Abstract
The ultimate goal of antitumor vaccines is to develop memory CD8+ cytotoxic T lymphocytes (CTLs), which are critical mediators of antitumor immunity. We previously demonstrated that the ovalbumin (OVA)-specific CD4+ T cell-based (OVA-TEXO) vaccine generated using OVA-pulsed dendritic cell (DCOVA)-released exosomes (EXOOVA) stimulate CTL responses via IL-2 and costimulatory CD80 signaling. To assess the potential involvement of other costimulatory pathways and to define the key constituent of costimulation for memory CTL development, we first immunized wild-type (WT) C57BL/6 and gene-knockout mice with WT CD4+ OVA-TEXO cells or OVA-TEXO cells with various molecular deficiencies. We then assessed OVA-specific primary and recall CTL responses using PE-H-2Kb/OVA257–264 tetramer and FITC-anti-CD8 antibody staining by flow cytometry. We also examined antitumor immunity against the OVA-expressing B16 melanoma cell line BL6-10OVA. We demonstrated that CD4+ OVA-TEXO cells stimulated more efficient CTL responses compared to DCOVA. By assessing primary and recall CTL responses in mice immunized with OVA-TEXO or with OVA-TEXO lacking the costimulatory molecules CD40L, 4-1BBL or OX40L, we demonstrated that these costimulatory signals are dispensable for CTL priming by OVA-TEXO. Interestingly, CD40L, but not 4-1BBL or OX40L, plays a crucial role in the development of functional memory CTLs against BL6-10OVA tumors. Overall, this work suggests that a novel CD4+ T cell-based vaccine that is capable of stimulating long-term functional CTL memory via CD40L signaling may represent a novel, efficient approach to antitumor vaccination.
Keywords: antitumor immunity, CD40L, memory CTL, T cell-based vaccine
Introduction
Vaccines that are capable of stimulating CD8+ cytotoxic T lymphocyte (CTL) responses and memory have been extensively studied in an effort to control pathogen-induced diseases and tumor development. Stimulation of T-cell responses by antigen-presenting cells (APCs) involves at least two signaling events: one elicited by T-cell receptors (TCRs) recognizing peptide-major histocompatibility complexes (pMHC) and another triggered by costimulatory molecules (e.g., CD40, CD80, 4-1BBL and OX40L)1 associated with the immunological synapse between the APC and the CD8+ T cell,2 a multiprotein signaling complex responsible for controlling T-cell activation.1,3 APCs also provide an additional polarization signal (signal 3), such as IL-12,4,5 which selectively drives the development of type I or type II immunity, each characterized by a distinct microenvironment promoting a particular set of effector mechanisms.4,6 Following the recognition of foreign antigen (Ag), CD8+ T cells undergo three distinct developmental phases: (i) a proliferation (primary) phase, in which naive CD8+ T cells undergo autonomous clonal expansion and develop into effector CTLs; (ii) a contraction phase, in which approximately 95% of the effector CTLs undergo activation-induced cell death through apoptosis, allowing the remaining approximately 5%–10% of the CTLs to develop into memory CTLs; and (iii) a maintenance phase, in which memory CTLs survive for a prolonged duration. Unlike their naive counterparts, memory CTLs display enhanced responses to subsequent Ag encounters, undergoing rapid proliferation and having heightened effector functions during recall responses.
