Abstract
During the last several years, research has produced a significant amount of knowledge concerning the characteristics of human γδ T lymphocytes. Findings regarding the immune functions of these cells, particularly their natural killer cell-like lytic activity against tumor cells, have raised expectations for the therapeutic applications of these cells for cancer. Pharmaceutical companies have produced selective agonists for these lymphocytes, and several teams have launched clinical trials of γδ T cell-based cancer therapies. The findings from these studies include hematological malignancies (follicular lymphoma, multiple myeloma, acute and chronic myeloid leukemia), as well as solid tumors (renal cell, breast and prostate carcinomas), consisting of samples from more than 250 patients from Europe, Japan and the United States. The results of these pioneering studies are now available, and this short review summarizes the lessons learned and the role of γδ T cell-based strategies in the current landscape of cancer immunotherapies.
Keywords: cancer, cell therapy, gamma–delta lymphocytes, immunotherapy, lymphoma, trials
Introduction
Since the discovery of γδ T cells in the late 1980s, a significant amount of knowledge has accumulated regarding human γδ T lymphocytes. The unconventional immune functions of these cells, and notably, their histocompatibility leukocyte antigen-unrestricted cytotoxic activity against malignant cells have raised expectations for their use in therapeutic applications for cancer. In addition, because various selective agonists for human γδ T lymphocytes have been synthesized, several clinical trials involving γδ T cell-based therapies in cancer patients have been launched around the world. The investigations included patients with follicular lymphoma, multiple myeloma and acute myeloid leukemia, as well as nonhematological malignancies, such as renal cells, breast and prostate carcinomas, totaling nearly 250 patients in Europe, Japan and the United States. By 2012, results have been available from most of these pioneering studies, and some conclusions can now be drawn. This review summarizes these studies and considers their results in the greater landscape of current cancer immunotherapies.
Rationale for harnessing γδ cells in cancer immunotherapy
Human γδ T lymphocytes comprise several subsets of cells defined by their T-cell receptor (TCR), the most prominent of which in circulating blood is composed of cells expressing the V gamma 9 V delta 2 TCR (hereafter referred to as Vγδ9Vγδ2 γδ T cells). This subset of lymphocytes is not present in mice, restraining preclinical studies either to xenografts in immunodeficient mice or nonhuman primate models. The primary reasons for involving γδ cell activation in cancer therapies will be summarized below only briefly, as this topic has recently been reviewed.1,2,3,4
First, the hallmark characteristic of T lymphocytes expected to interact with cancer cells is their capacity to infiltrate tumors. Accordingly, tumor-infiltrating gamma delta T lymphocytes (γδ TILs) were detected in a broad spectrum of malignancies.5,6,7 γδ TILs, primarily of the Vγ9Vδ2 γδ T cell type, were reported in renal cell carcinomas, albeit with controversial prognostic values.7,8,9,10 Nasopharyngeal carcinoma patients carry Vγ9Vδ2 γδ T lymphocytes that are functionally impaired in blood11 but are able to infiltrate and reduce xenografted nasopharyngeal tumors in mice.12 Likewise, orthotopic xenografts of breast tumors13 or bladder carcinomas14 in mice infused with human TCRVγ9Vδ2+ lymphocytes also show strong tumor infiltration by γδ TILs and subsequent arrest of tumor growth. In a B-cell depletion assay from cynomolgus monkeys treated with a phosphoantigen plus interleukin-2 (IL-2) and rituximab, endogenous cytotoxic γδ T cells were enriched in lymph nodes in which CD20+ B cells had been depleted.15 Radiolabeling and imaging experiments (our unpublished results) have confirmed the observed γδ cell accumulation at tumor sites, illustrating the capacity of these cells to infiltrate tumor sites in vivo. Additionally, when autologous transplants of In111-radiolabeled Vγ9Vδ2 T cells were tracked in 18 patients with advanced solid tumors, the radiolabeled cells trafficked predominantly to the lungs, liver and spleen, as well as to metastatic tumor sites outside of these organs in some patients.16 In breast, ovarian, prostate and colorectal cancers, γδ TILs also included cells that were negative for the Vγ9Vδ2 TCR.5,6,7 Vδ1+ γδ TILs are present in melanoma, and the infiltration of these cells into necrotizing melanoma correlates with survival.17 Orthotopic xenografts of the HT29 colon cancer cells in mice infused with human TCRVδ2− γδ T lymphocytes also carried γδ TILs that impaired tumor development.18 In prostate and breast tumors, however, Vδ1+ γδ TILs are rather immunosuppressive for dendritic cells and T cells19 and favor the growth of the tumor through IL-10 release.20
The second hallmark of T lymphocytes expected to attack tumors is their histocompatibility leukocyte antigen-unrestricted cytotoxic capacity for cancer cells, as well as their ability to secrete adequate cytokines. In vitro, TCRVγ9Vδ2+ lymphocytes exert a potent cytotoxic activity against a broad spectrum of malignancies. Activated γδ T lymphocytes can kill Burkitt's and non-Hodgkin lymphoma (NHL) of both B-cell and T-cell types, such as follicular lymphoma, diffuse large B-cell lymphoma, mantle cell lymphoma, B-cell chronic lymphocytic leukemia, anaplastic large-cell lymphoma and acute lymphocytic leukemia.21 These cells also kill acute myeloid leukemia cells,22 chronic myeloid leukemia cells23 and multiple myeloma cells.24,25 Activated γδ T lymphocytes are also capable of killing a broad spectrum of solid malignancies, while sparing normal tissues. For example, although activated γδ T lymphocytes from patients with glioblastoma multiforme appear anergized, these cells can kill corresponding target cells in vitro.26,27 Furthermore, these cells are also capable of killing metastatic renal cell carcinomas, mammary carcinomas, prostate carcinomas and colorectal carcinomas, again while sparing the normal, untransformed cells (reviewed in Ref. 28). The various pathways and cytokines involved in these cytolytic activities have been reviewed elsewhere29 and will not be discussed here.
