Abstract
Immunosuppressive mediators in tuberculosis pleurisy (pleural fluid (PF)) are associated with the course of disease, but they remain poorly defined. To study the local immune status of patients with tuberculosis pleurisy, we examined the effect of PF on the functions of T cells and the differentiation of Th1 cells. PF could inhibit the ability of T cells to produce cytokines. However, tumor-necrosis factor (TNF)-α derived from non-T cells was not impaired. Further analysis indicated that cell activation and cell cycle progression were also suppressed. Moreover, PF could inhibit Th1 cell differentiation. Importantly, we found that inhibitors of indoleamine 2,3-dioxygenase (IDO) and adenosine and neutralizing antibodies against IL-10 and transforming growth factor (TGF)-β could reverse cytokine production, suggesting that IDO, adenosine, IL-10 and Transforming growth factor–β1 in PF might take part in impairing T-cell functions. Taken together, our data demonstrate for the first time that several immunopathological factors participate in the downregulation of T-cell functions in local PF.
Keywords: suppressive factors, Th1 cytokine, Th1 differentiation, tuberculosis pleurisy
Introduction
In the clinical manifestations of tuberculosis, pleuritis is of particular interest because it can either resolve spontaneously within a few weeks or months, or may subsequently develop into a more serious form of tuberculosis.1 The pleural space is the site of naturally occurring tuberculous-associated inflammatory exudates.2, 3 The inflammatory process results in increased pleural vascular permeability,4 leading to the accumulation of fluid enriched in proteins and immunomodulatory factors.5 Thus, the pleural space provides an excellent model to study the immunological status at the site of Mycobacterium tuberculosis (Mtb) infection.
The local milieu that modulates T-cell functions is thought to be important. Previous studies have reported that immunosuppressive factors that counteract Th1 responses were dominant in bronchoalveolar lavage (BAL) cells6 as well as the BAL fluid7 of patients with tuberculosis. IL-10 and transforming growth factorTGF-β are two such potential deactivators of the immune responses. Moreover, increased levels of serum IL-10 and TGF-β were detected in tuberculosis patients, and the increased in vitro IL-10 and TGF-β secretion by the peripheral blood mononuclear cells (PBMCs) of tuberculosis patients in response to Mtb Ags also supports a role for these two immune suppressive mediators. These data support the idea that the immunosuppressive cytokines IL-10 and TGF-β downmodulate host anti-Mtb immunity.
In addition to IL-10 and TGF-β, we found that additional mediators that were not previously evaluated in pleural fluid (PF) might act to impair T-cell functions. Indoleamine 2,3-dioxygenase (IDO) expression is increased when inflammation occurs, which is induced by wounding and infection.8 IDO decreases the local concentration of free tryptophan9 while increasing the concentration of downstream metabolites,10 which leads to T-cell suppression. Additionally, adenosine is a signaling molecule that is generated at sites of tissue injury and inflammation to modulate inflammatory processes and immune responses.11 Clinical and experimental studies have indicated that adenosine levels are also elevated in the BAL fluid12 and exhaled breath condensate of asthmatics, where the magnitude of adenosine correlates with the magnitude of pulmonary inflammation.13, 14
In the present study, we focused on the effects of PF from patients newly diagnosed with tuberculosis on the functional ability of T cells from normal donors. We showed that PF could inhibit the functions of T cells, including cytokine production, cell activation, cell cycle progression and Th1 cell differentiation. Furthermore, we demonstrated that the application of 1-methyl-tryptophan (1-MT), caffeine, anti-IL-10 and anti-TGF-β neutralizing antibodies in PF could partially rescue T-cell functions.
Materials and methods
Subjects
A total of 31 patients (n=31; 20 men and 11 women) aged 17–92 years with newly diagnosed tuberculosis pleurisy at Chest Hospital of Guangzhou were enrolled in the study. All PF samples were obtained during diagnostic thoracocentesis before the initiation of chemotherapy and taken after permission from the patients. None of the subjects was receiving anti-tuberculous or steroid therapy at the time of the study. The diagnosis was based on positive cultures for Mtb, clinical and radiological features, and a good response to anti-tuberculosis treatment. Normal adults (n=35; 23 females and 12 men) aged 21–55 years were recruited at Zhongshan School of Medicine, Sun Yat-Sen University, Guangzhou, China. Umbilical cord blood from full-term newborn infants without infection was collected from the Secondary Affiliated Hospital of Sun Yat-Sen University. All individuals involved in this study provided adequate informed consent. The study was approved by the Medical School Review Board at Sun Yat-Sen University.
Monoclonal antibodies (mAbs)
The following antibodies were used for cell surface and intracellular staining: allophycocyanin-labeled anti-CD8, interferon (IFN)-γ-allophycocyanin, phycoerythrin (PE)-labeled anti-CD3, CD69-PE, IL-2-PE, peridinin chlorophyll protein-labeled anti-CD4, FITC-labeled anti-CD25, CD8-FITC, PE-cy7-conjugated tumor-necrosis factor (TNF)-α and isotype-matched control antibodies, which were purchased from BD Biosciences Pharmingen (San Jose, CA, USA). Purified anti-CD28 and anti-CD3 mAbs were also purchased from BD Biosciences Pharmingen.
Preparation of PF
PF was obtained by thoracocentesis from tuberculosis patients and centrifuged at 2000 r.p.m. for 10 min at room temperature. The cell-free PF was collected and stored at −80 °C before use.
Cell preparation
For the isolation of umbilical cord blood mononuclear cells (CBMCs), heparinized cord blood was mixed sufficiently with Dextran 500 solution (GE Healthcare Bio-Sciences, Uppsala, Sweden) and incubated at 37 °C in a 5% CO2 incubator to remove erythrocytes. After 30 min, CBMCs were obtained by Ficoll-Hypaque (Tianjin HaoYang Biological Manufacture, Tianjin, China) density gradient centrifugation. PBMCs obtained from healthy donors were isolated from heparinized venous blood by Ficoll-Hypaque density gradient centrifugation. The cells were suspended at a concentration of 2×106/ml in complete RPMI 1640 medium (Gibco, Grand Island, NY, USA) containing 10% heat-inactivated fetal calf serum (Sijiqing Biotech Co., Hangzhou, China), 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine and 50 µM 2-mercaptoethanol. All reagents were purchased from Gibco.