Dendritic cells (DCs) represent the most effective type of APC. DCs process exogenous Ags in their endosomal compartments, such as multivesicular endosomes,7 which can later fuse with the plasma membrane, releasing Ag-presenting vesicles called ‘exosomes'.8,9 Exosomes (EXOs) are 50–90 nm diameter vesicles containing Ag-presenting molecules, including MHC class I, MHC class II, CD1 and hsp70–90, tetraspan molecules (CD9, CD63 and CD81), adhesion molecules (CD11b and CD54) and costimulatory molecules (CD80 and CD86).10,11 Together, these molecules comprise the molecular machinery required for generating potent immune responses. Recently, we have shown that Con A-stimulated CD4+ T cells derived from ovalbumin (OVA)-specific TCR transgenic OTII mice can internalize OVA-pulsed, DC-released EXO (EXOOVA) via TCR/MHC and LFA-1/CD54 interactions. EXOOVA-loaded CD4+ T cells can be used as OVA-TEXO-cell vaccines capable of stimulating CD4+ T cell-independent CD8+ T-cell priming via pMHC-I-mediated targeting and via IL-2 and CD80 costimulatory signaling.12 Additional costimulations via 4-1BBL/4-1BB, OX40L/OX40 and CD40/CD40L interactions between DCs and CD8+ T cells have also been found to promote effector CD8+ T-cell responses during the priming phase13,14 and CD8+ Tm-cell development in the contraction phase.15,16,17,18 However, whether these costimulatory events are also involved in priming and memory development in OVA-TEXO-stimulated CD8+ T cells is currently not well understood.
In this study, we assessed the potential role of costimulatory molecules, including CD40L, 4-1BBL and OX40L, in OVA-TEXO vaccine-induced CD8+ CTL responses and memory development against OVA-expressing B16 melanoma in wild-type (WT) C57BL/6 mice.
Materials and methods
Reagents, cell lines and animals
The biotin-labeled or fluorescein isothiocyanate (FITC)-labeled antibodies (Abs) specific for CD11c (HL3), CD40L (MR1), CD80 (16-10A1), 4-1BBL (TKS-1), OX40L (RM134L) or H-2Kb/OVA257–264 (OVAI) (pMHC-I) (25D.1) were obtained from BD Pharmingen Canada Inc. (Missisauga, Ont., Canada) and Biolegend (San Diego, CA, USA). The depleting anti-CD20 (AISB12) and anti-plasmacytoid DC (120G8) Abs were obtained from eBioscience (San Diego, CA, USA). PE-labeled H-2Kb/OVA257–26412 tetramer was obtained from Beckman Coulter (Mississauga, Ont., Canada). Female WT C57BL/6 (B6), transgenic OTII and various gene knockout (KO) mice (except for 4-1BBL−/− mice, obtained from Amgen, Seattle, WA, USA) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Homozygous OTII/H-2Kb−/−, OTII/CD40L−/−, OTII/4-1BBL−/− and OTII/OX40L−/− mice were generated by backcrossing the designated gene KO mice onto the OTII background. All mice used in the experiments were 6–8 weeks old and were treated according to the animal care committee guidelines of the University of Saskatchewan.
DC and EXO preparation
The generation of bone marrow-derived mature OVA-pulsed DCs (DCOVA) (from WT C57BL/6 mice) in the presence of GM-CSF/IL-4 (20 ng/ml) has been described previously.11 DCOVA derived from 4-1BBL−/− and OX40L−/− mice were termed (4-1BBL−/−)DCOVA and (OX40L−/−)DCOVA, respectively. EXOs derived from the culture supernatants of DCOVA, (4-1BBL−/−)DCOVA and (OX40L−/−)DCOVA were termed EXOOVA, (4-1BBL−/−)EXOOVA and (OX40L−/−)EXOOVA, respectively.
CD4+ TEXO preparation
To generate active CD4+ T cells, spleen cells from OTII mice were cultured in RPMI 1640 medium containing IL-2 (20 U/ml) and Con A (1 µg/ml) for 3 days. The Con A-activated CD4+ T cells (ConA-T) were then purified using MACS anti-CD4 microbeads, as previously described.19 ConA-T cells derived from OTII/CD40L−/−, OTII/4-1BBL−/− and OTII/OX40L−/− mice were termed (CD40L−/−)T, (4-1BBL−/−)T and (OX40L−/−)T cells. ConA-T cells were incubated with EXOOVA (10 µg/1×106 T cells) at 37 °C for 3 h and were termed OVA-specific TEXO (OVA-TEXO).20 (CD40L−/−)T cells that had internalized EXOOVA were termed (CD40L−/−)TEXO, and (4-1BBL−/−)T and (OX40L−/−)T cells that had internalized (4-1BBL−/−)EXOOVA and (OX40L−/−)EXOOVA were termed (4-1BBL−/−)TEXO and (OX40L−/−)TEXO, respectively.
