Abstract
T helper 2 (Th2) polarization is a major pathological feature in allergic diseases; its etiology is not fully understood. This study aims to elucidate the adjuvant effect of the microbial product-derived small peptides in the initiation of antigen-specific Th2 polarization. In this study, a clinical survey of patients with chronic rhinosinusitis (CRS) and food allergy (FA) was carried out. The Staphylococcal enterotoxin B (SEB)-derived small peptides (Ssps) were examined in the human stool extracts. The formation of Ssp/antigen adducts was tested in a protein–protein combination assay. The bone marrow-derived dendritic cells (BMDCs) were employed to test the role of Ssp/ovalbumin (OVA) adducts in the dendritic cell (DC) maturation. A mouse model was developed to test the role of Ssp/OVA adducts in the initiation of Th2 polarization in the intestine. The results showed that 54 (18.2%) patients with FA were diagnosed among 296 patients with SEB+ CRS; only eight (2.9%) FA patients were identified among 272 patients with SEB− CRS. Ssps were detected in the stool protein extracts from FA patients with SEB+ CRS, but not in those with SEB− CRS. Ssp/OVA adducts induced DC maturation, speeded up DC migration, activated CD4+ T cells in the regional lymph nodes and induced skewed Th2 polarization in the local tissue. We conclude that patients with SEB+ CRS are prone to suffering from FA. SEB can be degraded to Ssps in the gastrointestinal tract. The Ssps can bind macromolecular antigens to form adducts to promote the antigenicity of the antigens and induction of the antigen-specific Th2 polarization and inflammation in the local tissue.
Keywords: intestine, food allergy, hapten, microbial products, immunization
Introduction
The prevalence of allergic disease has rapidly increased over the past several decades. The pathogenesis of allergy is unclear. One of the major pathological features of allergy is the skewed antigen-specific T helper 2 (Th2) polarization in the local tissue.1 How the local tissue is sensitized to specific antigens has not yet been fully understood. Based on animal model studies, the ingestion of a purified antigen, such as ovalbumin (OVA), generally induces the immune tolerance to the specific antigen,2 while exposure to a mixture of an antigen and an adjuvant induces sensitization to the specific antigen.3 Therefore, it seems that the presence or absence of adjuvants is a checkpoint for the induction of immune tolerance or sensitization; the underlying mechanisms have not yet been fully elucidated.
Adjuvants are substances that can promote immune responses. They can be mixed with vaccines to strengthen the immune reaction in the recipients to a specific antigen.4 Aluminum hydroxide is an adjuvant that has been used in human vaccination as well as animal studies.5,6,7 Other reported adjuvants include tetanus, diphtheria, pertussis vaccine and Staphylococcal enterotoxin B (SEB), for example. One of the mechanisms by which adjuvants facilitate the immunization is that adjuvants can act as a depot for the specific antigen, presenting the antigen over a relatively long time, thus sustaining the providing antigenic stimulation before the body clears the antigen. On the other hand, haptens also act to promote the induction of immune responses.8
Some small molecules can covalently attach to protein molecules with large molecular weight; these small molecules are designated haptens; the large protein molecules are named carriers.9,10 Such a process may result in upregulation of the antigenicity of the carriers, or both of the carriers and haptens. A number of haptens have been recognized relating the pathogenesis of allergic diseases including some plant derivatives (such as urushiol11), some drugs (such as penicillin12) and chemicals (such as trinitrobenzene sulfonic acid13). The role of hapten in the animal model of allergic dermatitis has been well recognized,14 in which haptens fulfill the role of adjuvants. As aforementioned, some bacterial products are used as adjuvants in the establishment of animal models of allergic diseases.3,7,15 Whether the microbial products act in the same way as hapten is unknown. We previously observed that patients with chronic rhinosinusitis (CRS) and S. aureus infection were prone to suffer from immune disorders in the intestine.16,17 CRS is a common disease; the S. aureus infection is common in CRS. High levels of SEB were detected in the sinuses of patients with CRS.17,18 To understand the mechanism of sinusitis-derived SEB in the induction of intestinal allergy, we performed a clinical survey and found that among SEB+ CRS patients, significantly more food allergy (FA) patients were observed. The SEB-derived small peptides (Ssps) were identified in the stool of patients with both FA and CRS; the Ssps had a strong adjuvant property in the induction of intestinal allergy in an animal model.