Third, two synthetic molecule drugs, phosphoantigen BrHPP and zoledronate, have been shown to selectively activate human TCRVγ9Vδ2+ lymphocytes in clinical trials. The in vitro bioactivity of BrHPP (EC50) is 24/nM for TCRVγ9Vδ2+ T-cell clones and peripheral blood mononuclear cells,30 and this drug is produced as Good Manufacture Practice grade for use in humans under the name Phosphostim/IPH1101 (Innate Pharma, France). Zoledronate, a third generation aminobisphosphonate inhibitor of farnesyl pyrophosphate synthase that has been used for osteolysis (Novartis, Switzerland), induces bioaccumulation of endogenous phosphoantigens. This drug therefore activates TCRVγ9Vδ2+ T-cell clones and peripheral blood mononuclear cell with an in vitro bioactivity (EC50) of 3/nM,31 but also targets all bioactivities relying upon farnesylated proteins. Other second generation aminobisphosphonates, such as pamidronate, alendronate ibandronate and risedronate, share the same spectrum of biological activities as zoledronate, albeit with overall greater EC50 values (equal to 0.2, 0.05 and 0.02, respectively), compared to zoledronate (EC50 of 0.01/µM).30 The in vitro and in vivo proliferation of TCRVγ9Vδ2+ T cells activated by phosphoantigens or aminobisphosphonates requires an exogenous supply of IL-2.
Results from cancer clinical trials based on activated γδ T lymphocytes
Our current knowledge of the in vivo bioactivity of human γδ T cells in cancer essentially relies upon in vivo activation of patients with phosphoantigens/IL-2 or aminobisphosphonates/IL-2 and on the adoptive transfer of autologous γδ T lymphocytes preactivated ex vivo with the aforementioned molecules.
NHL of the B-cell type is highly sensitive to γδ T cell-mediated lytic activity; therefore, a preliminary pilot study by Wilhelm's team examined toxicity, in vivo activation of γδ T cells and antilymphoma efficacy of pamidronate/IL-2 in 19 patients with relapsed/refractory low-grade NHL or multiple myeloma.32 The first group (10 patients) received pamidronate (90 mg/3 h intravenous (i.v.) on day 1), followed by IL-2 (from day 3 to day 8 by continuous 24-h i.v. infusions of increasing dosages from 0.25 to 3 million IU/m2). Neither γδ T-cell activation nor response to treatment was determined in this group. Therefore, a second group consisting of nine patients was preselected for an analysis of the in vitro γδ T-cell response to pamidronate/IL-2. These patients received pamidronate (as above), followed by increasing dosages of IL-2 (0.25–2 million IU/m2, 6-h i.v. bolus infusions from day 1 to 6). In this group, significant in vivo responses by γδ T cells were observed (five patients, 55% of total), with three patients achieving objective responses (33%, partial response). The authors thus demonstrated that pamidronate administered with low-dose IL-2 is well tolerated and induces a specific γδ cell amplification; furthermore, the authors found that the clinical response to this treatment observed in the patients, i.e., stabilization or partial response, is linked to γδ T-cell proliferation in vivo. These clinical data were the first to demonstrate the therapeutic potential of γδ cells in patients with B-cell cancers.
Following the aforementioned trial from Germany, a second study was launched in Palermo by Dieli's group. This team administered zoledronate (4 mg i.v., 15-min infusion every 3 weeks for 3 months) to nine cancer patients with bone metastases (three females with breast cancer, six males with prostate cancer) to determine if the bisphosphonate alone affected maturation of circulating effector Vγ9Vδ2 cells.33 The results of this study showed that zoledronate induced the in vivo development of Vγ9Vδ2 cells into the interferon-γ (IFN-γ)-producing effector subset, which is known to provide stronger antitumor responses in preclinical studies.
As indicated by its partial responses to treatment with recombinant IL-2 or IFN-α, metastatic renal cell carcinoma is resistant to conventional treatments but is quite immunogenic and sensitive to the immune system. Therefore, a pilot study on the effects of zoledronate and IL-2 was conducted in the United States by Malkovsky's group in 12 patients with metastatic renal cell carcinoma.34 This trial sought to determine whether activation of the patients' TCRVγ9Vδ2+ lymphocytes improved clinical outcomes. For six patients, the regimen consisted of three successive weekly cycles of intravenous injections of zoledronate (4 mg, day 1) with IL-2 (7 million IU/m2/day, from day 1 to day 5). For three patients, the regimen consisted of four successive weekly cycles of i.v. injections of zoledronate (4 mg, day 1) with subcutaneous (s.c.) administration of IL-2 (1 million U/m2/day, from day 1 to day 5). For three patients, this regimen was amended to include dose escalation of zoledronate. All patients also took a supplement of oral calcium and vitamin D during the regimen and had received premedication with oral acetaminophen. Adverse events, primarily of grade 1–2, which are typical of IL-2 monotherapy, were recorded in all patients, but no partial or complete responses were observed. Furthermore, the treatment induced a marked decrease in the in vitro TCRVγ9Vδ2+ T-cell response for the majority of the patients, with only one patient demonstrating in vivo proliferation of the targeted γδ lymphocytes 8 days after the first cycle.
