Abstract
Understanding the defense mechanisms of the host of an organism is important for infection control. In previous studies, we demonstrated that interferon-α (IFN-α), but not IL-12, was produced by human peripheral blood mononuclear cells infected with varicella-zoster virus (VZV). Here, we investigated what kind of cell(s) and which signal molecule(s) are involved in IFN-α production. Using cell isolation and ELISA, we found that plasmacytoid dendritic cells (pDCs) were responsible for IFN-α production during VZV infection. We also found that Toll-like receptor 9 (TLR9) was involved in VZV-induced IFN-α production because inhibitory CpG oligodeoxynucleotide inhibited IFN-α production. UV-inactivated VZV-induced IFN-α production was lower than that of active VZV, indicating another TLR9-independent pathway. Further studies demonstrated that double-stranded RNA-dependent protein kinase, but not DNA-dependent protein kinase was involved in VZV-induced IFN-α production. Together, these results suggest that pDCs play an important role in IFN-α production during VZV infection through TLR9-dependent and -independent pathways.
Keywords: IFN-α, mononuclear cells, plasmacytoid dendritic cell, TLR9, varicella-zoster virus
Introduction
Immunity can be broadly divided into two categories: innate immunity and adaptive immunity. Innate immunity was previously thought to be a nonspecific immune response, characterized by phagocytic and leukocytic engulfment and digestion of microorganisms. Now, innate immunity is considered specific, and, in addition to its role in the early phases of defense, it plays a key role in stimulating subsequent adaptive immunity.1, 2
The dendritic cell (DC) system, one of the major initiators of innate and adaptive immunity, recognizes pathogens, presents antigens and produces cytokines, provoking a strong host defense. At least two subsets of DCs were originally identified in peripheral blood, including myeloid DCs (mDCs) and plasmacytoid DCs (pDCs).3, 4, 5, 6 The mDCs express CD11c and CD1c (BDCA-1) antigens. In contrast, pDCs express CD123, BDCA-2 and BDCA-4 antigens.7, 8 They also express different patterns of pathogen recognition receptors and respond to different microbial antigens. The main function of pDCs appears to be the production of a large amount of type I interferons (IFNs) in response to infection.9, 10, 11, 12 Although pDCs produce a large amount of IFN-α in response to infections, other populations of peripheral blood cells such as monocytes, B cells and mDCs can also produce IFN-α under certain conditions. In addition, IFN-α can be produced in response to a wide variety of other substances. These include certain viruses, bacteria, double-stranded RNA (dsRNA), lipopolysaccharide and CpG oligodeoxynucleotide (ODN), as well as low-molecular-weight compounds, such as imiquimod. This range suggests that both the specific cell type and the signal molecules are very important in the production of IFN-α. The type of cells and the signal pathways involved in varicella virus infection remain unclear, and more evidence is needed.
Varicella-zoster virus (VZV), also known as human herpes virus 3, is a double-stranded, linear DNA virus.13 It causes chicken pox and establishes lifelong latency in the sensory ganglia. Reactivation of latent VZV results in herpes zoster. In previous studies, we showed that VZV infection induces an IFN-α-mediated Th1 reaction in human peripheral blood mononuclear cells (PBMCs).14, 15 We were interested in the mechanism of IFN-α induction in VZV-infected PBMCs, so in this study we investigated what kind of cell(s) and signal molecule(s) are involved in the production of IFN-α in human peripheral blood that is infected with varicella virus with the aim of discovering the mechanism of resistance to varicella virus in human hosts.
Material and methods
Preparation of PBMCs
Peripheral blood obtained from healthy adult volunteers was used in this study. Blood samples were collected after informed consent was obtained, and the institutional review board of this hospital approved this study. Peripheral blood was separated into leukocytes and red blood cells by 4.5% dextran sedimentation of red blood cells. Leukocytes were further separated into mononuclear cells and polymorphonuclear cells by density gradient centrifugation (Ficoll-Paque, Amersham Pharmacia, Biotech AB, Uppsala, Sweden), as in our previous description.16 After being washed, PBMCs were resuspended to 2×l06 cells/ml in RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum.
Reagents
A VZV (Varilrix; GlaxoSmithKline NZ Ltd, Auckland, New Zealand) vaccine containing a mean virus titer of 103.3 Plaque-forming units per dose was used as the source of VZV. The vaccine was developed from the live attenuated Oka strain, a Japanese clinical isolate, by further modification in MRC-5 human diploid cells. This produces an attenuated, clinically unapparent varicella infection in susceptible subjects and subsequent production of varicella-specific antibodies. UV light-inactivated VZV was prepared using a UV crosslinker 1 J/cm2 for 5 min at 4 °C.