Materials and methods
Human subjects
This study was carried out between 2005 and 2010. The hospitals where we recruited the patients, the diagnosis of CRS and the ethic statements are presented in the Supplementary Material.
Mice
BALB/c mice (6- to 8-week-old) were purchased from Charles River Laboratories (Saint-Constant, QC, Canada). TLR2−/− and C57BL/6J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained in a specific pathogen-free environment. The experimental procedures were approved by the Animal Care Committee at McMaster University.
Detection of Ssps in human stool samples
The stool samples were collected from patients with FA and CRS. The diagnostic procedures are presented in the Supplementary Material. The demographic data are presented in Supplementary Table 1. About 1 g of stool was collected from each subject. The stool was processed by the sulfate ammonium precipitation assay. Bio-Rad protein assay was used to determine the protein content. The usage of human specimens in the study was approved by the Human Research Ethic Committee at our universities.
Detection of SEB in the sinuses
The maxillary sinus punctuations via the inferior meatus were performed under local anesthesia. Proteins were extracted from the suctions from the sinuses and were further analyzed for the levels of SEB by ELISA.
Ammonium sulfate precipitation of protein from stool samples
The stool samples were resuspended in 0.1 M phosphate-buffered saline at 1 g/10 ml, shaked at 4 °C for 20 min and centrifuged at 17 500 r.p.m. for 15 min, and then the supernatant was collected and added saturated ammonium sulfate to saturation and then sterling slowly at 4 °C overnight. The precipitated pellets were resuspended in phosphate-buffered saline. The protein content was determined by a Bio-Rad protein assay.
Western blotting
A total of 50 µg denatured proteins was separated in 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and transferred onto nitrocellulose membrane. The membranes were then incubated with the primary antibodies (0.5–1 µg/ml) or isotype IgG (using as a negative control) overnight at 4 °C. Reactions were developed using the Pierce ECL chemiluminescence substrate kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). Results were recorded with X-ray film. Bands were semiquantified by densitometry. The intensity of the specific band was normalized to the intensity of the β-actin band.
SEB peptide sequencing
The peptide of SEB identified by Western blotting was cut from the gel and sequenced by mass spectrometry for the amino acid sequence with published procedures.19
Partial gastric digestion of SEB
A mimic gastric digestion was performed for SEB. Digestion mixtures consisted of 0.1 ml SEB (100 µg/ml) and 50 µl 0.4 M HCl with 8 g/l of NaCl and 6.8 g/l of pepsin. Digestion was performed at 37 °C for 15 min with shaking. The digestion was stopped with 40 µl of 0.3 M Na2CO3.
Some experimental procedures are presented in the Supplementary Material.
Results
SEB+ CRS correlates with FA
S. aureus infection is common in CRS.20 SEB is one of the virulence factors of S. aureus. Our previous studies indicate that SEB is associated with the pathogenesis of intestinal immune disorders.3,16 Thus, we carried out a clinical study to investigate the relation between CRS-derived SEB and FA. The results showed that 52.11% (296/568) CRS patients had SEB (635.74±157.43 pg/mg protein from sinus suction sample) in the secretions of the sinuses (group A); SEB was below the detectable levels in the other 272 (47.89%) patients (group B). Fifty-four patients in group A were diagnosed FA (54/296; 18.24%), while only eight patients in group B had FA (8/272; 2.94%), which was significantly less than that in group A (P<0.01; by χ2 test). The data imply that the sinusitis with S. aureus infection may contribute to the pathogenesis of FA.