Because prostate cancer patients harbor zoledronate-responsive γδ cells,35 a phase I clinical trial in 18 patients with metastatic hormone-refractory prostate cancer was performed by Dieli's group.36 Subjects were randomized into two groups, which received either zoledronate alone (group A: 4 mg i.v. every 21 days) or zoledronate with IL-2 (group B: identical dose of zoledronate, plus 0.6 million IU IL-2 s.c. immediately after zoledronate). All patients also received calcium supplements and vitamin D daily, and the treatment was performed for 1 year. Toxicity in the patients was moderate, including a transient flu-like syndrome 3 days after the first administration of zoledronate/IL-2 (67% of subjects) or zoledronate alone (22%), as reported earlier for multiple myeloma and NHL patients.32 A significant clinical response, including higher survival, was observed in group B during the 1-year follow-up. In addition, this outcome correlated with greater numbers of blood γδ T cells and with increased rates of effector memory TCRVγ9Vδ2+ T cells (i.e., γδ cells producing IFN-γ, perforin and TNF-related apoptosis-inducing ligand (TRAIL)). Using an identical treatment regimen as the zoledronate/IL-2 combination trial, a phase I trial was conducted by the same group in 10 therapeutically terminal, advanced metastatic breast cancer patients. With results from three γδ cell-responsive patients reaching either disease stabilization (two patients) or partial remission (one patient), the authors could correlate the clinical outcomes to blood γδ T-cell numbers.37
In France, using the synthetic phosphoantigen BrHPP (IPH1101), Bennouna and colleagues38 conducted a phase I trial in 28 patients with solid tumors to determine the maximum-tolerated dose and safety of this drug when combined with low doses of IL-2. Patients received BrHPP alone (1 h i.v. infusions with increasing dosages from 200 to 1800 mg/m2) during cycle 1 and then received BrHPP in combination with IL-2 (1 million IU/m2, s.c. from day 1 to day 7) during subsequent cycles (day 1, every 3 weeks). Pharmacodynamic data from the resulting 109 treatment cycles demonstrated that BrHPP induces in vivo γδ T-cell amplifications, for which the magnitudes depend upon the dose of phosphoantigen and presence of IL-2. The treatment was generally well tolerated at most doses, causing little dose-limiting toxicity, except for the appearance of cytokine release syndrome following the first infusion of the highest dose (1800 mg/m2) in two patients. Given these findings, the conditions for safe and efficient use of BrHPP/IL-2 in patients have now been determined.
Given BrHPP's safety profile, its in vivo bioactivity and its use in a preclinical study with BrHPP/IL-2 and rituximab in NHL patients,15 a multicentric phase II study using the drug was launched by Innate Pharma in relapsed follicular lymphoma patients. This study involved 45 follicular lymphoma patients who had previously received (up to four) previous lines of therapy using rituximab, at least once. The primary objective was to assess the efficacy of BrHPP compared to that of rituximab alone, which is 40% of overall responses (Davis, JCO, 2000). The secondary objective was to establish the pharmaco-dynamics (PD) of BrHPP in these patients. Patients received rituximab (375 mg/m2, 4 times, weekly) on days 0, 7, 14 and 21, BrHPP (750 mg/m2, 3 times, every 3 weeks) plus IL-2 (8 M IU daily for 5 days, every 3 weeks) on days 8, 29 and 50, and IL2 alone (identical dose) on days +1, +2, +3 and +4 after each BrHPP/IL-2 injection. Side effects were easily managed in most patients. The treatment induced strong and specific amplification of TCRVγ9Vδ2+ T lymphocytes in 39 of the 45 patients, although this response peaked 1 week after the first injection of BrHPP and further declined upon subsequent injections. However, these cells showed phenotypic and functional maturation into Th1 and cytotoxic effector memory cells expressing FcγRIIIa (CD16). Accordingly, the release of Th1 cytokines (IFN-γ, tumor necrosis factor-α), as well as rituximab-mediated antibody-dependent cell cytotoxicity (ADCC) activity, was maximal after the second and third injections of BrHPP. In parallel, natural killer cell numbers were slightly increased by the treatment, whereas no other IL2-responsive subset, including regulatory T cells (Tregs), was quantitatively affected. In addition to these pharmacodynamics, the overall clinical results could be drawn from 38 evaluable patients. This group consisted of 10 patients (26% of total) with complete response (CR) and led to 17 overall response rate (ORR) (45%). In addition, the therapeutic efficacy correlated with biological markers, with the greatest benefits being observed in patients with low follicular lymphoma international progression index (FLIPI) (Figure 1). Therefore, the combination of BrHPP and rituximab in immunotargeted therapy produces very encouraging results, particularly for follicular lymphoma patients with unfavorable FcγRIIIa gene polymorphisms (F/F or V/F, 95% of the patients), with very manageable side effects overall. Although such promising results would require large-scale, randomized clinical trials to validate this exciting new concept, these studies demonstrate the enormous anticancer potential of γδ cell-based therapies in combination with therapeutic antibodies.
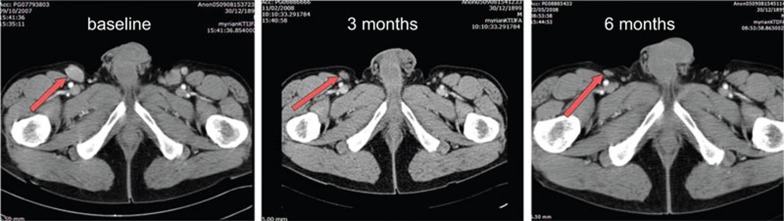
Figure 1.
Representative computed tomography (CT) scan showing BrHPP/RTX efficacy in an follicular lymphoma patient (age 55, high lactacte dehydrogenase (LDH), low follicular lymphoma international progression index (FLIPI)) who relapsed 20 months after a rituximab course.
