biol Endocrinol. 2009;7:16. doi: 10.1186/1477-7827-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simone N, Silano M, Castellani R, Di Nicuolo F, D'Alessio MC, Franceschi F, et al. Anti-tissue transglutaminase antibodies from celiac patients are responsible for trophoblast damage via apoptosis in vitro. . Am J Gastroenterol. 2010;105:2254–2261. doi: 10.1038/ajg.2010.233. [DOI] [PubMed] [Google Scholar]
- Boscolo S, Lorenzon A, Sblattero D, Florian F, Stebel M, Marzari R, et al. Anti transglutaminase antibodies cause ataxia in mice. PLoS One. 2010;5:e9698. doi: 10.1371/journal.pone.0009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Niro R, Sblattero D, Florian F, Stebel M, Zentilin L, Giacca M, et al. Anti-idiotypic response in mice expressing human autoantibodies. Mol Immunol. 2008;45:1782–1791. doi: 10.1016/j.molimm.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Smart CJ, Trejdosiewicz LK, Howdle PD. Specific circulating anti-gliadin IgG-class antibody does not mediate intestinal enteropathy in gliadin-fed mice. Int Arch Allergy Immunol. 1992;97:160–166. doi: 10.1159/000236112. [DOI] [PubMed] [Google Scholar]
- Freitag T, Schulze-Koops H, Niedobitek G, Melino G, Schuppan D. The role of the immune response against tissue transglutaminase in the pathogenesis of coeliac disease. Autoimmun Rev. 2004;3:13–20. doi: 10.1016/S1568-9972(03)00054-5. [DOI] [PubMed] [Google Scholar]
- Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol. 2009;9:858–870. doi: 10.1038/nri2670. [DOI] [PubMed] [Google Scholar]
- Myrsky E, Syrjänen M, Korponay-Szabo IR, Mäki M, Kaukinen K, Lindfors K. Altered small-bowel mucosal vascular network in untreated coeliac disease. Scand J Gastroenterol. 2009;44:162–167. doi: 10.1080/00365520802400875. [DOI] [PubMed] [Google Scholar]
- Haroon ZA, Hettasch JM, Lai TS, Dewhirst MW, Greenberg CS. Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis. Faseb J. 1999;13:1787–1795. doi: 10.1096/fasebj.13.13.1787. [DOI] [PubMed] [Google Scholar]
- de Kauwe AL, Chen Z, Anderson RP, Keech CL, Price JD, Wijburg O, et al. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J Immunol. 2009;182:7440–7450. doi: 10.4049/jimmunol.0900233. [DOI] [PubMed] [Google Scholar]
- Marietta E, Black K, Camilleri M, Krause P, Rogers RS, 3rd, David C, et al. A new model for dermatitis herpetiformis that uses HLA-DQ8 transgenic NOD mice. J Clin Invest. 2004;114:1090–1097. doi: 10.1172/JCI21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai E, Cherñavsky A, Pedreira S, Smecuol E, Vazquez H, Niveloni S, et al. Bone-specific antibodies in sera from patients with celiac disease: characterization and implications in osteoporosis. J Clin Immunol. 2002;22:353–362. doi: 10.1023/a:1020786315956. [DOI] [PubMed] [Google Scholar]
- Naiyer AJ, Shah J, Hernandez L, Kim SY, Ciaccio EJ, Cheng J, et al. Tissue transglutaminase antibodies in individuals with celiac disease bind to thyroid follicles and extracellular matrix and may contribute to thyroid dysfunction. Thyroid. 2008;18:1171–1178. doi: 10.1089/thy.2008.0110. [DOI] [PubMed] [Google Scholar]
- Sategna-Guidetti C, Franco E, Martini S, Bobbio M. Binding by serum IgA antibodies from patients with coeliac disease to monkey heart tissue. Scand J Gastroenterol. 2004;39:540–543. doi: 10.1080/00365520410008764. [DOI] [PubMed] [Google Scholar]
- McQueen F, Elliott B. B cell lymphoproliferation and organ-directed self-recognition to explain autoimmunity: back to the past. Med Hypotheses. 2010;75:328–333. doi: 10.1016/j.mehy.2010.03.015. [DOI] [PubMed] [Google Scholar]