Isolation of T-cell subsets
CD3+ T cells were negatively isolated from freshly isolated PBMCs using a cocktail of biotin-conjugated antibodies against CD14, CD16, CD19, CD36, CD56, CD123 and glycophorin A (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were then passed through a magnetic column for collection of T cells. Labeled cells were collected and used as CD3-depleted cells. The purity of T cells, assessed by flow cytometry (FACS Calibur; Becton Dickinson, San Jose, CA, USA), exceeded 98%. CD4+ T cells were negatively isolated from CBMCs using a biotin-antibody cocktail (anti-CD8, anti-CD14, anti-CD16, anti-CD19, anti-CD36, anti-CD56, anti-CD123, anti-TCRγ/δ and anti-glycophorin A) (Miltenyi Biotec). Unlabeled cells were collected as CD4+ T cells. The purity of CD4+ T cells was assessed by flow cytometry and exceeded 96%.
Cell culture conditions
PBMCs were incubated with or without anti-CD3 (0.2 µg/ml) or anti-CD3 plus anti-CD28 (1 µg/ml) in the presence or absence of different percentages (from 50 to 0.4%) of PF in a 96-well plate at a concentration of 4×105 cells/well in triplicate. Supernatants of the cell cultures were collected on days 1 and 3 and processed for enzyme-linked immunosorbent assay (ELISA) detection. Purified T cells (2×105 cells/well) were stimulated with anti-CD3 (0.2 µg/ml) plus anti-CD28 (1 µg/ml), and non-T cells (2×105 cells/well) were activated with bacille Calmette–Guérin (20 µg/ml) in a 96-well plate in triplicate. PF was added at final concentrations of 0.4%, 1.6%, 6.3%, 25% and 50% into cell cultures. The plates were incubated at 37 °C with 5% CO2. Cell-free supernatants were harvested and assessed for cytokine production by ELISA. The percentages of PF were calculated by the volume ratio (v/v, %) of the total culture conditions.
Th1 cell differentiation conditions
For the induction of Th1 cells, purified CD4+ T cells from CBMCs were cultured with anti-CD3 and anti-CD28 plus hIL-12 (5 ng/ml) and anti-hIL-4 (2 µg/ml) in the presence or absence of 25 or 6.3% PF. Cells were collected at day 5, washed and restimulated in triplicate with phorbol 12-myristate 13-acetate (PMA, 20 ng/ml; Sigma-Aldrich, St Louis, MO, USA) plus ionomycin (1 µg/ml; Sigma-Aldrich) for 1 and 3 days. In addition, 2×105 cells/well differentiated Th1 cells were restimulated with PMA plus ionomycin in the presence or absence of either 25 or 6.3% PF. Culture supernatants were harvested and analyzed for the production of IL-2 and TNF-α at day 1 and IFN-γ at day 3 by ELISA.
ELISA
The supernatants were harvested and assayed for IFN-γ, IL-2 and TNF-α production by ELISA according to the manufacturer's protocol (BD Pharmingen, San Diego, CA, USA). The detection limit of the IL-2, TNF-α and IFN-γ assay kits were 7.8, 7.8 and 9.4 pg/ml, respectively.
Cell surface and intracellular cytokine staining
For surface staining, cells were washed twice with phosphate-buffered saline (PBS) buffer containing 0.1% bovine serum albumin and 0.05% sodium azide, followed by incubation with fluorochrome-conjugated mAbs to cell surface markers at 4 °C in the dark for 30 min. Cell samples were washed twice and fixed in 1% paraformaldehyde before acquisition. To determine intracellular cytokine production, brefeldin A (10 µg/ml; Sigma-Aldrich) was added to cultures during the final 6 h of the experiment. Following stimulation, cells were washed, fixed with 4% paraformaldehyde, permeabilized and stained for the intracellular cytokines in PBS buffer containing 0.1% saponin for 30 min at 4 °C. After intracellular staining, cells were washed and resuspended in PBS. Flow cytometry was performed using a BD FACSCalibur cytometer. Lymphocytes were gated on forward and side scatter profiles and analyzed using FlowJo software (Treestar, San Carlos, CA, USA).
Cell cycle analysis
PBMCs were cultured with medium or anti-CD3 plus anti-CD28 in the presence or absence of 25% PF. After 3 days, cells were harvested and washed with a buffer solution containing sodium citrate, sucrose and dimethyl sulfoxide. Then the cells were trypsinized for 10 min at room temperature and then treated with trypsin inhibitor and RNase buffer for another 10 min. A cold (2–8 °C) propidium iodide stain solution was used to stain nuclear DNA. After gentle mixing, the staining procedure was carried out in the dark for 10 min. Flow cytometry was performed using a BD FACSCalibur cytometer and data were analyzed.
Neutralization experiment
PBMCs were cocultured with anti-CD3 and 6.3% PF in the presence or absence of neutralizing autobodies or inhibitors. The following reagents were added: IDO inhibitor 1-methyl-tryptophan (100 µM; Sigma-Aldrich), Selective A2A adenosine receptor antagonist caffeine (50 µg/ml; Sigma-Aldrich), anti-IL-10 mAb (10 µg/ml; R&D Systems, Minneapolis, MN, USA) and anti-TGF-β mAb (5 µg/ml; R&D Systems).
Statistical analysis
Statistical significance was determined with the two-tailed Student's t-test, with a P value of less than 0.05 considered statistically significant.