Ssps bind to macromolecular protein antigens
Based on the consideration that the CRS-derived SEB might be swallowed down to the gastrointestinal tract, we measured the SEB levels in stool samples of the 51 FA patients with SEB+ CRS. The results showed that high levels of SEB (ranged from 35.5 to 228.4 pg/mg protein; averaged 108.5±26.4 pg/mg protein) were detected in those patients. In the stool samples obtained from the 45 non-CRS FA patients, the levels of SEB (from 0 to 18.6 pg/mg protein; averaged as 1.5±3.1 pg/mg protein) were significantly lower than patients with SEB+ CRS (P<0.01; by t-test).
The swallowed SEB may be degraded by the proteolytic enzymes in the gastrointestinal tract. We then collected stool samples from patients with both FA and CRS. Proteins were extracted from the stool samples and analyzed by Western blotting. The results showed that SEB was detected at 31 kDa in samples of stool and sinuses in patients with FA and CRS, but not in those with non-CRS and FA or healthy subjects. In addition, another positive band with a molecular weight about 2.5 kDa was detected in the samples from patients with both FA and SEB+ CRS, which was also detected in samples treated with gastric fluid assay (Figure 1a). The results imply that the CRS-derived SEB may be degraded into small peptides while passing through the gastrointestinal tract.
Figure 1.
Ssp binding assay. (a) Protein extracts were prepared from the stool or sinus discharge of patients with chronic sinusitis and food allergy. The samples were analyzed by Western blotting. The immune blots show SEB-positive staining. Source of samples: lanes 1 and 2: patients with food allergy and sinusitis; lane 3: patients with food allergy, no sinusitis; lane 4: healthy subjects; lane 5: gastric fluid assay; lane 6: isotype staining control. (b) The bars indicate the Ssp/OVA binding assay. SEB: SEB (2 ng/ml) was added. The data represent three experiments. The data in (b) were presented as mean±s.d. *P<0.05, compared with ‘SEB' group. OD, Optical density; OVA, ovalbumin; SEB, Staphylococcal enterotoxin B; Ssp, SEB-derived small peptide.
We noted that the molecular weight in several SEB-positive bands from the stool samples was about 2.5 kDa (Figure 1a). In contrast to most protein antigens, these short peptides of SEB can be designated ‘small' peptides. According to the definition of hapten, haptens are small molecules that can attach a large molecular carrier to form adducts.9 We postulated that the Ssps might have the properties of the hapten to bind macromolecular proteins covalently. To test the hypothesis, we analyzed the peptide at 2.5 kDa by mass spectrometry. The results showed that the peptide consisted of 21 amino acids, which corresponded to the 1–21 amino acids in the sequence of SEB. This Ssp was then synthesized (Peptide 2.0, Chantilly, VA, USA). Using OVA as a model antigen, we carried out a protein–protein combination assay. The results showed that the Ssp had an affinity to bind OVA (Figure 1b). The results from Ssp were further confirmed by Western blotting (Supplementary Figure 1).
Ssp/Ag adducts promote dendritic cell (DC) maturation
We next observed if Ssps facilitated the antigen uptake by DCs. Based on the properties of Ssps in binding macromolecular proteins, as shown in Figure 1b and Supplementary Figure 1, using OVA as a model antigen, we added OVA or Ssps/OVA adducts to the culture of bone marrow-derived dendritic cell (BMDC) at 37 °C for 6 h. As shown by flow cytometry, BMDCs did absorb the non-conjugated OVA, but at a low rate. In contrast, the absorption of Ssp/OVA by BMDC was significantly increased in an Ssp dose-dependent manner. Since SEB can activate the Toll-like receptor (TLR) 2,21,22 we wondered if TLR2 was involved in the absorption of Ssp/OVA. In separate experiments, we prepared BMDCs from TLR−/− mice and then exposed to Ssp/OVA adducts. Indeed, the absorption of Ssp/OVA was downregulated to the control level (Supplementary Figure 2).