The following reagents were purchased: from Sigma (Sigma-Aldrich, St. Louis, MO), lipopolysaccharide (from Escherichia coli Serotype 0111:B4), SB239063 and 2-aminopurine (2AP); from Calbiochem (Calbiochem, San Diego, CA), U0126, Wedelolactone, 4,5-dimethoxy-2-nitrobenzaldehyde (DMNB) and SP600125; from eBioscience (eBioscience, San Diego, CA), Toll-like receptor 2 (TLR2), TLR3 and TLR4 blocking antibodies. ODNs were synthesized by MB Mission Biotech (Taipei, Taiwan). The stimulatory CpG-ODN 2216 contains the sequence 5′-GGG GGA CGA TCG TCG GGG GG-3′. The inhibitory CpG-ODN contains the sequence 5′-TTT AGG GTT AGG GTT AGG GTT AGG G-3′. The underlined bases were thioate stabilized.17
Induction of cytokines by PBMCs and in vitro IFN-α production-blocking test
PBMCs (2×106 cells/ml) were cocultured with and without VZV at 1∶20 (v/v) or stimulatory CpG ODN 2216 (5 µg/ml) in 24-well culture plates with a total solution of 1 ml. The cells were then incubated in a humidified atmosphere of 5% CO2 at 37 °C. Supernatants collected from parallel cultures of non-stimulated and antigen-stimulated PBMCs were aliquoted and stored at −80 °C until studied. We collected the supernatants 48 h after culture for measurement of IFN-α production using an ELISA (Endogen, Inc., Woburn, MA, USA). For the in vitro IFN-α production blocking test, PBMCs (2×106 cells/ml) were pre-incubated with and without wedelolactone 10 µM (IκB kinase (IKK) inhibitor), SB239063 10 µM (p38 inhibitor), U0126 10 µM (extracellular signal-regulated kinase (ERK) inhibitor), SP600125 10 µM (c-Jun N-terminal kinase (JNK) inhibitor), 2AP 50 µM and 1 mM (dsRNA-dependent protein kinase (PKR) inhibitor), or DMNB 10 µM and 100 µM (DNA-dependent protein kinase (DNA-PK) inhibitor) for 30 min or inhibitory CpG ODN (100 µg/ml) for 60 min and then cocultured with and without VZV (or UV-inactivated VZV) at 1∶20 (v/v) in 24-well culture plates with a total volume of 1 ml.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test
The assay was performed using MTT. Live but not dead cells reduced MTT to a blue formazan product using mitochondrial dehydrogenases. Briefly, 106 cells/well were seeded in 96-well culture plates and cultured with and without various chemical reagents. The MTT (Sigma) solution was prepared in RPMI without phenol red. Following exposure of the cells to the reagents, MTT solution was added, the cells were incubated for 3 h at 37 °C, and then dimethyl sulfoxide (Sigma) was added. Absorbency was then measured at 550 nm with an ELISA reader (Dynatech, Marmes-la-Coquette, France).
Western analysis
PBMCs (107 cells) cocultured with and without VZV at 37 °C were pelleted and lysed in 100 µl of sodium dodecyl sulfate buffer (0.5% sodium dodecyl sulfate, 0.05 M Tris 7.5 and 1 mM dithiothreitol) at different time points. Twenty micrograms of cell extract was resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis, and the protein gel was transferred to a nitrocellulose membrane. Monoclonal antibodies against human phospho-PKR (p-PKR), total PKR (Cell Signaling Technology, Inc., Beverly, MA, USA) and actin (Zymed Laboratories Inc., South San Francisco, CA, USA) were used as first antibodies to recognize p-PKR, total PKR and actin. An anti-rabbit IgG peroxidase-labeled antibody (Amersham; dilution, 1∶2000 in 5% milk) was used to visualize the first antibodies.
Cell subset isolation and IFN-α production
Positively selected helper T cells, cytotoxic T cells, natural killer cells, B-cells, pDCs and mDCs were isolated from PBMCs by labeling cells with CD4, CD8, CD56, CD19, BDCA-4 and BDCA-1 isolation kits (Miltenyi, Bergisch Gladbach, Germany), respectively, followed by positive magnetic sorting via an autoMACS (Miltenyi) magnetic cell separation instrument according to the manufacturer's instructions. The purity of isolated cells was further confirmed by flow cytometry and all samples were revealed to be more than 90% pure. A different population of cells was then suspended at 2×l06 cells/ml and cocultured with and without VZV at 1∶20 (v/v) for 48 h. The culture supernatants were collected and subjected to IFN-α detection.
Intracellular cytokine detection by fluorescence-activated cell sorting (FACS)
The intracellular cytokine detection procedure was performed according to the BD Fixation/Permeabilization Solution Kit with BD GolgiPlug manual. In brief, PBMCs were stimulated with or without VZV for 16 or 24 h, as indicated. The BD GolgiPlug reagent was added 8 h before staining for intracellular cytokines. At the indicated time points, cells were treated with autologous serum for 15 min at 4 °C to block Fc receptors. Then the surface was stained with phycoerythrin (PE)-labeled anti-CD4, anti-CD8, anti-CD14, anti-CD19, anti-CD56 antibodies (Abs) (BD Bioscience, Immunocytometry systems, San Jose, CA, USA), or biotin-labeled anti-CD1c (Miltenyi) and then fixed for 20 min in Cytofix/Cytoperm solution at 4 °C. Following fixation with the fixation/permeabilization solution, cells were washed twice and then resuspended in 100 µl of Perm/Wash buffer. The fixed and permeabilized cells were then stained with sheep Abs specific for human IFN-α (Endogen, Inc., Woburn, MA, USA). After a 1-h incubation at 4 °C, the cells were washed twice in permeabilization buffer and then stained with FITC-labeled anti-sheep Abs. For CD1c staining, another 25 µl of PE-Cy5-labeled streptavidin (0.2 µg/ml, final; BD PharMingen) was added, followed by another washing. Cells were incubated at room temperature for 30 min and then washed twice by centrifugation. For pDC staining, PBMCs were surface stained with FITC-labeled lineage cocktail (Lin), which contained antibodies to CD3, CD14, CD16, CD19, CD20, CD56 (BD Bioscience), PE-Texas Red-labeled anti-HLA-DR (Immunotech, A Beckman Coulter Company, Marseille, France), and PE-Cy5-labeled anti-CD123 (BD Bioscience). The pDCs were determined with Lin−/HLA-DR+/CD123bright. After fixation and permeabilization, cells were stained with sheep Abs specific for human IFN-α followed by PE-labeled anti-sheep Abs. Flow cytometry was performed using a FACS Calibur instrument (BD Biosciences) and analyzed using the accompanying CellQuest Pro software.
Statistics
Data presented are expressed as mean±standard error. Statistical comparisons were calculated by Student's t-test. A P value of <0.05 was regarded as significant. All statistical tests were performed using SPSS 12.0 for Windows XP (SPSS, Inc., Chicago, IL, USA).