Results
Suppressive effect of PF on the production of cytokines by PBMCs
To determine whether suppressive factors existed in PF, we first tested whether cytokine production by PBMCs can be inhibited when PBMCs are cultured with PF. In the presence of anti-CD3, PBMCs produce IFN-γ, IL-2 and TNF-α. When various concentrations of PF were added, the production of IFN-γ, IL-2 and TNF-α was reduced in a dose-dependent manner (Figure 1a). PBMCs almost lost the capacity to produce cytokines when exposed to 25% PF. The suppressive effect of PF was also significant at a concentration of 6.3%. Subsequently, to explore whether PF could also inhibit the production of cytokines when PBMCs were cultured with a stronger stimulation, we incubated PBMCs with anti-CD3 plus anti-CD28 in the presence of various concentrations of PF and found that PF could also reduce the production of IFN-γ, IL-2 and TNF-α in a dose-dependent manner (Figure 1b).
Figure 1.
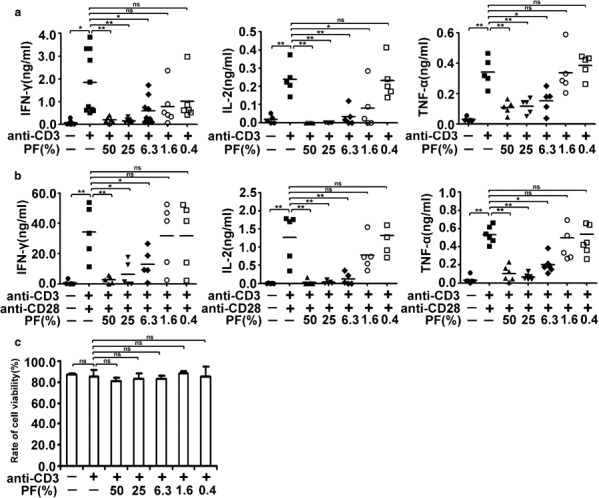
PF from tuberculosis patients inhibits cytokine production by PBMCs in a dose-dependent manner. PBMCs were stimulated with anti-CD3 (0.2 µg/ml) (a) or anti-CD3 plus anti-CD28 (1 µg/ml) (b) in the presence or absence of various concentrations of PF. The levels of IL-2 and TNF-α were detected at day 1, and IFN-γ was analyzed at day 3. Each dot indicates the average of triplicates from each donor, and small horizontal bars represent the mean. *P<0.05; **P<0.001; ns, no significance. (c) Effect of PF on the viability of cells. PBMCs were cultured as described in (a). Cells were resuspended and viability was estimated by means of trypan blue staining. Dead cells were counted and the viability of cells was calculated and displayed. The results are representative of four independent experiments. IFN, interferon; PBMC, peripheral blood mononuclear cell; PF, pleural fluid; TNF, tumor-necrosis factor.
Because PF vigorously suppressed the production of cytokines, we wondered whether the inhibitory action was due to toxic effects. To test this idea, PBMCs were incubated with anti-CD3 plus different concentrations of PF for 5 days. The numbers of living cells and dead cells were determined by trypan blue staining. No changes in cell viability were displayed when PMBCs were cultured with PF (from 50 to 0.4%) or without PF (Figure 1c). These results might suggest that the suppressive effect of PF on T cells was not due to a toxic effect.
PF directly inhibits T-cell cytokine production
To clarify the effect of PF on the production of cytokines by cell subsets, PBMCs were stimulated with or without anti-CD3, or anti-CD3 plus anti-CD28 in the presence or absence of 25% PF and analyzed by FACS. Addition of 25% PF into the cultures markedly reduced the production of cytokines by CD4+ and CD8+ T cells (data not shown). Furthermore, T cells purified from total PBMCs were stimulated with anti-CD3 plus anti-CD28 in the presence or absence of 25% PF, and the production of cytokines was detected by FACS. After stimulation with anti-CD3 plus anti-CD28, CD4+ T cells expressed IFN-γ (0.5%), IL-2 (0.59%) and TNF-α (1.31%). After incubation with 25% PF, the expression of cytokines was reduced (0.03% IFN-γ+, 0.01% IL-2+ and 0.03% TNF-α+; Figure 2a). The same pattern for cytokine expression by CD8+ T cells is shown in Figure 2b.
Figure 2.
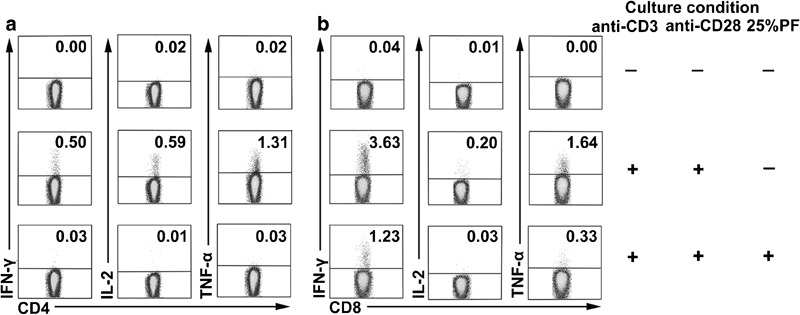
Suppressive effect of PF on cytokine production by subsets of T cells. Purified T cells were stimulated with or without anti-CD3 and anti-CD28 in the presence or absence of PF. T cells were harvested and stained. Subpopulations of CD4+ T cells (a) and CD8+ T cells (b) were analyzed for IFN-γ, IL-2 and TNF-α expression. Numbers in the quadrants indicate the percentages of IFN-γ, IL-2 and TNF-α. Data are representative of seven separate experiments. IFN, interferon; PF, pleural fluid; TNF, tumor-necrosis factor.