To observe the effect of Ssp/OVA adducts on DC migration, BMDCs were generated and exposed to Ssp/OVA adducts in the culture for 48 h. As shown by flow cytometry, the levels of the maturation marker, CD80, CD83, CD86 and MHC II, were increased significantly. To elucidate the effect of TLR2 on the Ssp/OVA adduct-induced DC maturation, a batch of BMDC was isolated from TLR2−/− mice, and exposed to Ssp/OVA adducts. Indeed, the TLR2-deficient BMDCs showed similar results to control BMDCs (Figure 2). We also assessed the levels of IL-12 in the culture. The results showed that the Ssp/OVA adducts did not increase the production of IL-12 by the BMDCs (data not shown).
Figure 2.
Ssp/OVA adducts facilitate DC maturation. BMDCs were exposed to Ssp, or OVA, or Ssp/OVA adducts in the culture for 48 h. The BMDCs were collected and intracellularly stained with indicated antibodies and analyzed by flow cytometry. The histograms indicate the frequencies of CD80+, CD83+, CD86+ and MHC II+ cells in the BMDCs. *, BMDCs were isolated from TLR−/− mice; the rest BMDCs were isolated from B6 mice (the littermates). The data represent three experiments. BMDC, bone marrow-derived dendritic cell; DC, dendritic cell; MHC, major histocompatibility complex; OVA, ovalbumin; Ssp, Staphylococcal enterotoxin B-derived small peptide; TLR, Toll-like receptor.
Ssp/OVA adducts modulate immune responses
To determine whether the Ssp/OVA adducts could modify DC's functions in vivo, we treated naive B6 mice or TLR2-deficient mice by gavage-feeding with Ssp/OVA adducts or OVA alone. The mice were killed on the next day; the Mesentery lymph node (MLN) were excised; the FITC+ DCs in MLN were analyzed by flow cytometry. The results showed that 3.37%±1.2% OVA-containing DCs were counted in the MLN of mice fed with OVA alone (Supplementary Figure 3a), significantly more in mice fed with Ssp/OVA adducts (9.16%±2.5% Supplementary Figure 3b), and significantly less in TLR2-deficient mice (2.64%±1.1% Supplementary Figure 3c). The results indicate that Ssp/OVA adducts can promote the DC migration to the draining lymph nodes after capturing the antigen. TLR2 plays a critical role in the process.
Next we fed mice with OVA or Ssp/OVA adducts daily for 5 days. As analyzed by flow cytometry, the frequency of Th2 cells was increased while the frequencies of Th1 cells and Foxp3+ T regulatory cells were decreased significantly in the MLNs, which did not occur in TLR2-deficient mice (Figure 3). The results indicate that the Ssp/OVA can promote the Th2 polarization in the MLN instead of inducing immune tolerance.
Figure 3.
Ssp/OVA adducts modulate the immune response in the MLN. The dot plots indicate the frequencies of CD4+IL-4+ T cells (a), CD4+IFN-γ+ T cells (b) and CD4+CD25+Foxp3+ Tregs (c) in mouse MLN. a5, b5 and c5 are isotype controls. The bars in a6, b6 and c6 indicate the summarized data in the dot plots (mean±s.d.). *P<0.01, compared with panel 1. The data represent five experiments. IFN, interferon; MLN, Mesentery lymph node; OVA, ovalbumin; Ssp, Staphylococcal enterotoxin B-derived small peptide.