Results
TLR9-dependent and -independent signals were involved in VZV-induced IFN-α production by PBMCs
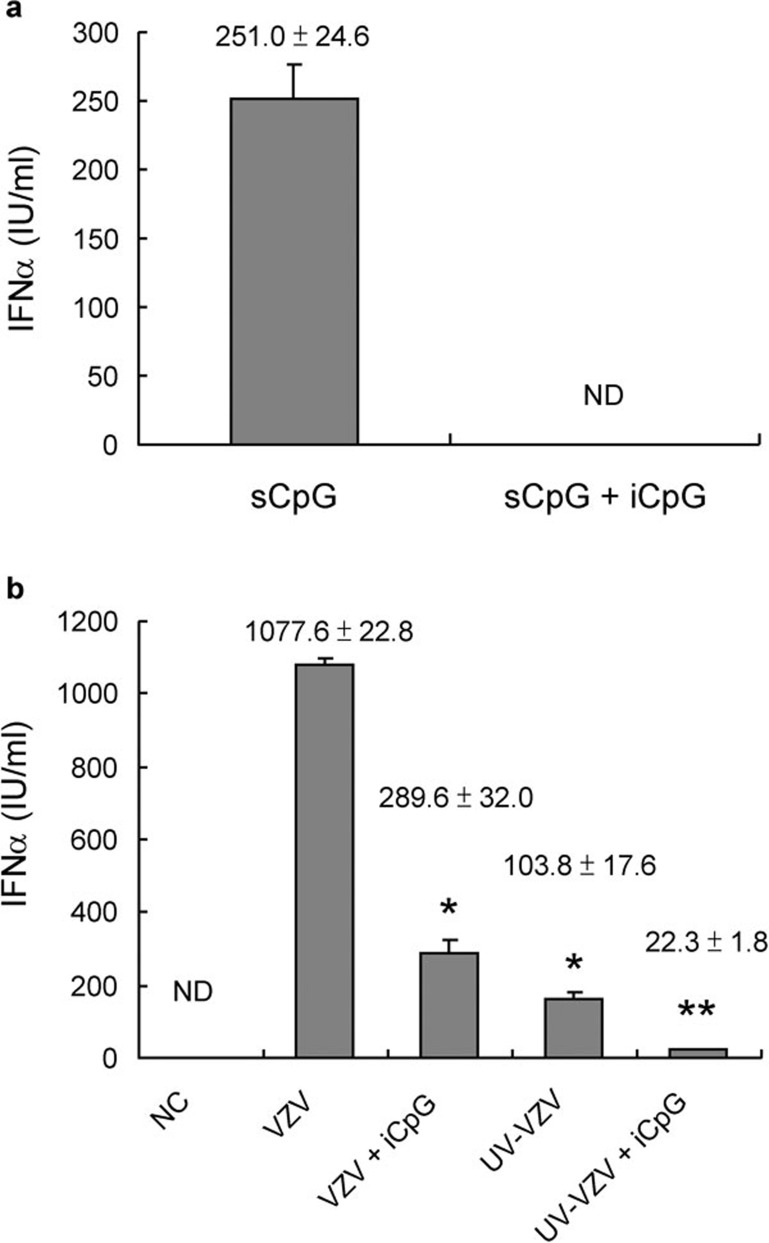
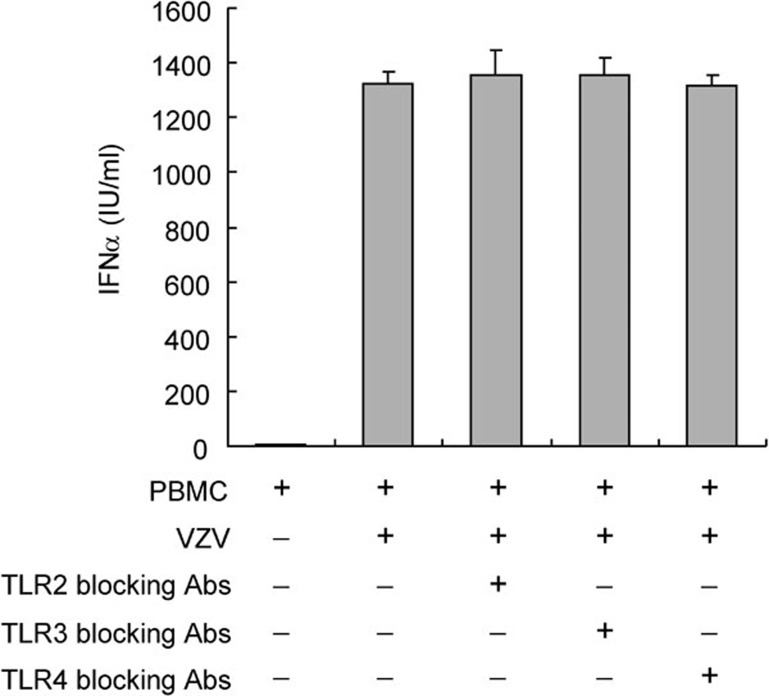
In a previous study, we tested the time course of IFN-α production.14 We found that PBMCs produce large amounts of IFN-α 48 h after VZV infection. Therefore, we used this time point for IFN-α detection in the following experiments. Studies were first performed to investigate whether PBMCs produce IFN-α upon VZV stimulation similar to other DNA viruses, through TLR9. Because inhibitory CpG ODN is a specific TLR9 inhibitor, it inhibited the IFN-α production induced by stimulatory CpG ODN (Figure 1a). As shown in Figure 1b, inhibitory CpG ODN partly inhibited the IFN-α production induced by VZV. This implies that VZV partially induces PBMCs to produce IFN-α through the TLR9 receptor-dependent pathway. UV light-inactivated VZV was then used to see if viral replication was needed for IFN-α production. We found that UV light-inactivated VZV induced less IFN-α than active VZV. The lowest IFN-α production took place after PBMCs were treated with UV light-inactivated VZV and inhibitory CpG ODN (Figure 1b). These data suggest that both TLR9-dependent and -independent signals are involved in VZV-induced IFN-α production by PBMCs. Viral replication was necessary in the VZV-induced TLR9-independent pathway. TLR2, TLR3 and TLR4 were not involved in the VZV-induced IFN-α production because TLR2, TLR3 and TLR4 blocking antibodies failed to block IFN-α production (Figure 2).