To check whether PF could suppress both T-cell and non-T-cell subsets, we compared the reactivity of T cells with non-T cells in the presence of PF by ELISA. T cells and T cell-depleted cells were purified from the same donor. T cells were stimulated with anti-CD3 and anti-CD28, and non-T cells were activated with bacille Calmette–Guérin in the presence of different concentrations of PF separately. As illustrated in Figure 3a–c, PF displayed a dose-dependent inhibitory effect on IFN-γ, IL-2 and TNF-α production by T cells. However, exposure of non-T cells to different concentrations of PF did not impair the production of TNF-α (Figure 3d). These results further confirmed that the reduction of cytokines by PF was not due to a toxic effect, but might be attributed to some immunosuppressive factors in PF, which could directly suppress T cells.
Figure 3.
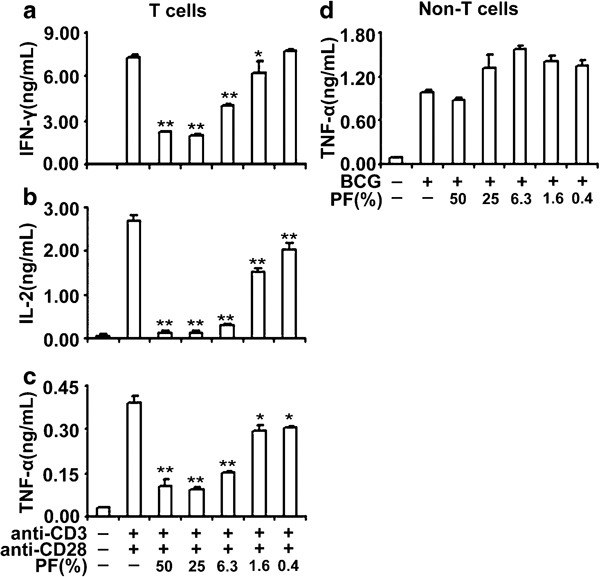
Distinct effects of PF on purified T cells and non-T cells. Purified T cells were cultured with or without anti-CD3 plus anti-CD28 in the presence or absence of different concentrations of PF. The levels of IFN-γ (a), IL-2 (b) and TNF-α (c) were detected by ELISA. Non-T cells were stimulated with BCG (20 µg/ml) in the presence or absence of various ratios of PF. ELISA analysis of the levels of TNF-α (d) in the culture supernatants. Data are representative of three experiments with similar results. *P<0.05; **P<0.001. BCG, bacille Calmette–Guérin; IFN, interferon; PF, pleural fluid; TNF, tumor-necrosis factor.
PF inhibits activation of CD4+ and CD8+ T cells
To further elucidate the functional status of PF-exposed cells, we examined the effect of PF on cell activation. PBMCs were stimulated with anti-CD3 or anti-CD3 plus anti-CD28 in the presence or absence of 25% PF, and the expression of cell activation molecules was detected by FACS. Anti-CD3 and anti-CD3 plus anti-CD28 were able to activate cells, while the addition of PF to the cultures significantly reduced the expression of CD69 and CD25 (data not shown). To further ascertain whether PF had a direct suppressive effect on T-cell activation, purified T cells were activated with anti-CD3 plus anti-CD28 in the presence or absence of 25% PF. The results showed high levels of CD69 expression following the stimulation of anti-CD3 plus anti-CD28 (39.8% of CD4+ T cells and 24% of CD8+ T cells). The addition of 25% PF could significantly suppress CD69 expression (2.03% of CD4+ T cells and 0.93% of CD8+ T cells; Figure 4a). As illustrated in Figure 4b, 43.9% of CD4+ T cells and 22.3% of CD8+ T cells expressed CD25, whereas treatment with 25%PF reduced the expression of CD25 (15.9% of CD4+ T cells and 0.24% of CD8+ T cells).
Figure 4.
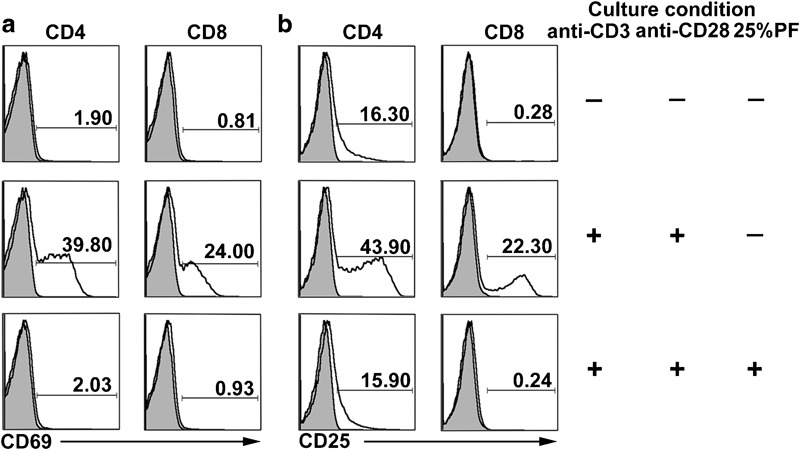
PF markedly inhibits cell activation. Purified T cells were cultured with or without anti-CD3 and anti-CD28 in the presence or absence of 25% PF. FACS analysis for the expression of CD69 (a) and CD25 (b) on CD4+ and CD8+ T cells was performed. Isotype controls are shown as grey areas, and expression of activation markers are depicted as solid lines. Data are representative of five experiments with similar results. PF, pleural fluid.
PBMCs display anomalous cell cycle arrest following PF treatment
In addition to investigating cell activation, we also assessed whether PF could disturb cell cycle progression. As illustrated in Figure 5a, control cells arrested normally, whereas cells stimulated with anti-CD3 plus anti-CD28 exhibited a significant retention within S phase (19.54 %) at 72 h post-treatment. In contrast, PF-exposed cells exhibited compromised S-phase arrest (2.57%). The average percentages of each phase were shown in the pie chart (Figure 5b).
Figure 5.