Exposure to Ssp/antigen adducts induce sensitization in the intestine
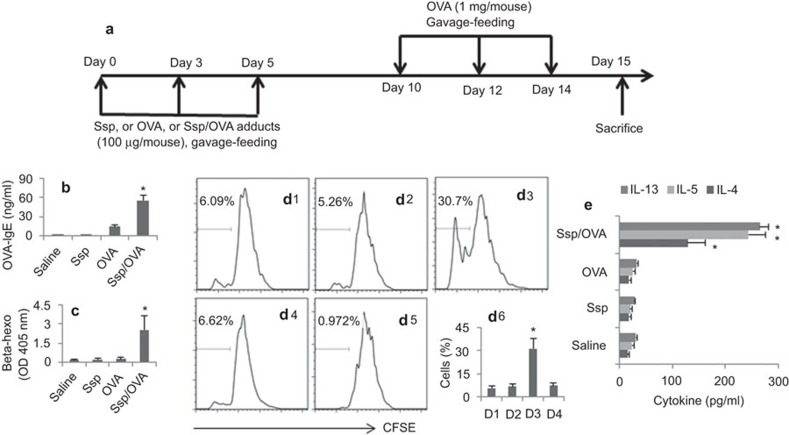
To further characterize the properties of the Ssp/OVA adducts, we treated BALB/c mice with the Ssp/OVA adducts following the procedure in Figure 4a. The treatment with Ssp/OVA adducts resulted in intestinal sensitization to the OVA manifesting increase in serum OVA-specific IgE (Figure 4b) and β-hexosaminidase activity (Figure 4c), and skewed antigen-specific Th2 responses (Figure 4d and e) in the small intestine of mice treated with Ssp/OVA adducts, but not in those treated with saline (naive control), or Ssp alone, or OVA alone.
Figure 4.
Ssp/OVA adducts induce intestinal sensitization. B6 mice were treated with the procedures as depicted in (a). The bars in (b) and (c) indicate the serum levels of OVA-specific IgE (b) and β-hexosaminidase activity (beta-hexo; c). (d) The histograms indicate the T cell proliferation. d1, mice were treated with saline. d2, mice were treated with OVA. d3, mice were treated OVA/Ssp adducts. d4, TLR2-deficient mice were treated OVA/Ssp adducts. d5 is a staining control. d6, the bars indicate the summarized data of d1–d4. (e) The bars indicate the cytokines levels of IL-13, IL-5 and IL-4 in the culture supernatants of the cells in (d) (sampled at the end of the culture; determined by ELISA). The y axis indicates the treatments in mice. Each group consisted of six mice. The data represent six experiments. *P<0.01, compared with saline group. OVA, ovalbumin; Ssp, Staphylococcal enterotoxin B-derived small peptide; TLR, Toll-like receptor.
Discussion
In the present study, we observed that CRS patients with S. aureus infection were prone to suffer from FA. One of the enterotoxins from S. aureus, SEB, was degraded into small peptides while passing through the gastrointestinal tract. The small peptides derived from SEB (we designated these as Ssps) could bind to other macromolecular proteins, such as OVA, to form adducts. The Ssp/OVA adducts could be captured by DCs, facilitate DC maturation, promote DC migration, modulate T cell responses in the MLNs and induce intestinal sensitization in mice.
SEB is one of the enterotoxins secreted by S. aureus; the latter is a common pathogen colonizing on the surface of the body. SEB may be ingested into the digestive tract via contaminated food, or mixing in the discharge of rhinosinusitis to be swallowed down to the gastrointestinal tract. Our previous studies3,16 indicate that concurrent exposure to SEB and food antigen can induce the skewed antigen-specific Th2 inflammation in the intestine, in which T cell immunoglobulin mucin domain (TIM)-4 plays a critical role in the induction of skewed Th2 polarization.3,23 Others found that mixed SEB and food antigens can compromise the established oral tolerance and induce the food antigen-specific Th2 inflammation in the intestine.24 In addition, SEB involvement in the pathogenesis of allergic disorders in the airway and skin has been well recognized.25,26 The present study has expanded the existing knowledge about the role of SEB in the pathogenesis of allergic disorders; the degraded SEB has a strong adjuvant effect on the induction of intestinal sensitization.