Cancer immunotherapy trials with autologous γδ T cells have been investigated in parallel by Japanese, Australian and French groups. The French company Innate Pharma has conducted a phase I study in 10 patients with metastatic renal cell carcinoma39 to determine the maximum-tolerated dose of autologous TCRVγ9Vδ2+ γδ T cells and the safety of these cells as a therapeutic product. Patients received a 1-h i.v. infusion of their own TCRVγ9Vδ2+ γδ T lymphocytes during treatment cycle 1, after which the patients were given the lymphocytes in combination with a low dose of IL-2 (s.c., 2 million IU/m2 from day 1 to day 7) in the two subsequent cycles at 3-week intervals. The dose of TCRVγ9Vδ2+ γδ T cells was increased from 1×109 cells to as high as 8×109 cells. Immunomonitoring demonstrated that the injected cells were initially cleared from the blood and reappeared at the conclusion of the IL-2 administration. Dose-limiting toxicity (characterized by disseminated intravascular coagulation) occurred in one patient at a dosage of 8×109 cells; other adverse events included gastrointestinal disorders, flu-like symptoms, and hypotension with tachycardia, resulting from the co-administration of lymphocytes and IL-2. These results suggest that this cell-based therapy is well tolerated and therapeutically interesting, as six patients showed stabilized disease following this treatment.
In parallel with these studies, Kobayashi's group at Tokyo Women's Hospital investigated seven patients with advanced renal cell carcinoma engrafted with autologous γδ T cells.40 Patients received from 1 to 400 million of their autologous γδ cells preactivated using phosphoantigens (by i.v. infusion every week, 6–12 times with IL-2 at 0.7 million IU/patient, with i.v. infusion conducted over 2 h). All patients had IL-2 related non-severe adverse events during the therapy, indicating that the treatment was quite well tolerated, and four out of the seven patients showed a significant in vivo γδ T-cell expansion and production of IFN-γ in response to phosphoantigen in vitro. The clinical benefit was moderate, however, as only three out of the seven patients (42%) presented slower tumor growth and all of the patients died.
More recently, however, the same team reported a far better outcome for a patient with lung metastasis following a radical nephrectomy for a renal cell carcinoma that was unresponsive to IFN-α. This patient received the following treatment: zoledronate (4 mg i.v. over 30 min) simultaneously with IL-2 (1.4 million IU i.v. over 2 h) and phosphoantigen-preactivated autologous γδ cells (from 0.3×109 to 3.5×109 cells, i.v.) on day 0, followed daily by an identical dose of IL-2 over days 1–4. This monthly cycle was repeated over a period of 6 months. Although no serious adverse events occurred, this immunotherapy yielded much greater γδ T-cell counts and IFN-γ production in vivo, along with complete remission of the patient, which is currently ongoing 2 years following the study without treatment. Therefore, the authors concluded that γδ cell-based immunotherapy is a clinically beneficial and safe therapeutic option for patients with advanced renal cell carcinoma, whose rates of circulating γδ cells might constitute a favorable prognostic indicator.41
In parallel, Medinet and the Seta Clinic Group in Tokyo also validated the technical feasibility of large-scale, zoledronate-based γδ T-cell expansion for cell-based therapy. These groups examined the effects of i.v. injection of 2×109–4×109 cells/patient in six healthy volunteers, 10 patients with advanced non-small cell lung cancers, five patients with bone metastases from prostate or breast cancer, four patients with lung metastases from colon cancer42 and six multiple myeloma patients.43 This team further enrolled 25 additional patients carrying various solid tumors who had not undergone treatment with statins (blockers of endogenous phosphoantigen biosynthesis) for zoledronate-activated TCRVγ9Vδ2+ γδ T cell-based immunotherapy. Two of these patients received γδ cells 3 times, five patients 4 times, one patient 5 times and 17 patients 6 times. Overall, γδ cells from only three individuals were insufficiently amplified (<108 cells) by the process, whereas cells from all of the other patients expanded efficiently enough for the reinfusion. The authors also reported that patients who had been pre-treated with cells or concurrently treated with cells and either zoledronate or chemotherapy (or both) generally had suppressed γδ cell proliferation. Although the tumor response could not be assessed in all patients, six out of the 25 enrolled showed disease stabilization.44
Non-small cell lung cancer cells are also recognized and killed by human TCRVγ9Vδ2+ γδ T cell; therefore, 10 non-small cell lung cancer patients were enrolled to receive autologous γδ T-cell immunotherapy 3–12 times, every 2 weeks. One serious adverse event (grade 3 pneumonia) was recorded, and neither a complete response nor stabilization was observed. However, the ‘functional assessment of cancer therapy–biologic response modifier scores' of all but one patient were either stable or improved during immunotherapy. Furthermore, six patients were alive at the end of the observation (240–850 days), demonstrating the potential of γδ cell therapy for this condition.45 Likewise, Nicol's team in Brisbane, Australia recently reported that when autologous transplants of In111-radiolabelled TCRVγ9Vδ2+ γδ T cells were tracked in 18 patients with advanced stages of various solid tumors, the cells trafficked predominantly to the lungs, liver and spleen in all patients but also to metastatic tumor sites in some patients. No dose-limiting toxicity was reported in this study, and three out of the 18 patients had clinical responses while continuing their previously ineffective chemotherapy. These observations led the authors to conclude that zoledronate-activated TCRVγ9Vδ2+ γδ T cells offer clinical benefits when administered in combination with other drugs.16
Lesson I: advances in γδ T-cell cancer immunotherapies
Safety of γδ T-cell activation in patients
The aforementioned pioneering trials have defined conditions for the safe use of phosphoantigens and zoledronate for the activation of γδ T cells in patients. Flu-like symptoms, but no γδ cell expansion, are generally induced with low doses of stimuli (e.g., 200–1800 mg/m2 BrHPP) without IL-2, while higher doses of stimuli co-injected with IL-2 increase the level of γδ cell expansion and concomitantly increase the frequency of drug-related toxicity and severity of adverse events. The grade 3 and 4 severity of adverse events that has recurrently been reported is characterized by thrombophlebitis, thrombosis, hyperglycemia, hypocalcemia, chest and musculoskeletal pain, gastritis, myocardial infarction and renal creatinine toxicity. When these drugs are administered concurrently with IL-2, the maximum tolerable doses for BrHPP and zoledronate are 1500 and 4 mg/m2 (every 28 days), respectively. The latter treatment induces febrile symptoms comparable to those induced by BrHPP, but these symptoms are accompanied by calcium release toxicity and osteonecrosis of the jaw in rare cases.