Figure 1.
TLR9-dependent and -independent signals involved in VZV-induced IFN-α production. (a) The IFN-α production of PBMCs induced by TLR9 agonist (sCpG) was inhibited by iCpG. (b) The IFN-α production of PBMCs induced by VZV was partially inhibited by inhibitory CpG and UV light inactivation. (sCpG: 5 µg/ml; iCpG: 100 µg/ml) (UV crosslinker 1 J/cm2×5 min) *P<0.01 VZV versus VZV+iCpG and VZV versus UV-VZV, **P<0.05 VZV+iCpG versus UV-VZV+iCpG and UV-VZV versus UV-VZV+iCpG. Data presented were calculated from six replicated experiments. iCpG, inhibitory CpG; IFN, interferon; NC, negative control; ND, non-detectable; PBMC, peripheral blood mononuclear cell; sCpG, stimulatory CpG; TLR, Toll-like receptor; VZV, varicella-zoster virus.
Figure 2.
TLR2, TLR3 and TLR4 are not involved in VZV-induced IFN-α production. PBMCs (2×106 cells/ml) were pre-incubated with and without TLR2, TLR3 or TLR4 blocking antibodies (1 µg/ml) for 30 min and then cocultured with VZV. Supernatants collected 48 h after stimulation were subjected to IFN-α detection. The IFN-α production of PBMCs induced by VZV was not inhibited by TLR2, TLR3 or TLR4 blocking antibodies. Abs, antibodies; IFN, interferon; PBMC, peripheral blood mononuclear cell; TLR, Toll-like receptor; VZV, varicella-zoster virus.
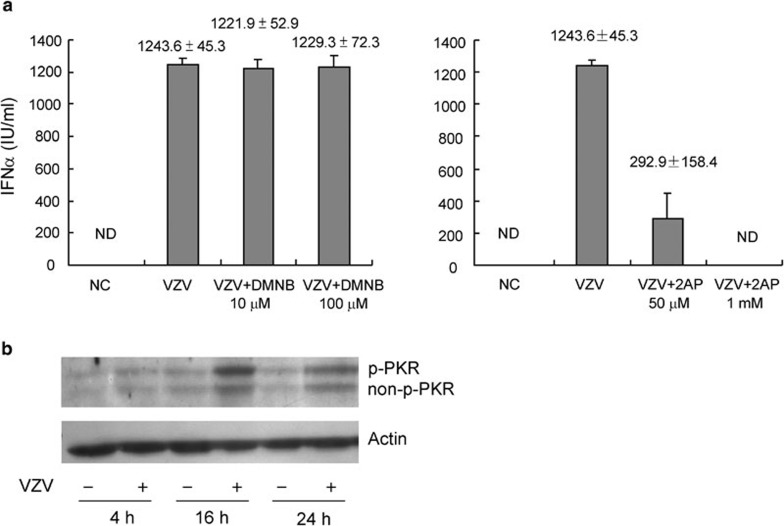
PKR but not DNA-PK was involved in IFN-α production following TLR9-independent VZV infection
PKR is involved in the induction of IFN-α by dsRNA and many other viral infections.18, 19, 20 To discover whether PKR or DNA-PK was involved in IFN-α production, PBMCs stimulated with VZV were cocultured with and without 2AP (PKR inhibitor) or DMNB (DNA-PK inhibitor) for 48 h. We found that 2AP, but not DMNB, could block VZV-induced IFN-α production (Figure 3a). An MTT test was performed under the same conditions to exclude drug toxicity, and cell viability was not decreased by the addition of 2AP or DMNB (data not shown). In another experiment, PBMCs collected at different time points after stimulation with and without VZV were subjected to western blot analysis. Non-p-PKR and p-PKR were both increased as PBMCs were stimulated with VZV for 16 and 24 h (Figure 3b). Thus, PKR, but not DNA-PK, was involved in IFN-α production by PBMCs upon VZV infection.
Figure 3.
PKR but not DNA-PK was involved in IFN-α production by PBMCs upon VZV infection. (a) PBMCs were pre-incubated with and without DMNB or 2AP for 30 min and then cocultured with VZV. Supernatants were collected 48 h after stimulation and subjected to detection of IFN-α. The IFN-α production of PBMCs induced by VZV could be partially inhibited by 2AP, but not by DMNB. Data presented were calculated from six replicated experiments. (b) Proteins of PBMCs collected at different time points after stimulation with and without VZV were subjected to western blot. Total PKR and p-PKR were increased as PBMCs were stimulated with VZV. Plots represent three replicated experiments. DMNB, 4,5-dimethoxy-2-nitrobenzaldehyde; DNA-PK, DNA-dependent protein kinase; IFN, interferon; NC, negative control; ND, non-detectable; PBMC, peripheral blood mononuclear cell; PKR, double-stranded RNA-dependent protein kinase; p-PKR, phospho-PKR; VZV, varicella-zoster virus; 2AP, 2-aminopurine.