It is known that most tissues in the body have proteases that can degrade proteins. The proteases are rich in the intestine. Thus, it is logical to infer that the ingested microbial products, such as SEB, can be degraded in the gastrointestinal tract. Indeed, our results confirmed the inference by showing that SEB was degraded into a number of small peptides in the protein extracts which were isolated from stool of patients with CRS and FA. Considering that both haptens and SEB are involved in the pathogenesis of allergy,25,26,27 we deducted that SEB might be degraded in the intestine before it is involved in the development of sensitization; the degraded SEB, or the Ssps, were peptides with low molecular weight as shown by the present data, which implicated that the Ssps might act as a hapten. The subsequent experimental results proved the deduction; the Ssps could bind to a macromolecular model antigen, OVA, in a dose-dependent manner. However, the whole molecule of SEB did not bind OVA to form adducts.
DCs have the capacity to initiate the immune response by capturing, processing, presenting antigens and producing the costimulatory molecules. DCs express all the described TLRs that can recognize the stimulation of microbes and their products. In the present study, the BMDCs expressed high levels of MHC II and the costimulatory molecules upon exposure to Ssps or Ssp/OVA adducts. This fact indicates that the BMDCs recognize the stimulation of Ssps. The knockdown of TLR2 abrogated the maturation of BMDCs, indicating that TLR2 mediated the stimulation of Ssp to the BMDCs. DCs have a unique feature that upon capture of an antigen, DCs mature quickly and do not capture other antigens. The forming adducts of Ssp and OVA can overcome this characteristic of DC; both OVA and Ssp can be captured by DCs simultaneously. Thus, the Ssp-activated DCs get the opportunity to boost the process of the captured antigen and Ssp concurrently.
The interaction between CD80/CD86 (on DCs) and CD28 (on CD4+ T cells) can trigger a T cell immune response, which may occur in the draining lymph nodes. Our data show that after stimulation by Ssp/OVA adducts, the BMDCs not only matured quickly, but also migrated to the MLNs; this phenomenon implicates that these DCs may present the antigens to CD4+ T cells in the MLNs. The naive CD4+ T cells can be dictated to develop to either Th1 or Th2 cells. In the present study, abundant OVA-specific Th2 cells were detected in the MLNs and in the lamina propria, indicating that the exposure to Ssp/OVA adducts can induce the antigen-specific Th2 polarization in the intestine. The reexposure to the specific antigens resulted in the Th2 pattern intestinal inflammation. The remaining question here is by what mechanism the Ssp/OVA adducts only induce the skewed Th2 response, but not the Th1 response. The proposed answer may be that exposure to SEB induces DCs to produce TIM-4.28,29,30 When the mature DCs present the antigen/major histocompatibility complex II complex to T cells, the TIM-4 is also presented to the T cells. The interaction of TIM-4 and TIM-1 (on T cells) can drive naive CD4+ T cells to differentiate into Th2 cells.3,23
In summary, the present data indicate that patients with SEB+ CRS are prone to FA; the ingested SEB can be degraded to small peptides (Ssp) in the intestine. The Ssps have the properties of haptens to form adducts with macromolecular antigens. Exposure to the Ssp/antigen adducts can induce DC maturation, initiate CD4+ T cell responses in the intestine and induce the antigen-specific Th2 cell polarization and inflammation in the intestine.
Author contributions
SBY, XC, TLL, TL, BQW, JBW, DFT, ZW, MQX, SQQ and ZGL collected samples, performed the experiments and revised the paper. SBY organized the clinical survey. PCY designed the project, supervised the experiments and wrote the paper.
Acknowledgments
This study was supported by grants from the Canadian Institute of Health Research (CIHR, #191063; #220058) and Natural Sciences, Engineering Research Council of Canada (NSERC, #371268). Dr PC Yang holds a New Investigator Award of CIHR (#177843).
The authors do not have any conflict of interest in this paper.