The pharmacodynamics of phosphoantigens administered to humans has been established
When injected i.v. into non-human primates, BrHPP can activate circulating γδ cells, despite its extremely short half-life (only a few minutes long).46 The half-life of zoledronate, in contrast, is far longer (146 h). Whichever stimulus is utilized to induce γδ T cells, the kinetics of the ensuing proliferation of γδ T cells in human blood typically peaks between days 6 and 8, and the amplitude of the proliferative response directly relates to the presence of IL-2 and the dose of BrHPP or bisphosphonate. Most specifically in regard to these lymphocytes, the antigen-driven activation of these cells leads to progressively decreased responses to subsequent stimulations, or tachyphylaxis.38,46
Dependence on IL-2 for γδ cell activation in vivo
The use of IL-2 in cancer immunotherapy has been widely debated, owing to this cytokine's intrinsic toxicity. In spite of this toxicity, several conditions can be treated with low-dose IL-2-based regimens, such as renal and prostate carcinomas. Therefore, the aforementioned protocols have carefully determined the optimal doses and formulations for the use of this cytokine in therapies harnessing γδ T cells, resulting in limited IL-2-related adverse events. In these trials, IL-2 also elevated the frequency of Tregs, which can inhibit γδ T-cell responses or even induce regulatory γδ T cells, according to in vitro experiments.47,48 The reverse situation, however, might also occur in these patients, as phosphoantigen-activated γδ T cells can overcome Tregs49 and TGF-β immunosuppressive functions.50 The above trials demonstrated that Tregs were not significantly induced by these regimens involving only low doses of IL-2, and they did not offer evidence of Treg-based γδ T-cell immunosuppression. Therefore, although the utilization of IL-2 in these trials was a concern, its use did not hamper clinical responses. However, this issue highlights the need for investigating new methods that will activating γδ T cells using IL-2-free protocols.
Lesson II: remaining issues
Autologous γδ T lymphocyte-based cell therapy is now feasible and safe but technically more demanding than PAG/IL-2 injection in patients. The advantage of this type of therapy is the ability to produce highly controlled amplification ex vivo, opening the possibility of further modifying the growing cells throughout the culture process, by supplementation of the cytokine supply, for example. Furthermore, this method theoretically opens the possibility of expanding the cells more efficiently ex vivo for hyporesponsive patients in which PAG/IL2 would otherwise fail to induce a strong response. The manufacturing process has specific Good Manufacture Practice constraints to respect, but protocols for large-scale expansions (>1000-fold) of TCRVγ9Vδ2+ γδ T cells are now available, albeit costly. Using well-defined protocols, it is now possible to produce batches of several billion (2×109 to 1010) γδ cells from blood samples within 2 weeks. For autologous transplantation, however, the produced batches of cells must meet certain requirements for cell purity, including frequency of TCR γδ cells (>70% appears to be a reasonable threshold).39,51 These thresholds might lessen the number of batches available to reinject into patients, as the batches might not meet the requirements either in cell number or cell purity. Although these are obvious technical limitations, such autologous γδ cell infusions are safe and well tolerated, as they induce an identical spectrum of symptoms as those induced by phosphoantigens alone (as mentioned above), but not those of zoledronate. Despite these limitations, autologous γδ T lymphocyte-based cell therapy demonstrates signs of clinical efficacy, demonstrating the therapeutic potential of this method in cancer immunotherapy.
Activation-induced γδ T-cell anergy has repeatedly been reported in most, if not all, in vivo studies in patients. Similar observations had been made in nonhuman primates, through either activation with BrHPP/IL2 alone15,46 or in antituberculous vaccine trials.52 The underlying molecular basis of this process still remains unknown but could conceivably be attributed to inadequate activation conditions. This condition could possibly result from absent signals delivered to the γδ cells during the activation process, from suboptimal exposure to phosphoantigens or from TCRVγ9Vδ2+ γδ T cell-intrinsic physiological characteristics not found in αβ T cells. Our lack of knowledge on this issue represents an obvious limitation to the broader use of γδ cells in cancer therapy. Indeed, most currently available trials demonstrate evidence of some clinical benefits in patients, but these benefits most likely result from ‘one shot' in vivo responses by γδ T cells.
Immunoescape by cancer cells remains an important issue for γδ cell-based strategies. Indeed, a growing body of evidence demonstrates that cancer cells develop an ability to avoid checkpoints introduced by the immune system and that tumors differ in regard to the pathways used to avoid their own immune-mediated destruction.
It has been suggested that tumor progression could be thwarted by γδ cell-induced responses. This concept was recently validated by a clinical study on the treatment of 21 adult patients with advanced renal cell carcinoma, malignant melanoma and acute myeloid leukemia with zoledronate/IL-2 (low-dose). This phase I/II trial underwent a total of 58 treatment cycles to assess the safety, pharmacodynamics and antitumor activity of the treatment. In addition to reporting standard levels of safety, the authors reported significant γδ cell activation (quantitated by IFN-γ production) and expansion in all evaluable patients but no objective responses in either of the cohorts with solid tumors. By contrast, two patients with acute myeloid leukemia achieved objective tumor responses, namely partial remission. Most interestingly, Kunzmann and colleagues noticed an unexpected increase in serum VEGF in response to zoledronate/IL-2 and found that this spike correlated with a lack of clinical response.53 Therefore, the authors concluded that γδ cell-derived VEGF induced by the treatment can limit clinical outcomes by harnessing innate tumor immunity. Transcriptome profiling of TCRVγ9Vδ2+ γδ T lymphocytes revealed that these cells upregulate expression of VEGF upon activation,54 raising a special consideration for angiogenesis-dependent solid tumors.