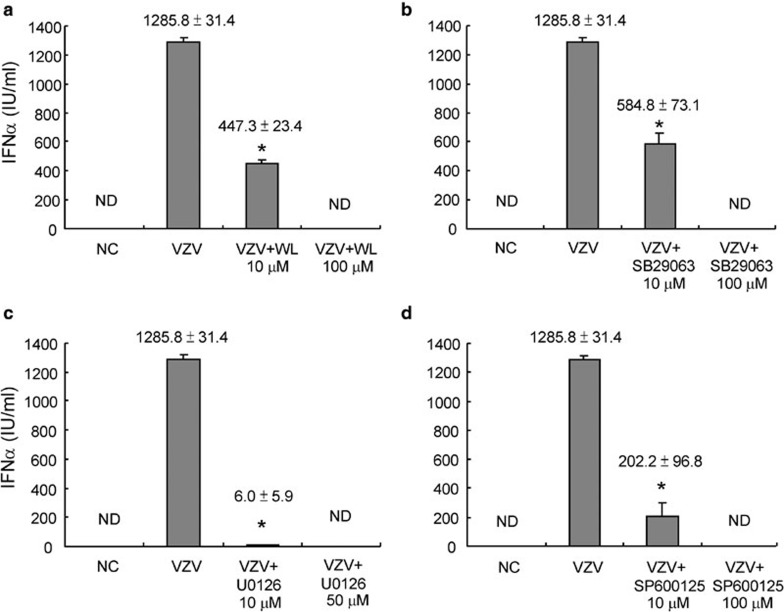
The induction of IFN-α by VZV needed activation of NF-κB and other mitogen-activated protein kinases (MAPKs)
Experiments were then performed to explore whether NF-κB and other MAPKs were also important for the induction of IFN-α after VZV infection. PBMCs were stimulated with VZV with and without IKK, p38, ERK and JNK inhibitors for 48 h. Then the supernatant was subjected to IFN-α detection. It was found that the IFN-α production induced by VZV infection could be partially blocked by IKK, p38, ERK and JNK inhibitors at lower doses, but totally blocked by these inhibitors at higher doses (Figure 4). This finding suggests that they are all major mediators in VZV-induced IFN-α secretion.
Figure 4.
The transcription factor NF-κB and other MAPKs were all important for IFN-α production by PBMCs induced by VZV. PBMCs (2×106 cells/ml) were pre-incubated with and without WL (10 µM), SB239063 (10 µM), U0126 (10 µM) or SP600125 (10 µM) for 30 min and then cocultured with VZV. Supernatants were collected 48 h after stimulation. The culture supernatants were subjected to detection of IFN-α using a commercial ELISA kit. The IFN-α production of PBMCs induced by VZV could be partially inhibited by selective (a) IKK, (b) p38, (c) ERK and (d) JNK inhibitors. *P<0.01 VZV versus VZV+inhibitor. Data presented were calculated from six replicated experiments. ERK, extracellular signal-regulated kinase; IFN, interferon; IKK, IκB kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; NC, negative control; ND, non-detectable; PBMC, peripheral blood mononuclear cell; VZV, varicella-zoster virus; WL, wedelolactone.
An MTT test was performed under the same conditions to exclude drug toxicity, and cell viability was not decreased by these MAPK inhibitors (data not shown). This suggests that NF-κB and other MAPKs are all very important for IFN-α production by PBMCs after VZV infection.
pDCs were responsible for IFN-α production in PBMCs during VZV infection
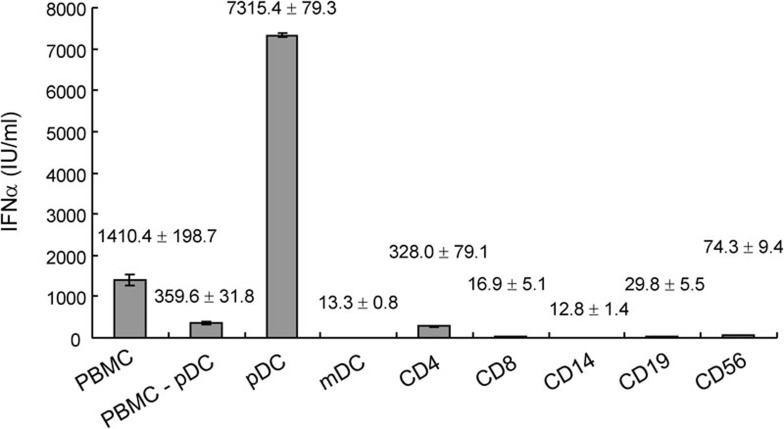
Studies were then performed to discover what kind of cell(s) play a major role in the IFN-α production of PBMCs upon VZV stimulation. First, different cell populations in PBMCs were isolated with a magnetic cell separation instrument and stimulated with VZV for 48 h. The supernatant was then harvested for IFN-α detection. From the ELISA data, we found that pDCs were responsible for the largest IFN-α production (Figure 5).
Figure 5.
pDC response for IFN-α production in PBMCs during VZV infection with ELISA detection. Different cell populations in PBMCs were isolated with autoMACS system, suspended at 2×106 cells/ml and stimulated with VZV for 48 h. The supernatant was collected and subjected to IFN-α detection with ELISA. The pDCs were responsible for the largest IFN-α production in PBMCs. Data presented were calculated from six replicated experiments. IFN, interferon; PBMC, peripheral blood mononuclear cell; pDC, plasmacytoid dendritic cell; VZV, varicella-zoster virus.