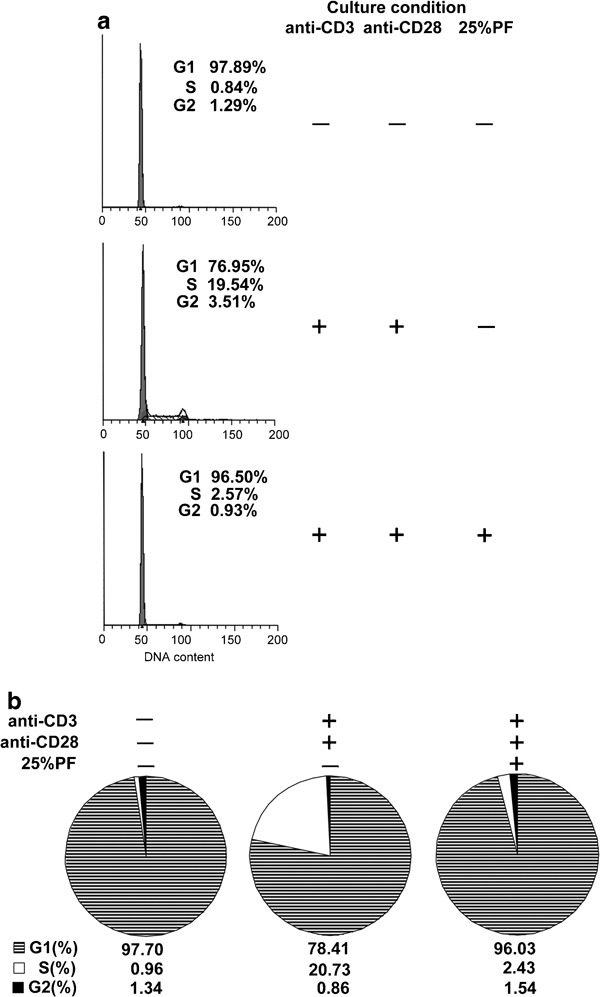
PF vigorously blocks the G1/S phase of the cell cycle. PBMCs were incubated with or without anti-CD3 plus anti-CD28 in the presence or absence of 25% PF for 2 days. The cells were harvested and subjected to cell cycle analysis. (a) The percentages of cells in the different phases of the cell cycle were determined by FACS. (b) Means from three independent experiments are shown. The stripes and black filled represent the G1 and G2 phases, respectively, while the blank space depicts S phase. PBMC, peripheral blood mononuclear cell; PF, pleural fluid.
PF inhibits Th1 cell differentiation and cytokine production
To examine whether treatment of CD4+ T cells from CBMCs exposed to PF during primary culture altered functional responses, we cultured naive CD4+ T cells in Th1-polarizing condition (anti-CD3 plus anti-CD28, hIL-12 and anti-hIL-4) in the presence or absence of 25 or 6.3% PF. After 5 days, cells were collected, washed and restimulated with PMA plus ionomycin. Th1 cell development was notably blunted when 25% PF was added to Th1 cell differentiation culture conditions. Th1 cell development was impaired to a small extent in the presence of 6.3% PF (Figure 6a). Consistent with FACS analysis, ELISA results showed that naive CD4+ T cells cultured in Th1-polarizing condition plus 25% PF significantly reduced the production of IFN-γ, IL-2 and TNF-α (P<0.001). Addition of 6.3% PF to Th1-polarizing conditions suppressed IFN-γ, IL-2 and TNF-α production to a small extent (Figure 6c). These results established the idea of a direct suppressive impact of PF on the Th1 cell differentiation process.
Figure 6.
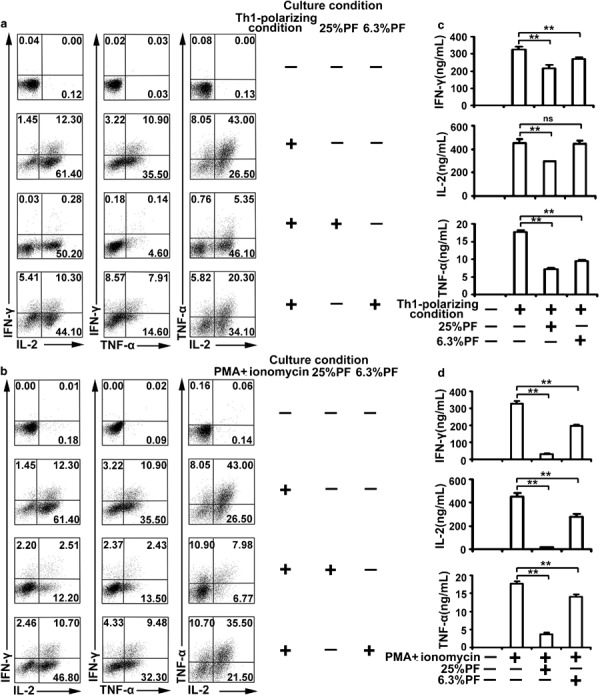
PF inhibits the cell differentiation and functions of Th1 cells. (a) Purified CD4+ T cells from CBMCs were cultured with anti-CD3 and anti-CD28 plus hIL-12 (5 ng/ml) and anti-hIL-4 (2 µg/ml) in the presence or absence of PF for 5 days. Cells were harvested, washed and restimulated with PMA (20 ng/ml) plus ionomycin (1 µg/ml). FACS was used to analyze the expression of IFN-γ, IL-2 and TNF-α. Data are representative of three experiments with similar results. (b) The cells stimulated with anti-CD3 and anti-CD28 plus hIL-12 and anti-hIL-4 (Th1-polarizing condition) were harvested, washed and restimulated with PMA plus ionomycin in the presence or absence of PF. The expression of IFN-γ, IL-2 and TNF-α was analyzed by FACS. Data are representative of 10 experiments with similar results. (c, d) The levels of IFN-γ, IL-2 and TNF-α in culture supernatants were measured by ELISA. Data are representative of 10 experiments with similar results. *P<0.05; **P<0.001; ns, no significance. CBMC, cord blood mononuclear cell; IFN, interferon; PF, pleural fluid; PMA, phorbol 12-myristate 13-acetate; TNF, tumor-necrosis factor.