Immunoevasion strategies that specifically protect against γδ cell functions can involve diverse cellular and molecular mediators (reviewed in Refs. 55 and 56) which have the potential to have impacted the outcome of the above trials. For example, tumor-derived PGE2 is a potent γδ T-cell immunosuppressive mediator,57,58 but this variable was neither considered nor monitored in the aforementioned assays. The potent immunosuppressor TGF-β, which is frequently secreted by human cancers, is capable of repressing cytotoxic immune responses, although the bioactivity of this molecule can be counterbalanced by augmenting its activation with phosphoantigens.50 There are currently few case reports, however, depicting a tumor cell relapse following a therapeutic response to γδ T cell-based immunotherapy. Nevertheless, the aforementioned trials only involved patients with advanced and relapsing metastatic renal cancers, prostate cancer and B-cell lymphomas. Such evolved tumor cells carry massive genetic alterations and are therefore able to suppress all steps of immune action, namely, the recruitment of, engagement by and function of cytolytic cells.59,60,61,62 Additional tumor-extrinsic criteria, such as the genotype of the patient, can also impact the clinical outcome. Efficacy of the treatment in the relapsing follicular lymphoma patients was expected to result from the enhancement of ADCC by rituximab/BrHPP/IL-2 co-administration. Indeed, polymorphisms in the IgG Fc receptor FcγRIIIa gene strongly affect the efficacy of ADCC in patients undergoing rituximab-based therapies63 and the individual pharmacokinetics for each patient.64 Therefore, genotyping patients and determining their cancer biomarkers prior to the use of γδ cell-based protocols could provide patients with the most appropriate options. A primary effort in this direction is the integration of γδ cell-based therapies and drugs for which ADCC-based therapeutic activity is independent of FcγRIIIa polymorphisms, such as the more recent anti-CD20 antibodies.
The future of γδ T-cell cancer immunotherapies
To be successful, cancer immunotherapies involving γδ cells will require updated protocols that limit anergy and the use of drugs able to overcome immunoescape. Although the former contingency is currently an open issue, the second is already well underway. Indeed, ongoing therapies against cancer immunoescape include nonspecific immune stimulation by IL-2, IFN-α, cyclophosphamide, TLR agonists (e.g., imiquimod and resiquimod), the BCG vaccine and biomolecules such as the anti-CD25 mAb or the IL-2-diphtheria toxin conjugate. By inducing immunogenic cell death during chemotherapy, anthracyclins might also present this bioactivity.65 Ongoing and recently completed clinical trials against cancer immunoescape also involve monoclonal antibodies blocking PD1, PDL1 and CTLA4, while several other cancer vaccines and cell transfer strategies are also underway. In addition, several new therapeutic monoclonal antibodies against tumor cell antigens are now rapidly progressing through phase I-III trials, and some therapeutic successes will undoubtedly emerge with these new drugs. Therefore, novel regimens that combine such drugs with γδ cell-based strategies, either with phosphoantigens or with γδ T cells, are currently being considered and investigated by translational research labs around the world. These avenues will undoubtedly represent the next frontier for immunotherapeutic innovations in cancer research.
References
- Meraviglia S, Caccamo N, Guggino G, Tolomeo M, Siragusa S, Stassi G, et al. Optimizing tumor-reactive γδ T cells for antibody-based cancer immunotherapy. Curr Mol Med. 2010;10:719–726. doi: 10.2174/156652410793384150. [DOI] [PubMed] [Google Scholar]
- Kabelitz D. Human γδ T lymphocytes for immunotherapeutic strategies against cancer. F1000 Med Rep. 2010;2:45. doi: 10.3410/M2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann V, Wilhelm M. Adjuvant zoledronic acid for breast cancer: mechanism of action. Lancet Oncol. 2011;12:991–992. doi: 10.1016/S1470-2045(11)70252-2. [DOI] [PubMed] [Google Scholar]
- Hannani D, Ma Y, Yamazaki T, Dechanet-Merville J, Kroemer G, Zitvogel L. Harnessing γδ T cells in anticancer immunotherapy. Trends Immunol. 2012;33:199–206. doi: 10.1016/j.it.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, et al. Vγ9Vδ2T cell response to colon carcinoma cells. J Immunol. 2005;175:5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- Viey E, Laplace C, Escudier B. Peripheral γδ T-lymphocytes as an innovative tool in immunotherapy for metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2005;5:973–986. doi: 10.1586/14737140.5.6.973. [DOI] [PubMed] [Google Scholar]
- Dechanet J, Merville P, Berge F, Bone-Mane G, Taupin JL, Michel P, et al. Major expansion of γδ T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J Infect Dis. 1999;179:1–8. doi: 10.1086/314568. [DOI] [PubMed] [Google Scholar]
- Viey E, Lucas C, Romagne F, Escudier B, Chouaib S, Caignard A. Chemokine receptors expression and migration potential of tumor-infiltrating and peripheral-expanded Vγ9Vδ2 T cells from renal cell carcinoma patients. J Immunother. 2008;31:313–323. doi: 10.1097/CJI.0b013e3181609988. [DOI] [PubMed] [Google Scholar]
- Inman BA, Frigola X, Harris KJ, Kuntz SM, Lohse CM, Leibovich BC, et al. Questionable relevance of γδ T lymphocytes in renal cell carcinoma. J Immunol. 2008;180:3578–3584. doi: 10.4049/jimmunol.180.5.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng BJ, Ng SP, Chua DT, Sham JS, Kwong DL, Lam CK, et al. Peripheral γδ T-cell deficit in nasopharyngeal carcinoma. Int J Cancer. 2002;99:213–217. doi: 10.1002/ijc.10326. [DOI] [PubMed] [Google Scholar]