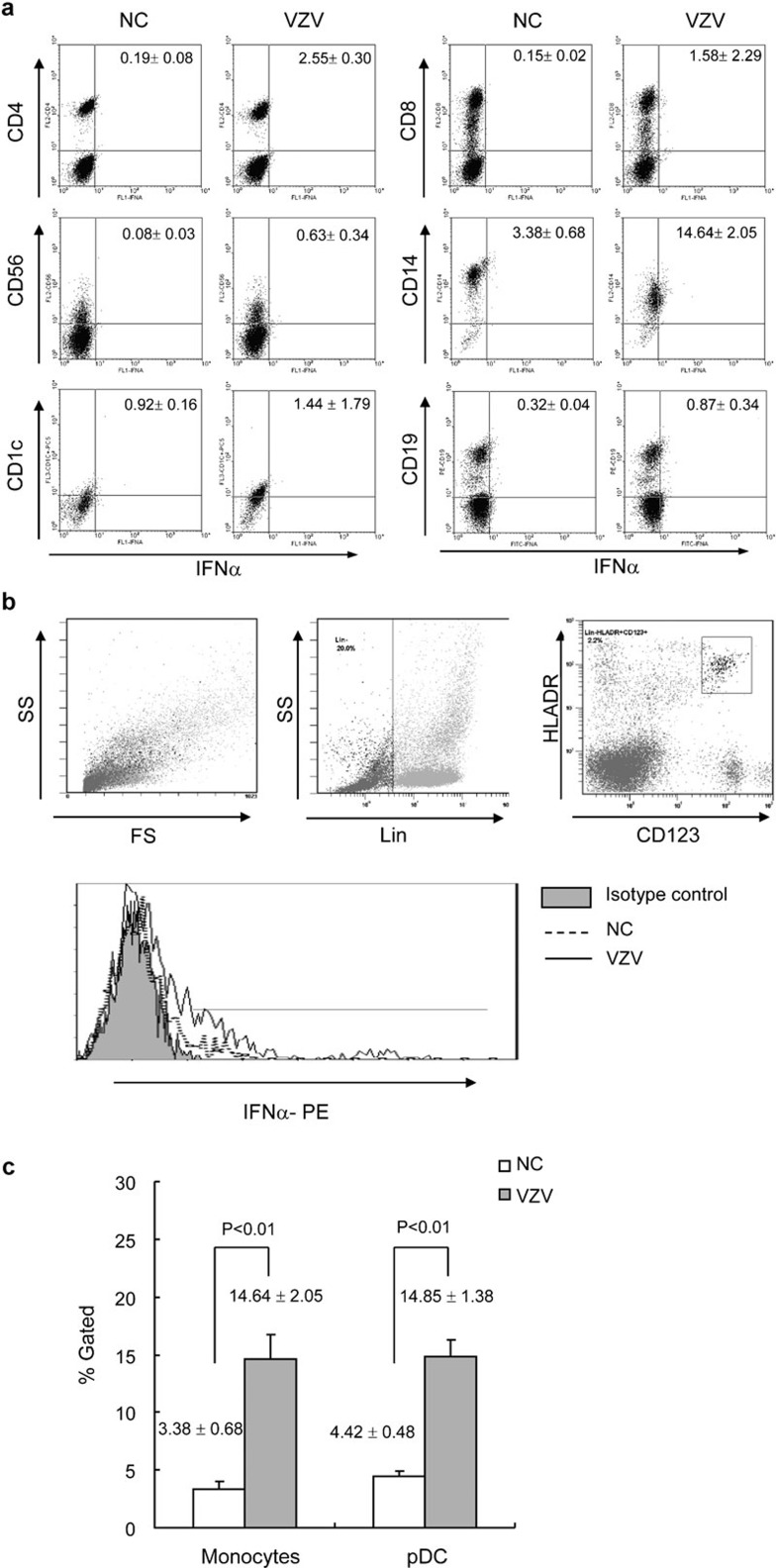
Sorting PBMCs into individual populations prior to stimulation removed the opportunity for different kinds of cells to interact. In an effort to be physiologically oriented, we used an intracellular cytokine staining approach to directly determine which cell types expressed IFN-α in response to VZV stimulation in PBMCs. pDCs and mDCs were identified based on Lin−/HLA-DR+/CD123bright and CD1c expressions, respectively. Figure 6 illustrates the VZV-stimulated IFN-α expression in FACS-gated CD4+, CD8+ T cells, natural killer cells (CD56+), monocytes (CD14+), mDCs (CD1c+) (Figure 6a) and pDCs (Lin−/HLA-DR+/CD123bright) (Figure 6b) harvested 24 h after VZV infection. The intracellular cytokine staining indicated that pDCs and monocytes were responsible for the majority of IFN-α production upon stimulation with VZV (Figure 6). Thus, it seemed as though monocytes could not produce IFN-α independently during VZV stimulation.
Figure 6.
VZV-induced IFN-α expressed by innate and adaptive immune cell populations. (a) PBMCs were stimulated with and without VZV for 16 h, BD GolgiPlug reagent was added for another 8 h, and then samples were stained with PE- or PC5-labeled Abs specific for the indicated cell surface markers, permeabilized, and then stained with sheep Abs specific for human IFN-α, followed by FITC-labeled anti-sheep Abs. The FACS plots shown represent the VZV-induced IFN-α staining in the indicated gated cell types. The cells shown on the dot plots of CD14 were gated on monocytes and on PBMCs by forward sideward scatter characteristics. Unstimulated control stains showed <3.5% cytokine staining in all cell types examined. The values shown in the upper right quadrant indicate the percentage of cytokine-positive cells. The results presented are representative of six replicated experiments. (b) The pDCs were determined with Lin−/HLA-DR+/CD123bright. After fixation and permeabilization, cells were stained with sheep Abs specific for human IFN-α, followed by PE-labeled anti-sheep Abs. (c) Plots illustrate IFN-α expression levels in VZV-stimulated gated monocytes (CD14+) and pDCs (Lin−/HLA-DR+/CD123bright) from six replicated experiments. Abs, antibodies; FACS, fluorescence-activated cell sorting; IFN, interferon; NC, negative control; PBMC, peripheral blood mononuclear cell; pDC, plasmacytoid dendritic cell; PE, phycoerythrin; VZV, varicella-zoster virus.
Discussion
Different antigen-presenting cell subsets express different receptors for microbial products. This variation enables the innate immune system to initiate responses to a large variety of microbes and generate different immune responses to a single microbe, thus increasing the chances of successfully controlling a microbial invasion. TLRs play an essential role in the recognition of microbial components. Recent studies have shown that TLRs detect specific molecules, including lipopolysaccharide (detected by TLR4), bacterial lipoproteins, lipopolytricholic acids (detected by TLR2), flagellin (detected by TLR5), the unmethylated CpG ODNs (oligodeoxynucleotides containing unmethylated CpG motifs) of bacteria and viruses (detected by TLR9), dsRNA (detected by TLR3) and single-stranded viral RNA (detected by TLR7).21, 22, 23, 24 In addition to ligand specificity, individual TLRs differ in their expression patterns and the signal transduction pathways they activate. It has been demonstrated that monocytes preferentially express TLRs 1, 2, 4, 5, 6, 7 and 8, whereas pDCs express TLRs 1, 6, 7 and 9. The mDCs express TLRs 1, 2, 3, 5, 6, 7 and 8. A given DC population will respond only to the pathogens for which they have corresponding TLRs.25 This specificity suggests that DC populations are specialized to deal with certain types of pathogens. Furthermore, signaling through a certain TLR can result in a distinct outcome, even if another TLR is triggered by the same ligand.