To detect whether PF could inhibit differentiated Th1 cells, we cultured Th1 cells with PMA plus ionomycin in the presence or absence of PF. Th1 cells produce high levels of IFN-γ, IL-2 and TNF-α when stimulated with PMA plus ionomycin. Notably, the production of IFN-γ, IL-2 and TNF-α was substantially inhibited by PF. Moreover, addition of 6.3% PF could partially impair Th1 cytokine production (Figure 6b). We confirmed the result by assessing the levels of IFN-γ, IL-2 and TNF-α by ELISA (Figure 6d). All together, these results indicated that PF could inhibit Th1 cell differentiation and cytokine production.
Multiple immunosuppressive factors are present in PF
Our findings clearly indicated that one or more immunosuppressants in PF acted to dampen T-cell functions. To better characterize the suppressive factors, we heated PF at 100 °C for 3 min, which resulted in some degree of loss of activity. These results suggested that the inhibitory effect was mediated by both heat-labile and -stable factors (data not shown).
To further elucidate the inhibitory mechanisms, PBMCs were incubated with anti-CD3 and 6.3% PF with inhibitors against IDO (1-MT), a selective A2A adenosine receptor antagonist (caffeine) and neutralizing autobodies to IL-10 and TGF-β. The individual blockade of IDO, adenosine, IL-10 and TGF-β could significantly reverse IFN-γ production (Figure 7a). Application of 1-MT, caffeine and anti-IL-10 led to a comparable and significant increase in IL-2 production (Figure 7b). Addition of caffeine and anti-IL-10 could partially reverse TNF-α production, while blockade of IDO or TGF-β restored the expression of TNF-α to a non-significant lesser extent (Figure 7c). Overall, these data suggested that application of 1-MT, caffeine, anti-IL-10 and anti-TGF-β could partially reverse cytokine production, indicating that multiple mechanisms, mediated by factors such as IDO, adenosine, IL-10 and TGF-β in PF, may lead to the suppressive effect on T cells.
Figure 7.
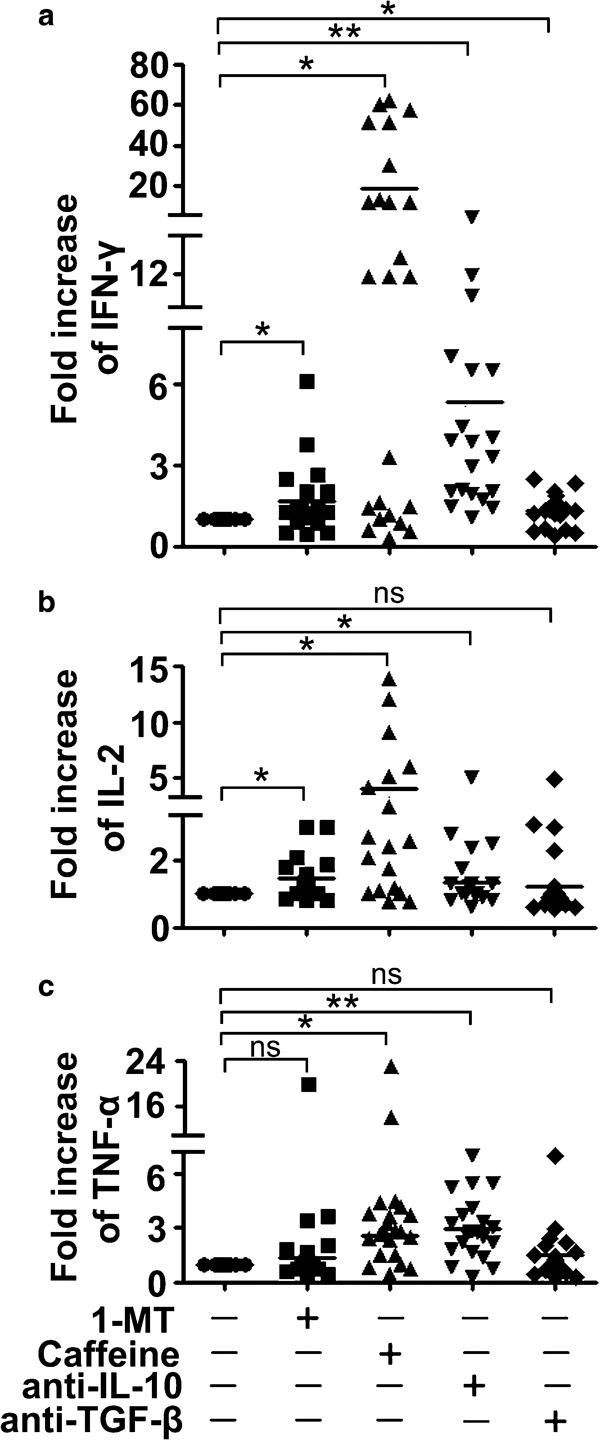
Multiple inhibitory pathways can be blocked to reverse cytokine production. PBMCs were cocultured with anti-CD3 and PF. The IDO inhibitor 1-MT (100 µM), the adenosine A2A receptor antagonist caffeine (50 µg/ml), anti-IL-10 mAb (10 µg/ml) and anti-TGF-β mAb (5 µg/ml) were added to the culture condition at the time of onset separately. The levels of IL-2, TNF-α and IFN-γ were detected by ELISA. The fold increase (relative to the stimulation with anti-CD3 and PF, set as 1) of IFN-γ (a), IL-2 (b) and TNF-α (c) production is shown. The graphs display the combined results with each symbol representing the mean value from a single donor and horizontal bars representing the mean value of all data points. *P<0.05; **P<0.001; ns, no significance. IDO, indoleamine 2,3-dioxygenase; IFN, interferon; mAb, monoclonal antibody; 1-MT, 1-methyl-tryptophan; PBMC, peripheral blood mononuclear cell; PF, pleural fluid; TGF, transforming growth factor; TNF, tumor-necrosis factor.