Assessment of CTL responses by PE-tetramer staining
C57BL/6 or various gene KO mice (6/group) were injected intravenously (i.v.) with DCOVA, WT OVA-TEXO or OVA-TEXO with a specific molecular deficiency (3×106 cells/mouse). To assess recall responses, immunized mice were boosted i.v. with DCOVA (0.5×106 cells/mouse) on day 30 after the primary immunization. Six or four days after the primary immunization or the boost, peripheral blood samples from the mice were incubated with PE-H-2Kb/OVA257–264 and FITC-anti-CD8 Ab for 30 min at room temperature, and then analyzed by flow cytometry.
In vivo cytotoxicity assay
C57BL/6 mouse splenocytes were labeled with either high (3.0 µM, CFSEhigh) or low (0.6 µM, CFSElow) concentrations of CFSE. CFSEhigh and CFSElow cells were then pulsed with OVAI (OVA257–264, SIINFEKL) and irrelevant Mut1 (FEQNTAQP) peptides, which served as internal OVA-specific target cells and internal control target cells, respectively. These peptide-pulsed target cells were co-injected i.v. at a 1∶1 ratio into mice immunized with either OVA-TEXO or OVA-TEXO harboring a specific molecular deficiency (3×106 cells/mouse) 6 days after the immunization. Sixteen hours post-injection, the residual antigen-specific CFSEhigh and control CFSElow target cells remaining in the recipients' spleens were analyzed by flow cytometry.19
Animal studies
To examine antitumor protective immunity, WT C57BL/6 or CD40−/− mice (6/group) were injected i.v. with WT OVA-TEXO or OVA-TEXO with a specific molecular deficiency (3×106 cells/mouse). Thirty days after the immunization, mice were challenged i.v. with 0.5×106 OVA-expressing B16 melanoma BL6-10OVA cells.19 The mice were euthanized 3 weeks following tumor cell challenge, and the lung metastatic tumor colonies were counted in a blind fashion. Metastases on freshly isolated lungs appeared as discrete, black-pigmented foci in BL6-10OVA tumors. Metastatic foci that were too numerous to count were assigned an arbitrary value of >300.19