- Zheng BJ, Chan KW, Im S, Chua D, Sham JS, Tin PC, et al. Anti-tumor effects of human peripheral γδ T cells in a mouse tumor model. Int J Cancer. 2001;92:421–425. doi: 10.1002/ijc.1198. [DOI] [PubMed] [Google Scholar]
- Capietto AH, Martinet L, Fournie JJ. Stimulated γδ T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J Immunol. 2011;187:1031–1038. doi: 10.4049/jimmunol.1100681. [DOI] [PubMed] [Google Scholar]
- Yuasa T, Sato K, Ashihara E, Takeuchi M, Maita S, Tsuchiya N, et al. Intravesical administration of γδ T cells successfully prevents the growth of bladder cancer in the murine model. Cancer Immunol Immunother. 2009;58:493–502. doi: 10.1007/s00262-008-0571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S, et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–4884. doi: 10.1182/blood-2008-08-172296. [DOI] [PubMed] [Google Scholar]
- Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, et al. Clinical evaluation of autologous γδ T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105:778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialasiewicz AA, Ma JX, Richard G. α/β− and γ/δ TCR+ lymphocyte infiltration in necrotising choroidal melanomas. Br J Ophthalmol. 1999;83:1069–1073. doi: 10.1136/bjo.83.9.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud C, Bilhere E, Loizon S, Pitard V, Behr C, Moreau J, et al. Antitumor activity of γδ T cells reactive against cytomegalovirus-infected cells in a mouse xenograft tumor model. Cancer Res. 2009;69:3971–3978. doi: 10.1158/0008-5472.CAN-08-3037. [DOI] [PubMed] [Google Scholar]
- Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique Toll-like receptor signaling pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Ke Y, Kapp LM, Kapp JA. Inhibition of tumor rejection by γδ T cells and IL-10. Cell Immunol. 2003;221:107–114. doi: 10.1016/s0008-8749(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Sicard H, Al Saati T, Delsol G, Fournie JJ. Synthetic phosphoantigens enhance human Vγ9Vδ2 T lymphocytes killing of non-Hodgkin's B lymphoma. Mol Med. 2001;7:711–722. [PMC free article] [PubMed] [Google Scholar]
- Gertner-Dardenne J, Castellano R, Mamessier E, Garbit S, Kochbati E, Etienne A, et al. Human Vγ9Vδ2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immunol. 2012;188:4701–4708. doi: 10.4049/jimmunol.1103710. [DOI] [PubMed] [Google Scholar]
- D'Asaro M, La Mendola C, Di Liberto D, Orlando V, Todaro M, Spina M, et al. Vγ9Vδ2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol. 2010;184:3260–3268. doi: 10.4049/jimmunol.0903454. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- Burjanadze M, Condomines M, Reme T, Quittet P, Latry P, Lugagne C, et al. In vitro expansion of γδ T cells with anti-myeloma cell activity by Phosphostim and IL-2 in patients with multiple myeloma. Br J Haematol. 2007;139:206–216. doi: 10.1111/j.1365-2141.2007.06754.x. [DOI] [PubMed] [Google Scholar]
- Bryant NL, Suarez-Cuervo C, Gillespie GY, Markert JM, Nabors LB, Meleth S, et al. Characterization and immunotherapeutic potential of γδ T-cells in patients with glioblastoma. Neuro Oncol. 2009;11:357–367. doi: 10.1215/15228517-2008-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb LS., Jr γδ T cells as immune effectors against high-grade gliomas. Immunol Res. 2009;45:85–95. doi: 10.1007/s12026-009-8114-9. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, Pitters E, Zoller M. Potential of human γδ T lymphocytes for immunotherapy of cancer. Int J Cancer. 2004;112:727–732. doi: 10.1002/ijc.20445. [DOI] [PubMed] [Google Scholar]
- Bonneville M, O'Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- Belmant C, Decise D, Fournie JJ. Phosphoantigens and aminobisphosphonates: New leads targeting γδ T lymphocytes for cancer immunotherapy. Drug Discov Today. 2006;3:17–23. [Google Scholar]
- Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced γ,δ-T-cell proliferation and activation in vitro. . J Bone Miner Res. 2004;19:278–288. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, et al. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, et al. Induction of γδ T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. . Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- Lang JM, Kaikobad MR, Wallace M, Staab MJ, Horvath DL, Wilding G, et al. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1447–1460. doi: 10.1007/s00262-011-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoe M, Ogawa Y, Takeshita K, Morita J, Shichijo T, Fuji K, et al. Zoledronate stimulates γδ T cells in prostate cancer patients. Oncol Res. 2010;18:493–501. doi: 10.3727/096504010x12671222663638. [DOI] [PubMed] [Google Scholar]
- Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, et al. In vivo manipulation of Vγ9Vδ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennouna J, Levy V, Sicard H, Senellart H, Audrain M, Hiret S, et al. Phase I study of bromohydrin pyrophosphate (BrHPP, IPH 1101), a Vγ9Vδ2 T lymphocyte agonist in patients with solid tumors. Cancer Immunol Immunother. 2010;59:1521–1530. doi: 10.1007/s00262-010-0879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C, et al. Phase-I study of Innacell γδ, an autologous cell-therapy product highly enriched in γ9δ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, et al. Safety profile and anti-tumor effects of adoptive immunotherapy using γ-δ T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Tanaka Y, Nakazawa H, Yagi J, Minato N, Tanabe K. A new indicator of favorable prognosis in locally advanced renal cell carcinomas: γδ T-cells in peripheral blood. Anticancer Res. 2011;31:1027–1031. [PubMed] [Google Scholar]
- Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S, et al. Zoledronate facilitates large-scale ex vivo expansion of functional γδ T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10:842–856. doi: 10.1080/14653240802419328. [DOI] [PubMed] [Google Scholar]
- Abe Y, Muto M, Nieda M, Nakagawa Y, Nicol A, Kaneko T, et al. Clinical and immunological evaluation of zoledronate-activated Vγ9γδ T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956–968. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M, et al. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy. 2011;13:92–97. doi: 10.3109/14653249.2010.515581. [DOI] [PubMed] [Google Scholar]
- Nakajima J, Murakawa T, Fukami T, Goto S, Kaneko T, Yoshida Y, et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous γδ T cells. Eur J Cardiothorac Surg. 2010;37:1191–1197. doi: 10.1016/j.ejcts.2009.11.051. [DOI] [PubMed] [Google Scholar]
- Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M, et al. In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Kimmel B, Herrmann T, Einsele H, Wilhelm M. Inhibition of phosphoantigen-mediated γδ T-cell proliferation by CD4+ CD25+ FoxP3+ regulatory T cells. Immunology. 2009;126:256–267. doi: 10.1111/j.1365-2567.2008.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, et al. Cutting edge: TGF-beta1 and IL-15 induce FOXP3+ γδ regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183:3574–3577. doi: 10.4049/jimmunol.0901334. [DOI] [PubMed] [Google Scholar]
- Gong G, Shao L, Wang Y, Chen CY, Huang D, Yao S, et al. Phosphoantigen-activated Vγ2Vδ2 T cells antagonize IL-2-induced CD4+CD25+Foxp3+ T regulatory cells in mycobacterial infection. Blood. 2009;113:837–845. doi: 10.1182/blood-2008-06-162792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capietto AH, Martinet L, Cendron D, Fruchon S, Pont F, Fournie JJ. Phosphoantigens overcome human TCRVγ9+ γδ Cell immunosuppression by TGF-β: relevance for cancer immunotherapy. J Immunol. 2010;184:6680–6687. doi: 10.4049/jimmunol.1000681. [DOI] [PubMed] [Google Scholar]
- Salot S, Bercegeay S, Dreno B, Saiagh S, Scaglione V, Bonnafous C, et al. Large scale expansion of Vγ9Vδ2 T lymphocytes from human peripheral blood mononuclear cells after a positive selection using MACS ‘TCR γ/δ+ T cell isolation kit'. J Immunol Methods. 2009;347:12–18. doi: 10.1016/j.jim.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Cendron D, Ingoure S, Martino A, Casetti R, Horand F, Romagne F, et al. A tuberculosis vaccine based on phosphoantigens and fusion proteins induces distinct γδ and alphabeta T cell responses in primates. Eur J Immunol. 2007;37:549–565. doi: 10.1002/eji.200636343. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Smetak M, Kimmel B, Weigang-Koehler K, Goebeler M, Birkmann J, et al. Tumor-promoting versus tumor-antagonizing roles of γδ T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother. 2012;35:205–213. doi: 10.1097/CJI.0b013e318245bb1e. [DOI] [PubMed] [Google Scholar]
- Pont F, Familiades J, Dejean S, Fruchon S, Cendron D, Poupot M, et al. The gene expression profile of phosphoantigen-specific human γδ T lymphocytes is a blend of alphabeta T-cell and NK-cell signatures. Eur J Immunol. 2012;42:228–240. doi: 10.1002/eji.201141870. [DOI] [PubMed] [Google Scholar]
- Martinet L, Poupot R, Fournie JJ. Pitfalls on the roadmap to γδ T cell-based cancer immunotherapies. Immunol Lett. 2009;124:1–8. doi: 10.1016/j.imlet.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Capietto AH, Martinet L, Fournie JJ. How tumors might withstand γδ T-cell attack. Cell Mol Life Sci. 2011;68:2433–2442. doi: 10.1007/s00018-011-0705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet L, Jean C, Dietrich G, Fournie JJ, Poupot R. PGE2 inhibits natural killer and γδ T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem Pharmacol. 2010;80:838–845. doi: 10.1016/j.bcp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Martinet L, Fleury-Cappellesso S, Gadelorge M, Dietrich G, Bourin P, Fournie JJ, et al. A regulatory cross-talk between Vγ9Vδ2 T lymphocytes and mesenchymal stem cells. Eur J Immunol. 2009;39:752–762. doi: 10.1002/eji.200838812. [DOI] [PubMed] [Google Scholar]
- Challa-Malladi M, Lieu YK, Califano O, Holmes AB, Bhagat G, Murty VV, et al. Combined genetic inactivation of β2-microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20:728–740. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilaveanu LB, Sznol J, Aziz SA, Duchen D, Kluger HM, Camp RL. CD70 expression patterns in renal cell carcinoma. Hum Pathol. 2012;43:1394–9. doi: 10.1016/j.humpath.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegmann J, Junker K, Loncarevic IF, Michel S, Schimmel B, von Eggeling F. Immune escape for renal cell carcinoma: CD70 mediates apoptosis in lymphocytes. Neoplasia. 2006;8:933–938. doi: 10.1593/neo.06451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas K, Richmond A, Rayman P, Biswas S, Thornton M, Sa G, et al. GM2 expression in renal cell carcinoma: potential role in tumor-induced T-cell dysfunction. Cancer Res. 2006;66:6816–6825. doi: 10.1158/0008-5472.CAN-06-0250. [DOI] [PubMed] [Google Scholar]
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- Cartron G, Trappe RU, Solal-Celigny P, Hallek M. Interindividual variability of response to rituximab: from biological origins to individualized therapies. Clin Cancer Res. 2011;17:19–30. doi: 10.1158/1078-0432.CCR-10-1292. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]