Discussion
Tuberculosis pleurisy is an inflammatory process that leads to the accumulation of fluid enriched in cells, proteins and some immune mediators.2, 3 Patients with tuberculosis pleurisy have elevated levels of IFN-γ, IL-12, IL-6, IL-8 and IL-18. Th1 cytokines were associated with protective immunity against Mtb.15, 16, 17 Despite the heightened levels of inflammatory mediators, several immune factors that lead to suppression of T-cell functions have been identified in current research.18, 19 Thus, the balance between pro- and anti-inflammatory mediators seems to be crucial to the final outcome of tuberculosis.
Cells in pleurisy are mostly derived from circulating PBMCs, and they are strongly impaired in regard to various functions. We initially examined the effect of PF on cytokine production. Both individual and pooled PF had a similar impact on PBMCs. Dose–response studies showed that 25% PF entirely suppressed IFN-γ, IL-2 and TNF-α production by PBMCs under anti-CD3 or anti-CD3 plus anti-CD28 stimulation. Importantly, cell viability was not affected in the culture conditions. These data suggested that the inhibitory response was not due to a toxic effect. In addition, we found that PF could directly inhibit T-cell cytokine production, whereas non-T cells displayed no suppressive effect. These results indicated that some suppressive factors located within PF could impair T-cell functions. Moreover, PF displayed an inhibitory effect on cell activation and cell cycle progression.
Th1 cells have been linked to many chronic inflammatory disorders and evolved to enhance clearance of intracellular pathogens.20 Therefore, we checked the impact of PF on Th1 cell differentiation and function. Following the addition of PF to Th1 cell differentiation conditions, naive CD4+ T cells could not develop into Th1 cells, implying that PF could inhibit the progression of Th1 cell differentiation. In addition, PF could also vigorously inhibit differentiated Th1 cells from producing IFN-γ, IL-2 and TNF-α. These results clearly indicated that PF could not only impair T cells that are newly recruited from peripheral blood, but also influence local proliferation and differentiation of Th1 cells.
It seems that several immune factors can act to impair Th1 type-mediated inflammation. Such downmodulatory factors are actually anti-inflammatory molecules that are produced to protect against the tissue damage induced by excessive inflammation, but at the same time deregulate protective immunity.18 Our observations are supported by other investigators that relied on murine models,21 in vitro stimulated peripheral blood cells of patients22 or lung cells from induced sputum23 to show a suppressed immune status was associated with tuberculosis patients. In the current study, using pleurisy fluid from tuberculosis patients, we confirmed and further extended the prior research. We correlated these interesting findings with the clinical syndromes of our patients. Various symptoms, such as fever, night sweats, emaciation, weight loss and tissue damage, resembled the pathological effects of host immunity. The impairment of T-cell functions and prevention of Th1 cell differentiation could be recognized as important mechanisms of Mtb immune escape in patients.
IDO is induced by pathogens in infected host tissues.24 The overexpression of IDO can suppress immune responses by blocking T-cell proliferation locally,25 and its activity can be inhibited by the competitive inhibitor 1-MT.26 In our experiments, 1-MT could marginally restore IFN-γ production, suggesting that the suppressive effect of PF is mediated by IDO and additional soluble factors.
Under normal physiological conditions, the level of adenosine in the tissue microenvironment is relatively low.27 At the height of inflammation, the destruction of host tissue combined with damaged microcirculation and hypoxia leads to elevation of extracellular adenosine.28 Animal models of asthma,29 arthritis,30 sepsis,31 inflammatory bowel disease32 and wound healing33 have helped to elucidate the regulatory roles of adenosine in dictating the development and progression of disease. Adenosine potently inhibits a wide range of T-cell responses, including cell proliferation,34 synthesis of IL-2 and proinflammatory cytokines, such as IFN-γ and TNF-α,35 and some of the earliest steps of T-cell activation, which were reported to be mediated mainly by the adenosine A2A receptor. Therefore, it is plausible that such a mechanism may contribute to the negative effect of PF on T-cell functions. To confirm our hypothesis, we cocultured PF-exposed PBMCs with caffeine, an antagonist of A2A adenosine receptor, and found that caffeine could significantly restore the production of cytokines, suggesting that adenosine may be a key factor participating in the suppressive effect.
IL-10 and TGF-β, the two classical regulatory cytokines, are known to not only inhibit T-cell activity in response to Mtb,7 but also promote T-cell anergy possibly by downregulating the cell surface expression of the costimulatory and antigen-presenting molecules on Mtb-infected monocytes.9, 36 In addition to earlier reports that BAL fluids from a significant number of tested tuberculosis patients contained elevated IL-10 levels and bioactive TGF-β,7 recent reports showed that lung cells from induced sputum expressed significantly high levels of IL-10, TGF-βRI and TGF-βRII.37 In the present study, we found that the addition of anti-IL-10 or anti-TGF-β could partially reverse IFN-γ production, suggesting that local IL-10 and TGF-β in PF may promote a cytokine microenvironment where resident and newly recruited immune cells become refractory to appropriate activating signals.
Our findings demonstrated for the first time that some soluble factors in PF directly dampened normal T-cell differentiation and functions, and these results pointed out that the individual application of inhibitors against IDO, adenosine, IL-10 and TGF-β could partially rescue T-cell functions. Our research may help to shed light on the mechanisms regarding the suppressive characteristics of local immunity in patients in the face of Mtb invasion and represent the first preclinical evidence, to our knowledge, that subversion of the repressed condition may be of significant therapeutic value for the management of tuberculosis pleurisy.
Acknowledgments
This study was supported by the 115 grant (no. 2008ZX10003011), the National Nature Science Foundation of China (no. 30872300) and the National Key Basic Research Program of China (973; no. 2007CB512404).