In this study, we have investigated the IFN-α production pathway of PBMCs following stimulation by VZV infection. Results of intracellular staining, but not cell sorting, experiments showed that both pDCs and monocytes respond with IFN-α production. Because different cell types might operate dependently, monocytes may need other (unknown) signals for IFN-α production during VZV stimulation.
The pathogenesis of primary VZV infection was previously presumed to occur after infection of mononuclear cells in the regional lymph nodes about 4 to 6 days after inoculation of the respiratory mucosa.26 This infection is followed by a primary viremic phase, with seeding of the reticuloendothelial system. After virus replication, a secondary phase of viremia occurs at about day 9, resulting in VZV transport to the skin.27 The vesicular rash usually develops 10 to 21 days after exposure.28, 29 However, recent experiments in severely immunodeficient mice with human skin grafts support the idea that infected T cells have the potential to transport VZV to the skin during primary viremia. These studies also suggest that the prolonged interval between exposure and the appearance of varicella skin lesions reflects the time required for VZV to overcome an IFN-α-mediated innate immune barrier produced directly by epidermal cells.30, 31 In our study, we found that rapid activation of an IFN-α-mediated innate immune response produced by pDCs in PBMCs might contribute to the limited spread of viremia.
With regard to inhibitory CpG ODN and UV-inactivated VZV, we found that both TLR9-dependent and -independent signals were involved in VZV-induced IFN-α production by PBMCs. This phenomenon has also been observed in herpes simplex virus infection in a murine system.18, 32 More evidence of TLR-independent recognition of RNA and DNA has been noted recently. dsRNA has been shown to be recognized by the cytoplasmic helicase domain of the helicase protein retinoic acid-induced gene 1.33, 34, 35 In contrast to TLR-dependent IFN-α production in pDCs, retinoic acid-induced gene 1 plays an essential role in the antiviral response of many other cell types.36, 37 Although DNA could be sensed partly in a TLR-independent manner,32, 38, 39 the mechanisms of intracellular recognition of DNA have still not been fully clarified.
DNA-PK is a serine/threonine protein kinase. DNA-PK has been reported to be a signaling molecule for CpG DNA-induced immune responses.40, 41 Phosphorylation of IFN regulatory factor-3, one of the proteins involved in sensing viral infection and triggering the production of IFNs, by DNA-PK after Sendai virus infection has also been reported.42 However, recent reports have shown that DNA-PK is not essential for CpG DNA responses.43, 44 Our results were consistent with the latter reports, and DNA-PK seems to be dispensable for VZV-induced IFN-α production. In contrast to DNA-PK, PKR was involved in VZV-induced IFN-α production in PBMCs. Figure 3b shows two bands in the immunoblot of total PKR. The upper band should be p-PKR and the lower band non-p-PKR. With VZV stimulation, there was an obvious increase in abundance of both p-PKR and non-p-PKR. This suggests that both phosphorylated-PKR and non-phosphorylated-PKR are increased by VZV stimulation. Thus, VZV infection might influence the transcriptional or post-translational process of PKR expression. PKR is involved in the induction of IFN-α by dsRNA and many other viral infections, such as herpes simplex virus.18, 19, 20 It has been reported that replication and transcription of the viral genome of single-stranded viral RNA viruses and many DNA viruses often lead to the formation of cytosolic dsRNA.45, 46 Thus, dsRNA could induce PKR activation. Recently, 2AP was suggested to have suppressive effect on other kinases;47 therefore, further studies to explore the roles of PKR and other kinases in VZV infection are needed.
In a previous report, VZV was noted to activate IL-6 in monocytes and macrophages via TLR2.48 Our results, however, suggested a pathway different from the previously described TLR2 activation by VZV. We found that TLR2, TLR3 and TLR4 were not involved in the IFN-α production induced by VZV infection. The difference might be due to cell type-specific and cytokine-specific induction pathways. Similar discrepancies between different kinds of cytokine induction have also been reported in herpes simplex virus-infected murine macrophages.18 The reason for this cytokine specificity could be the differential expression of receptors or a different intrinsic ability to respond to stimuli from different antigens. However, the results of this study cannot exclude the role of TLR7 in VZV-infected pDC. The role of TLR7 in VZV-infected human pDC needs further studies.
In conclusion, we found that both TLR9-dependent and -independent signals were involved in VZV-induced IFN-α production. We also found that pDCs were the major cell type involved in IFN-α production in PBMCs. These findings have not been mentioned in previous reports. With an enhanced understanding of the defense mechanisms of a host against an organism, we can develop better strategies for infection control and vaccine development.
Acknowledgments
This study was supported in part by grants NSC 94-2314-B-182A-101 (H R Yu) and NSC 98-2314-B-182A-004-MY3 (H R Yu) from the National Science Council, Taiwan.