Statistical analysis
Statistical analyses were performed using the Student's t-test or the Mann–Whitney U test20 for comparison of variables from different groups. A value of P<0.05 or <0.01 was considered to be statistically significant or very significant.
Results and discussion
CD4+ OVA-TEXO cells stimulate efficient CD8+ T-cell responses
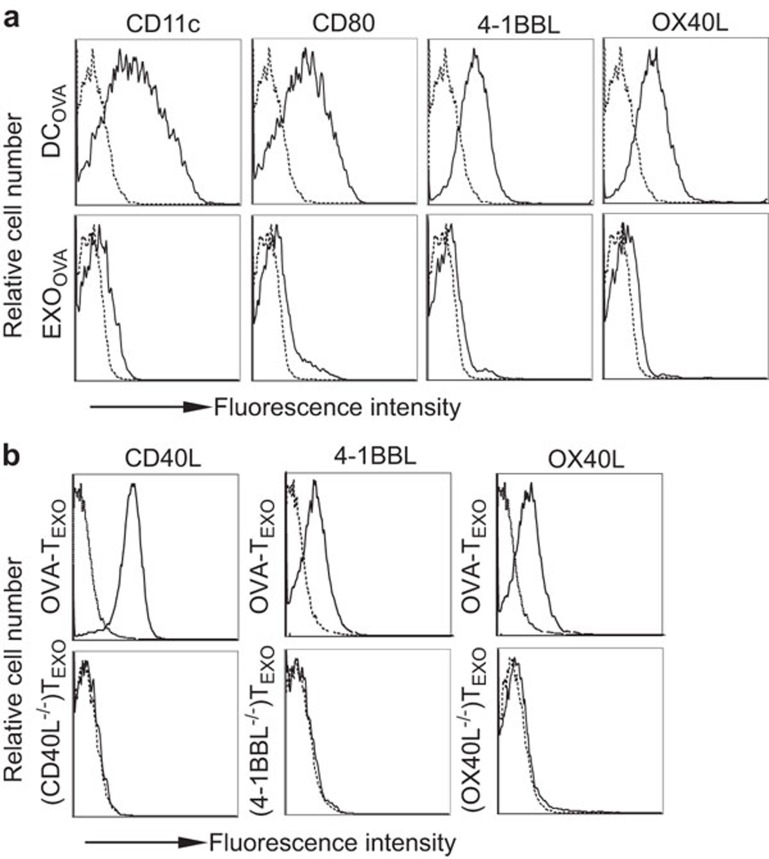
OVA-pulsed DCs (DCOVA expressed CD11c (a marker of DCs), the costimulatory molecules CD80, 4-1BBL, and OX40L and pMHC-I complexes, whereas DCOVA-released EXO (EXOOVA) displayed these molecules to a much lesser extent (Figure 1a). OVA-TEXO cells derived from ConA-stimulated OTII CD4+ T lymphocytes (ConA-T) that had internalized EXOOVA expressed 4-1BBL, OX40L and CD40L (Figure 1b) and secreted IFN-γ (approximately 2.6 ng/ml/106 cells/24 h) and IL-2 (approximately 2.1 ng/ml/106 cells/24 h) but did not secrete IL-4 or IL-10. To examine the stimulatory effect of WT OVA-TEXO, we immunized C57BL/6 mice with OVA-TEXO cells or DCOVA as a control. The efficiency of the CD8+ T-cell responses were then assessed using FITC-anti-CD8 Ab and PE-H-2Kb/OVA257–264 tetramer staining, followed by flow cytometry on day 6 post-immunization. We found that OVA-TEXO immunization induced an OVA-specific CD8+ T-cell response (2.30% of the total CD8+ T-cell population) in vivo that was more efficient than the DCOVA-stimulated CTL response (1.95% of the total CD8+ T-cell population) (Figure 2a).
Figure 1.
Flow cytometric analysis. (a) DCOVA and EXOOVA and (b) OVA-TEXO or OVA-TEXO with indicated molecular deficiencies were stained with a panel of specific Abs (solid lines) or isotype-matched irrelevant Abs (dotted lines) and analyzed by flow cytometry. One representative experiment of two is shown. Ab, antibody; DC, dendritic cell; EXO, exosome; OVA, ovalbumin.
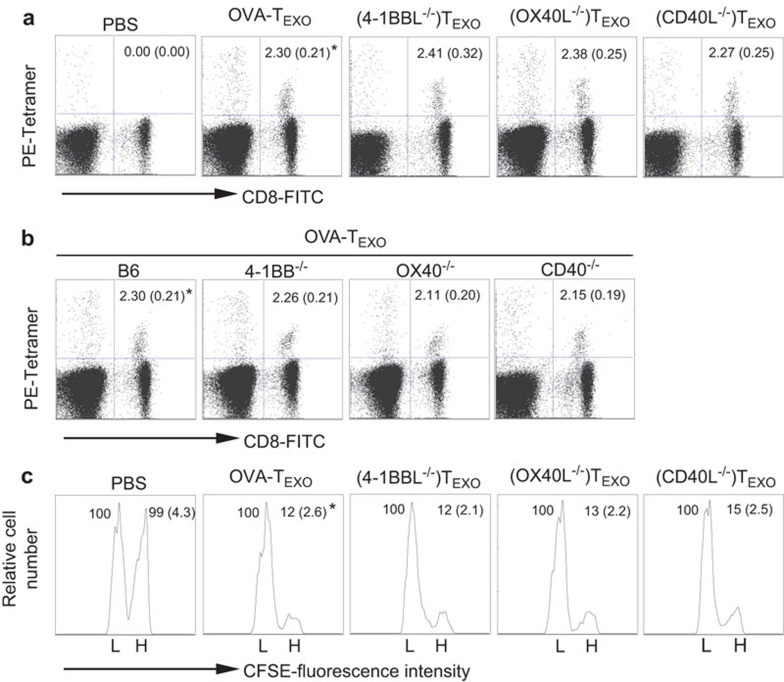
Figure 2.