Supplementary Information
References
- Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567–573. doi: 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- Miyagawa F, Gutermuth J, Zhang H, Katz SI. The use of mouse models to better understand mechanisms of autoimmunity and tolerance. J Autoimmun. 2010;35:192–198. doi: 10.1016/j.jaut.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PC, Xing Z, Berin CM, Soderholm JD, Feng BS, Wu L, et al. TIM-4 expressed by mucosal dendritic cells plays a critical role in food antigen specific Th2 differentiation and intestinal allergy. Gastroenterology. 2007;133:1522–1533. doi: 10.1053/j.gastro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Montomoli E, Piccirella S, Khadang B, Mennitto E, Camerini R, de Rosa A. Current adjuvants and new perspectives in vaccine formulation. Expert Rev Vaccine. 2011;10:1053–1061. doi: 10.1586/erv.11.48. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Rudin M, di Pietrantonj C. Adverse events after immunisation with aluminium-containing DTP vaccines: systematic review of the evidence. Lancet Infect Dis. 2004;4:84–90. doi: 10.1016/S1473-3099(04)00927-2. [DOI] [PubMed] [Google Scholar]
- Jung K. Safety and tolerability of immunotherapy using various updosing schedules of a new SCIT product with an optimised allergen/aluminium hydroxide ratio. Allergy. 2011;66:41–43. doi: 10.1111/j.1398-9995.2011.02632.x. [DOI] [PubMed] [Google Scholar]
- Yang PC, Jury J, Soderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104–114. doi: 10.2353/ajpath.2006.050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden JP, Dearman RJ, White JML, Basketter DA, Kimber I. The hapten-atopy hypothesis II: the ‘cutaneous hapten paradox'. Clin Exp Allergy. 2011;41:327–337. doi: 10.1111/j.1365-2222.2010.03684.x. [DOI] [PubMed] [Google Scholar]
- Sela M. Structural components responsible for peptide antigenicity. Appl Biochem Biotechnol. 2000;83:63–70. doi: 10.1385/abab:83:1-3:63. [DOI] [PubMed] [Google Scholar]
- Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Köhler A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Hu DL, Tagawa YI, Sekikawa K, Iwakura Y, Hanada K, et al. IFN-[gamma] and TNF-[alpha] are involved in urushiol-induced contact hypersensitivity in mice. Immunol Cell Biol. 2005;83:18–24. doi: 10.1111/j.1440-1711.2005.01310.x. [DOI] [PubMed] [Google Scholar]
- Meng X, Jenkins RE, Berry NG, Maggs JL, Farrell J, Lane CS, et al. Direct evidence for the formation of diastereoisomeric benzylpenicilloyl haptens from benzylpenicillin and benzylpenicillenic acid in patients. J Pharmacol Exp Ther. 2011;338:841–849. doi: 10.1124/jpet.111.183871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick LR, Meirelles K, Small JS, Puleo FJ, Koltun WA, Cooney RN. A new model of chronic hapten-induced colitis in young rats. J Pediatr Gastroenterol Nutr. 2010;50:240–250. doi: 10.1097/MPG.0b013e3181cb8f4a. [DOI] [PubMed] [Google Scholar]
- Veien NK. Systemic contact dermatitis. Int J Dermatol. 2011;50:1445–1456. doi: 10.1111/j.1365-4632.2011.05104.x. [DOI] [PubMed] [Google Scholar]
- Yang PC, Berin MC, Perdue MH. Enhanced antigen transport across rat tracheal epithelium induced by sensitization and mast cell activation. J Immunol. 1999;163:2769–2776. [PubMed] [Google Scholar]