References
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R. Control of adaptive immune responses by Toll-like receptors. Curr Opin Immunol. 2002;14:380–383. doi: 10.1016/s0952-7915(02)00343-6. [DOI] [PubMed] [Google Scholar]
- O'Doherty U, Peng UM, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, et al. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–2778. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YL, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Hagihara M, Okamoto A, Higuchi A, Tanabe A, Hirabayashi K, et al. Frequencies of dendritic cells (myeloid DC and plasmacytoid DC) and their ratio reduced in pregnant women: comparison with umbilical cord blood and normal healthy adults. Hum Immunol. 2003;64:1144–1151. doi: 10.1016/j.humimm.2003.08.342. [DOI] [PubMed] [Google Scholar]
- Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Feldman SB, Ferraro M, Zheng HM, Patel N, Gould-Fogerite S, Fitzgerald-Bocarsly P. Viral induction of low frequency interferon-alpha producing cells. Virology. 1994;204:1–7. doi: 10.1006/viro.1994.1504. [DOI] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Straus SE, Aulakh HS, Ruyechan WT, Hay J, Casey TA, Vande Woude GF, et al. Structure of varicella-zoster virus DNA. J Virol. 1981;40:516–525. doi: 10.1128/jvi.40.2.516-525.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HR, Chen RF, Hong KC, Bong CN, Lee WI, Kuo HC, et al. IL-12 independent polarization of Th1 reaction in human mononuclear cells with varicella-zoster virus infection. Eur J Immunol. 2005;35:3664–3672. doi: 10.1002/eji.200526258. [DOI] [PubMed] [Google Scholar]
- Yu HR, Chang JC, Chen RF, Chuang H, Hong KC, Wang L, et al. Different antigens trigger different Th1/Th2 reactions in neonatal mononuclear cells (MNCs) relating to T-bet/GATA-3 expression. J Leukoc Biol. 2003;74:952–958. doi: 10.1189/jlb.0902474. [DOI] [PubMed] [Google Scholar]
- Yang KD, Liou WY, Lee CS, Chu ML, Shaio MF. Effects of phenobarbitol on leukocyte activation: membrane potential, actin polymerization, chemotaxis, respiratory burst, cytokine production, and lymphocyte proliferation. J Leukoc Biol. 1992;52:151–156. doi: 10.1002/jlb.52.2.151. [DOI] [PubMed] [Google Scholar]
- Ashman RF, Lenert P. Structural requirements and applications of inhibitory oligodeoxyribonucleotides. Immunol Res. 2007;39:4–14. doi: 10.1007/s12026-007-0065-4. [DOI] [PubMed] [Google Scholar]
- Malmgaard L, Melchjorsen J, Bowie AG, Mogensen SC, Paludan SR. Viral activation of macrophages through TLR-dependent and -independent pathways. J Immunol. 2004;173:6890–6898. doi: 10.4049/jimmunol.173.11.6890. [DOI] [PubMed] [Google Scholar]
- Der SD, Lau AS. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc Natl Acad Sci USA. 1995;92:8841–8845. doi: 10.1073/pnas.92.19.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WM, Ostertag D, Li ZW, Chang L, Chen Y, Hu Y, et al. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity. 1999;11:721–731. doi: 10.1016/s1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Brunell PA, Straus SE, Krause PR. Recent advances in varicella-zoster virus infection. Ann Intern Med. 1999;130:922–932. doi: 10.7326/0003-4819-130-11-199906010-00017. [DOI] [PubMed] [Google Scholar]
- Lin TY, Huang YC, Ning HC, Hsueh C. Oral acyclovir prophylaxis of varicella after intimate contact. Pediatr Infect Dis. 1997;16:1162–1165. doi: 10.1097/00006454-199712000-00012. [DOI] [PubMed] [Google Scholar]
- Grose C. Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics. 1981;68:735–737. [PubMed] [Google Scholar]
- Ozaki T, Ichikawa T, Matsui Y, Nagai T, Asano Y, Yamanishi K, et al. Viremic phase in nonimmunocompromised children with varicella. J Pediatr. 1984;104:85–87. doi: 10.1016/s0022-3476(84)80596-x. [DOI] [PubMed] [Google Scholar]
- Ku CC, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. Varicella-zoster virus transfer to skin by T Cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med. 2004;200:917–925. doi: 10.1084/jem.20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CC, Besser J, Abendroth A, Grose C, Arvin AM. Varicella-Zoster virus pathogenesis and immunobiology: new concepts emerging from investigations with the SCIDhu mouse model. J Virol. 2005;79:2651–2658. doi: 10.1128/JVI.79.5.2651-2658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, et al. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci USA. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim MH. RIG-I: an essential regulator of virus-induced interferon production. J Hepatol. 2005;42:431–433. doi: 10.1016/j.jhep.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Wagner H, Bauer S. All is not Toll: new pathways in DNA recognition. J Exp Med. 2006;203:265–268. doi: 10.1084/jem.20052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202:1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W, Gong X, Li Z, Takabayashi K, Ouyang H, Chen Y, et al. DNA-PKcs is required for activation of innate immunity by immunostimulatory DNA. Cell. 2000;103:909–918. doi: 10.1016/s0092-8674(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Dragoi AM, Fu X, Ivanov S, Zhang P, Sheng L, Wu D, et al. DNA-PKcs, but not TLR9, is required for activation of Akt by CpG-DNA. EMBO J. 2005;24:779–789. doi: 10.1038/sj.emboj.7600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova AY, Trost M, Murray JM, Cantley LC, Howley PM. Interferon regulatory factor-3 is an in vivo target of DNA-PK. Proc Natl Acad Sci USA. 2002;99:2818–2823. doi: 10.1073/pnas.052713899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Kaisho T, Takeda K, Akira S. The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J Immunol. 2003;170:3059–3064. doi: 10.4049/jimmunol.170.6.3059. [DOI] [PubMed] [Google Scholar]
- Ishii KJ, Takeshita F, Gursel I, Gursel M, Conover J, Nussenzweig A, et al. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J Exp Med. 2002;196:269–274. doi: 10.1084/jem.20020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, et al. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Langland JO. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology. 1996;219:339–349. doi: 10.1006/viro.1996.0259. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Williams BR, Miller SD. Distinct roles of protein kinase R and Toll-like receptor 3 in the activation of astrocytes by viral stimuli. Glia. 2007;55:239–252. doi: 10.1002/glia.20450. [DOI] [PubMed] [Google Scholar]
- Wang JP, Kurt-Jones EA, Shin OS, Manchak MD, Levin MJ, Finberg RW. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J Virol. 2005;79:12658–12666. doi: 10.1128/JVI.79.20.12658-12666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]