CD40L signaling by OVA-TEXO is not involved in effector CD8+ T-cell priming. (a) C57BL/6 or (b) gene KO mice were immunized with DCOVA, WT OVA-TEXO or OVA-TEXO with indicated molecular deficiencies. Six days after the immunization, tail-blood samples were stained with PE-H-2Kb/OVAI peptide tetramer and FITC-anti-CD8 Ab and analyzed by flow cytometry. Values represent the mean percentage of OVA-specific CD8+ CTLs, with standard deviations in parentheses. *P>0.05 vs. cohorts of other groups (Student's t-test). (c) For in vivo cytotoxicity assays, OVA-specific CFSEhigh and irrelevant-peptide CFSElow splenocytes were injected i.v. into WT or gene KO OVA-TEXO-immunized C57BL/6 mice 6 days after immunization. Sixteen hours later, the percentages of residual CFSEhigh (H) and CFSElow (L) target cells remaining in the recipients' spleens were analyzed by flow cytometry. Values represent the mean percentage of CFSEhigh vs. CFSElow target cells remaining in the spleen. One representative experiment of two is shown. Ab, antibody; CTL, cytotoxic T lymphocyte; EXO, exosome; i.v., intravenously; KO, knockout; OVA, ovalbumin; WT, wild-type.
CD40L, 4-1BBL and OX40L signaling are dispensable for priming effector CD8+ CTL responses
We previously demonstrated that CD80 costimulation plays an important role in OVA-TEXO-initiated priming of CD8+ T-cell responses.12 To assess the potential role of other costimulatory molecules, such as CD40L, 4-1BBL and OX40L, we immunized C57BL/6 mice with (CD40L−/−)TEXO, (4-1BBL−/−)TEXO or (OX40L−/−)TEXO. We found that (CD40L−/−)TEXO-, (4-1BBL−/−)TEXO- and (OX40L−/−)TEXO-stimulated OVA-specific CD8+ T-cell responses (6 days post-immunization) were similar to responses observed in mice immunized with WT OVA-TEXO cells (2.30%) of total CD8+ T cells) (P>0.05) (Figure 2a). This finding indicates that CD40L, 4-1BBL and OX40L signaling are not involved in OVA-TEXO-induced CD8+ T-cell priming. This result was further confirmed by analyzing CTL priming in WT B6 and gene KO mice that had been immunized with WT CD4+ OVA-TEXO cells. Consistent with our earlier findings, there was also no significant difference between the WT B6 and gene KO groups (P>0.05) (Figure 2b). To assess the effector function of primed CD8+ T cells, we next performed an in vivo cytotoxicity assay. In this assay, OVAI peptide-pulsed/CFSEhigh and irrelevant Mut1 peptide-pulsed/CFSElow C57BL/6 mouse splenocytes (which served as OVA-specific and control target cells, respectively) were co-injected i.v. at a 1∶1 ratio into C57BL/6 mice that had been immunized with WT OVA-TEXO or OVA-TEXO carrying various molecular deficiencies six days after the immunization. Sixteen hours post-injection, the residual OVA-specific CFSEhigh and control CFSElow target cells remaining in the recipients' spleens were analyzed by flow cytometry. We found that 88% of the OVA-specific CFSEhigh target cells, but none of the irrelevant control Mut1 peptide-pulsed (CFSElow) target cells, were killed in the 16 h following the transfer of the target cells into WT OVA-TEXO-immunized mice (Figure 2c). This result indicates that OVA-TEXO can efficiently stimulate the differentiation of CD8+ T-cells into CTL effectors. Similar to WT OVA-TEXO, (CD40L−/−)TEXO, (4-1BBL−/−)TEXO and (OX40L−/−)TEXO cells also actively stimulated the differentiation of CD8+ T cells into CTL effectors (P>0.05) (Figure 2c), suggesting that CD40L, 4-1BBL and OX40L signaling by CD4+ OVA-TEXO cells is dispensable for priming effector CD8+ CTL responses.