- Liu T, Wang BQ, Zheng PY, He SH, Yang PC. Rhinosinusitis derived Staphylococcal enterotoxin B plays a possible role in pathogenesis of food allergy. BMC Gastroenterology. 2006;6:24. doi: 10.1186/1471-230X-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PC, Liu T, Wang BQ, Zhang TY, An ZY, Zheng PY, et al. Rhinosinusitis derived Staphylococcal enterotoxin B possibly associates with pathogenesis of ulcerative colitis. BMC Gastroenterology. 2005;5:28. doi: 10.1186/1471-230X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Shi P, Chen B, Shi G, Li H, Wang H. Superantigen-induced glucocorticoid insensitivity in the recurrence of chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg. 2011;145:717–722. doi: 10.1177/0194599811413859. [DOI] [PubMed] [Google Scholar]
- Kientz CE, Hulst AG, Wils ER. Determination of staphylococcal enterotoxin B by on-line (micro) liquid chromatography-electrospray mass spectrometry. J Chromatogr A. 1997;757:51–64. doi: 10.1016/s0021-9673(96)00661-9. [DOI] [PubMed] [Google Scholar]
- Feazel LM, Robertson CE, Ramakrishnan VR, Frank DN. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope. 2012;122:467–472. doi: 10.1002/lary.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandron M, Aries MF, Boralevi F, Martin H, Charveron M, Taieb A, et al. Age-related differences in sensitivity of peripheral blood monocytes to lipopolysaccharide and Staphylococcus aureus toxin B in atopic dermatitis. J Invest Dermatol. 2007;128:882–889. doi: 10.1038/sj.jid.5701112. [DOI] [PubMed] [Google Scholar]
- Mandron M, Ariès MF, Brehm RD, Tranter HS, Acharya KR, Charveron M, et al. Human dendritic cells conditioned with Staphylococcus aureus enterotoxin B promote TH2 cell polarization. J Allergy Clin Immunol. 2006;117:1141–1147. doi: 10.1016/j.jaci.2005.12.1360. [DOI] [PubMed] [Google Scholar]
- Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009;123:231–238. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Novo CA, Jedrzejczak-Czechowicz M, Lewandowska-Polak A, Claeys C, Holtappels G, van Cauwenberge P, et al. T cell inflammatory response, Foxp3 and TNFRS18-L regulation of peripheral blood mononuclear cells from patients with nasal polyps-asthma after staphylococcal superantigen stimulation. Clin Exp Allergy. 2010;40:1323–1332. doi: 10.1111/j.1365-2222.2010.03577.x. [DOI] [PubMed] [Google Scholar]
- Forbes-Blom E, Camberis M, Prout M, Tang SC, le Gros G. Staphylococcal-derived superantigen enhances peanut induced Th2 responses in the skin. Clin Exp Allergy. 2012;42:305–314. doi: 10.1111/j.1365-2222.2011.03861.x. [DOI] [PubMed] [Google Scholar]
- Ngatu NR, Okajima MK, Yokogawa M, Hirota R, Eitoku M, Muzembo BA, et al. Anti-inflammatory effects of sacran, a novel polysaccharide from Aphanothece sacrum, on 2,4,6-trinitrochlorobenzene-induced allergic dermatitis in vivo. Ann Allergy Asthma Immunol. 2012;108:117–122. doi: 10.1016/j.anai.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Liu T, He SH, Zheng PY, Zhang TY, Wang BQ, Yang PC. Staphylococcal enterotoxin B increases TIM4 expression in human dendritic cells that drives naive CD4 T cells to differentiate into Th2 cells. Mol Immunol. 2007;44:3580–3587. doi: 10.1016/j.molimm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Dorfman DM, Hornick JL, Shahsafaei A, Freeman GJ. The phosphatidylserine receptors, T cell immunoglobulin mucin proteins 3 and 4, are markers of histiocytic sarcoma and other histiocytic and dendritic cell neoplasms. Hum Pathol. 2010;41:1486–1494. doi: 10.1016/j.humpath.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzanet R, Meyers JH, Balasubramanian S, Slavik J, Kassam N, Dardalhon V, et al. TIM-4 expressed on APCs induces T cell expansion and survival. J Immunol. 2008;180:4706–4713. doi: 10.4049/jimmunol.180.7.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.