References
- Ferrer J. Pleural tuberculosis. Eur Respir J. 1997;10:942–947. [PubMed] [Google Scholar]
- Porcel JM. Tuberculous pleural effusion. Lung. 2009;187:263–270. doi: 10.1007/s00408-009-9165-3. [DOI] [PubMed] [Google Scholar]
- Light RW, Macgregor MI, Luchsinger PC, Ball WJ. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–513. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- Aleman M, de la Barrera SS, Schierloh PL, Alves L, Yokobori N, Baldini M, et al. In tuberculous pleural effusions, activated neutrophils undergo apoptosis and acquire a dendritic cell-like phenotype. J Infect Dis. 2005;192:399–409. doi: 10.1086/431680. [DOI] [PubMed] [Google Scholar]
- Saka H, Shimokata K.State of the art: treatment of malignant pleural and pericardial effusions Gan To Kagaku Ryoho 199724Suppl 3418–425.Japanese. [PubMed] [Google Scholar]
- Schwander SK, Torres M, Carranza CC, Escobedo D, Tary-Lehmann M, Anderson P, et al. Pulmonary mononuclear cell responses to antigens of Mycobacterium tuberculosis in healthy household contacts of patients with active tuberculosis and healthy controls from the community. J Immunol. 2000;165:1479–1485. doi: 10.4049/jimmunol.165.3.1479. [DOI] [PubMed] [Google Scholar]
- Bonecini-Almeida MG, Ho JL, Boechat N, Huard RC, Chitale S, Doo H, et al. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-beta) and analysis of TGF-beta receptors I and II in active tuberculosis. Infect Immun. 2004;72:2628–2634. doi: 10.1128/IAI.72.5.2628-2634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem Biophys Res Commun. 2005;338:20–24. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]
- Zeller JC, Panoskaltsis-Mortari A, Murphy WJ, Ruscetti FW, Narula S, Roncarolo MG, et al. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. J Immunol. 1999;163:3684–3691. [PubMed] [Google Scholar]
- Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV. Use of the A2A adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. . Biochem Pharmacol. 2003;65:493–501. doi: 10.1016/s0006-2952(02)01548-4. [DOI] [PubMed] [Google Scholar]
- Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Molnar VG, et al. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J. 2002;20:1393–1398. doi: 10.1183/09031936.02.00005002. [DOI] [PubMed] [Google Scholar]
- Huszar E, Horvath I, Barat E, Herjavecz I, Boszormenyi-Nagy G, Kollai M. Elevated circulating adenosine level potentiates antigen-induced immediate bronchospasm and bronchoconstrictor mediator release in sensitized guinea pigs. J Allergy Clin Immunol. 1998;102:687–691. doi: 10.1016/s0091-6749(98)70288-x. [DOI] [PubMed] [Google Scholar]
- Jalapathy KV, Prabha C, Das SD. Correlates of protective immune response in tuberculous pleuritis. FEMS Immunol Med Microbiol. 2004;40:139–145. doi: 10.1016/S0928-8244(03)00303-1. [DOI] [PubMed] [Google Scholar]
- Barnes PF, Lu S, Abrams JS, Wang E, Yamamura M, Modlin RL. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condos R, Rom WN, Liu YM, Schluger NW. Local immune responses correlate with presentation and outcome in tuberculosis. Am J Respir Crit Care Med. 1998;157:729–735. doi: 10.1164/ajrccm.157.3.9705044. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pando R, Orozco H, Aguilar D. Factors that deregulate the protective immune response in tuberculosis. Arch Immunol Ther Exp (Warsz) 2009;57:355–367. doi: 10.1007/s00005-009-0042-9. [DOI] [PubMed] [Google Scholar]
- Ho JL, Lapa ES. Promotion of a down-modulated lung immune state may be a strategy by M. tuberculosis to foster active disease and persistence. Discov Med. 2010;9:34–41. [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins HL, Kaufmann SH. The many faces of host responses to tuberculosis. Immunology. 2001;103:1–9. doi: 10.1046/j.1365-2567.2001.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahiratmadja E, Alisjahbana B, de Boer T, Adnan I, Maya A, Danusantoso H, et al. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect Immun. 2007;75:820–829. doi: 10.1128/IAI.00602-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha RA, Kotsimbos TC, Song YL, Menzies D, Hamid Q. IFN-gamma and IL-12 are increased in active compared with inactive tuberculosis. Am J Respir Crit Care Med. 1997;155:1135–1139. doi: 10.1164/ajrccm.155.3.9116999. [DOI] [PubMed] [Google Scholar]
- Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushley MJ, Tattersfield AE, Holgate ST. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. 1983. Br J Clin Pharmacol. 2004;58:S751–S758. doi: 10.1111/j.1365-2125.2004.02285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos MC, Yap JS, Desai A, Posadas I, McCrary CT, Cronstein BN. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 2000;43:656–663. doi: 10.1002/1529-0131(200003)43:3<656::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Law WR. Adenosine receptors in the response to sepsis: what do receptor-specific knockouts tell us? Am J Physiol Regul Integr Comp Physiol. 2006;291:R957–R958. doi: 10.1152/ajpregu.00412.2006. [DOI] [PubMed] [Google Scholar]
- Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Victor-Vega C, Desai A, Montesinos MC, Cronstein BN. Adenosine A2A receptor agonists promote more rapid wound healing than recombinant human platelet-derived growth factor (Becaplermin gel) Inflammation. 2002;26:19–24. doi: 10.1023/a:1014417728325. [DOI] [PubMed] [Google Scholar]
- Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- de Waal MR, Haanen J, Spits H, Roncarolo MG, Te VA, Figdor C, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AS, Lago PM, Boechat N, Huard RC, Lazzarini LC, Santos AR, et al. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol. 2009;183:718–731. doi: 10.4049/jimmunol.0801212. [DOI] [PubMed] [Google Scholar]