CD40L-, but not 4-1BBL- or OX40L-induced signaling, is required for functional CTL memory development
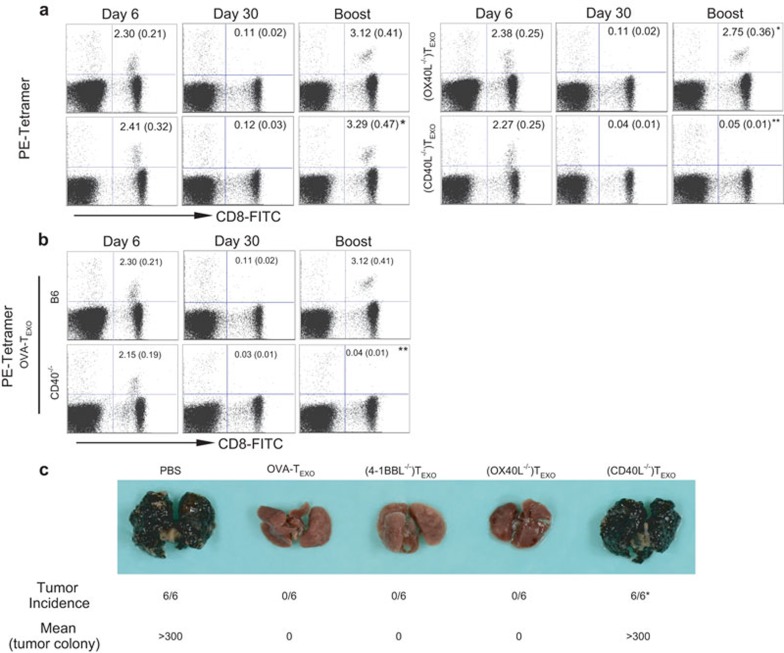
To assess whether CD8+ memory CTLs are functional, the mice were further boosted with DCOVA 30 days after the immunization, at a timepoint when the OVA-TEXO-stimulated effector CD8+ CTLs would have developed into memory T cells. We observed a 20-fold increase in boosted CD8+ T cells 4 days after the boost of DCOVA in mice previously immunized with OVA-specific OVA-TEXO, (OX40L−/−)TEXO and (4-1BBL−/−)TEXO cells (P>0.05) (Figure 3a). Interestingly, there was no increase in boosted CD8+ T cells in mice previously immunized with (CD40L−/−)TEXO (P<0.05) (Figure 3a), suggesting that CD40L-, but not 4-1BBL- or OX40L-signaling by CD4+ TEXO cells is required for functional CD8+ T-cell memory development. This observation was further confirmed by our finding that CD40−/− CTLs also had defects in their recall responses in CD40−/− mice that had previously been primed by WT OVA-TEXO (Figure 3b). To assess the antitumor immunity in these mice, we further challenged the immunized mice with the OVA-expressing B16 melanoma cell line BL6-10OVA 30 days after the primary immunization. We found that all OVA-TEXO-immunized mice were significantly more protected against BL6-10OVA tumor challenge compared to (CD40L−/−)TEXO-immunized mice (P<0.01) (Figure 3c), confirming the loss of functional CTL memory derived from (CD40L−/−)TEXO-immunization.
Figure 3.
CD40L signaling by OVA-TEXO is involved in CD8+ T-cell memory development. (a) Thirty days after immunization with WT OVA-TEXO or OVA-TEXO harboring indicated molecular deficiencies, the tail-blood samples of immunized C57BL/6 mice were analyzed for OVA-specific memory CTL responses by flow cytometry. (b) Thirty days after the immunization of C57BL/6 or CD40−/− mice with WT OVA-TEXO, the tail-blood samples of immunized mice were analyzed for OVA-specific memory CTL responses by flow cytometry. All of the above immunized mice were further boosted with DCOVA. Four days after the boost, the tail-blood samples of immunized mice were analyzed for OVA-specific memory CTL recall responses by flow cytometry. Values represent the mean percentage of OVA-specific CD8+ CTLs, with standard deviations in parentheses. *P>0.05 and **P<0.05 vs. cohorts of the OVA-TEXO group (a) or C57BL/6 mice (b) (Student's t-test). (c) The immunized mice were challenged with BL6-10OVA tumor cells at day 30 following the primary immunization, and mice were euthanized 3 weeks subsequent to challenge. Lungs with black tumor nodules are shown. *P<0.01 vs. cohorts of the OVA-TEXO group (Mann–Whitney U test). One representative experiment of two is shown. CTL, cytotoxic T lymphocyte; DC, dendritic cell; EXO, exosome; OVA, ovalbumin; WT, wild-type.
CD40L signaling by CD4+ T cells has been found to be important in licensing DCs to induce CTL responses via interactions between CD40 on DCs and CD40L on CD4+ T cells.21 In addition, Munroe et al.22 demonstrated a functional costimulation of T cells through CD40 via the induction of kinases and transcription factors. Furthermore, Bourgeois et al.23 reported that CD40 deficiency has a major impact on CTL memory responses, suggesting that help provided by CD4+ T cells during CD8+ T-cell responses may involve direct cell-cell interactions between CD4+ and CD8+ T cells in DC-CD4+–CD8+ T-cell clusters. However, these reports did not provide any direct in vivo evidence for the role of CD40L expressed on CD4+ T cells in CTL memory development. In this study, we demonstrate that although CD40L signaling by CD4+ OVA-TEXO cells is dispensable for CD8+ T-cell priming, this signaling event plays an important role in functional CD8+ T-cell memory development. The loss of the functional recall responses and antitumor immunity in C57BL/6 mice 30 days after the primary immunization with CD4+ (CD40L−/−)TEXO cells clearly indicates the critical role of CD40L signaling by CD4+ OVA-TEXO cells in long-term functional CTL memory development.
Dendritic cells (DCs are the most effective APCs and have been extensively used in tumor vaccines in clinical trials.24 Unfortunately, these attempts have demonstrated only limited efficacy, mainly due to the silencing effect imposed on DCs by the tumor-tolerogenic microenvironment.25 In addition, similar to many other types of vaccines, such as DNA, tumor lysates, tumor antigens or peptides, DC vaccines rely on CD4+ T cell-dependent CTL responses and antitumor immunity. In clinical practice, a wealth of data indicate that antitumor immunity directed against a wide variety of malignancies is suppressed in cancer patients.26 Suppressive CD4+ T cells, including Tr1, Th2 and natural CD4+25+ Tr cells, play a critical role in the mediation of the suppression.27,28 In cancer patients, enrichment of CD4+25+ Tr cell populations has been observed both in the circulation and in the tumor microenvironment.29,30
We previously showed that (i) the novel EXO-targeted OVA-TEXO cells were able to directly stimulate CTL responses in the absence of host CD4+ T-cell help and trigger more efficient CTL responses compared to DCOVA; (ii) pMHC-I, CD80 signaling and IL-2 signaling play important roles in CD4+ OVA-TEXO-stimulated CTL responses; and (iii) OVA-TEXO cells triggered OVA-specific CTL responses in C57BL/6 mice, even following the transfer of CD4+25+ Tr cells, by counteracting CD4+25+ Tr tolerance.12 In this study, we further demonstrate that CD4+ T cell-based OVA-TEXO vaccination stimulates functional long-term CTL memory via CD40L signaling, indicating that (i) the three signals required for OVA-TEXO to induce a T-cell response (pMHCI/TCR, costimulatory signal such as CD80/CD28 and CD40L/CD40, and IL-2) are distinct from the three signals required for DCs (pMHCI/TCR, CD80/CD28 and IL-12);4,5 and (ii) CD40L signaling plays an important role in OVA-TEXO-stimulated functional memory CTL development.
Overall, this work suggests that a novel CD4+ T cell-based vaccine capable of stimulating long-term functional CTL memory via CD40L signaling may represent a novel and efficient approach to antitumor vaccination.
Acknowledgments
This research was supported by research grants from the Canadian Institutes of Health Research and Canadian Breast Cancer Foundation. Yufeng Xie and Lu Wang were supported by Postdoctoral Fellowships from the Saskatchewan Health Research Foundation and the Scholarship Council of Chinese Education Ministry. We would also like to extend our appreciation to Mark Boyd for his help with the flow cytometry.
References
- Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2:227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- He T, Zong S, Wu X, Wei Y, Xiang J. CD4+ T cell acquisition of the bystander pMHC I colocalizing in the same immunological synapse comprising pMHC II and costimulatory CD40, CD54, CD80, OX40L, and 41BBL. Biochem Biophys Res Commun. 2007;362:822–828. doi: 10.1016/j.bbrc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- Padhan K, Varma R. Immunological synapse: a multi-protein signalling cellular apparatus for controlling gene expression. Immunology. 2010;129:322–328. doi: 10.1111/j.1365-2567.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B. Modulating TH1/TH2 responses with microbes, dendritic cells, and pathogen recognition receptors. Immunol Res. 2004;29:187–196. doi: 10.1385/IR:29:1-3:187. [DOI] [PubMed] [Google Scholar]
- Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- Kleijmeer MJ, Escola JM, UytdeHaag FG, Jakobson E, Griffith JM, Osterhaus AD, et al. Antigen loading of MHC class I molecules in the endocytic tract. Traffic. 2001;2:124–137. doi: 10.1034/j.1600-0854.2001.020207.x. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113 Pt 19:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Hao S, Bai O, Yuan J, Qureshi M, Xiang J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol. 2006;3:205–211. [PubMed] [Google Scholar]
- Hao S, Liu Y, Yuan J, Zhang X, He T, Wu X, et al. Novel exosome-targeted CD4+ T cell vaccine counteracting CD4+25+ regulatory T cell-mediated immune suppression and stimulating efficient central memory CD8+ CTL responses. J Immunol. 2007;179:2731–2740. doi: 10.4049/jimmunol.179.5.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wenger RH, Zhao M, Nielsen PJ. Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes. J Exp Med. 1997;185:251–262. doi: 10.1084/jem.185.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, et al. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- Mousavi SF, Soroosh P, Takahashi T, Yoshikai Y, Shen H, Lefrancois L, et al. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MG, Shen L, Rock KL. CD40 on APCs is needed for optimal programming, maintenance, and recall of CD8+ T cell memory even in the absence of CD4+ T cell help. J Immunol. 2008;180:4382–4390. doi: 10.4049/jimmunol.180.7.4382. [DOI] [PubMed] [Google Scholar]
- Boesteanu AC, Katsikis PD. Memory T cells need CD28 costimulation to remember. Semin Immunol. 2009;21:69–77. doi: 10.1016/j.smim.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J, Huang H, Liu Y. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol. 2005;174:7497–7505. doi: 10.4049/jimmunol.174.12.7497. [DOI] [PubMed] [Google Scholar]
- Xiang J, Qi Y, Chen Y. Inhibition of established tumor growth in syngeneic mice by local inoculation of engineered mouse myeloma cells secreting a recombinant fusion protein RM4/TNF. Cancer Gene Ther. 1997;4:353–358. [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Munroe ME, Bishop GA. A costimulatory function for T cell CD40. J Immunol. 2007;178:671–682. doi: 10.4049/jimmunol.178.2.671. [DOI] [PubMed] [Google Scholar]
- Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- Palucka K, Ueno H, Banchereau J. Recent developments in cancer vaccines. J Immunol. 2011;186:1325–1331. doi: 10.4049/jimmunol.0902539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM. Some active areas of DC research and their medical potential. Eur J Immunol. 2010;40:2085–2088. doi: 10.1002/eji.201040733. [DOI] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Anderson DE. Immune regulation in tumor-bearing hosts. Curr Opin Immunol. 2006;18:214–219. doi: 10.1016/j.coi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Gross S, Walden P. Immunosuppressive mechanisms in human tumors: why we still cannot cure cancer. Immunol Lett. 2008;116:7–14. doi: 10.1016/j.imlet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]