Abstract
Phenotypic and functional heterogeneity are the hallmarks of effector and memory T cells. Upon antigen stimulation, γδ T cells differentiate into two major types of memory T cells: central memory cells, which patrol the blood and secondary lymphoid organs, and effector memory cells, which migrate to peripheral tissues. γδ T cells display in vitro a certain degree of plasticity in their function that is reminiscent of that which is observed in conventional CD4 T cells. Similar to CD4 T cells, in which a plethora of specialized subsets affect the host response, γδ T cells may readily and rapidly assume distinct Th1-, Th2-, Th17-, TFH and T regulatory-like effector functions, suggesting that they profoundly influence cell-mediated and humoral immune responses. In addition to differences in cytokine repertoire, γδ T cells exhibit diversity in homing, such as migration to lymph node follicles, to help B cells versus migration to inflamed tissues. Here, we review our current understanding of γδ T-cell lineage heterogeneity and flexibility, with an emphasis on the human system, and propose a classification of effector γδ T cells based on distinct functional phenotypes.
Keywords: cytokines, effector and memory cells, γδ T cells, lineage-specifying factors, T-cell subsets
Introduction
Most human peripheral blood γδ T cells express a T-cell receptor (TCR) that consists of the Vγ9 and Vδ2 chains (here and thereafter referred to as Vγ9Vδ2 T cells) and that recognizes non-peptidic phosphorylated metabolites of isoprenoid biosynthesis that are produced by microorganisms and stressed cells.1 Once primed, γδ T cells migrate to follicles in order to help B cells to produce antibodies and to peripheral sites of antigen exposure to fight incoming pathogens by delivering the appropriate type of effector cell function.2 Upon activation, Vγ9Vδ2 cells can be skewed toward distinct effector functions depending on the polarizing cytokines that are present, similarly to CD4 helper T (Th) cells.3,4,5 Accordingly, under appropriate culture conditions, Vγ9Vδ2 cells divert from the typical Th1-like phenotype and polarize to Th2,5,6 Th17,7,8 TFH and T regulatory cells.9 Such a broad plasticity emphasizes the capacity of Vγ9Vδ2 cells to influence the nature of the immune response to different challenges.
Pioneering studies demonstrated that Th1 effector cells produce interferon (IFN)-γ, which promotes the clearance of viruses and intracellular bacteria, while Th2 cells produce IL-4, IL-5 and IL-13, which promote the clearance of extracellular parasites.10,11 Once the antigen is eliminated, central memory (TCM) and effector memory (TEM) T cells persist in the memory pool to provide systemic immune surveillance in lymphoid organs and in peripheral non-lymphoid tissues to react promptly in case of rechallenge.12 To accomplish this, T cells retain a memory not only of the cytokines to be produced, but also of the site where effector function will be needed. The distinction of Th1 and Th2 cells and of TCM and TEM subsets has provided the initial paradigms with which to understand the functional organization of the immune response.10,12
In contrast to CD4 T cells, γδ T cells default toward type 1 cytokine production and predominantly produce IFN-γ upon activation. However, as with αβ T cells, γδ T cells may differentiate into IFN-γ (γδ1)- and IL-4 (γδ2)-producing cells.5,6 Priming under Th1 conditions (IL-12 plus anti-IL-4 monoclonal antibodies) induces a Th1 profile that is characterized by increased secretion of IFN-γ and tumor-necrosis factor (TNF)-α, whereas Th2 conditions (IL-4) induce increased production of IL-4 (Th2 profile) by the γδ T cells. These results indicate that the major subset of human γδ T cells can be polarized into either a Th1 or a Th2 cytokine pattern depending on the cytokine milieu in which contact with the antigen occurs. However, the molecular mechanisms that underlie human γδ T-cell polarization have yet to be defined.
Th1 differentiation is promoted by IL-12 (and, in humans, also by type I IFN)13,14 and requires expression of the transcription factor T-bet, which mediates inheritable modifications of the IFN-γ gene,14 leading to its expression following antigenic stimulation. In contrast, Th2 differentiation is promoted by IL-4 and requires expression of the transcription factor GATA-3,15 which mediates inheritable modifications of the IL-4, IL-5 and IL-13 genes.16
In the mouse, the molecular basis for the default production of IFN-γ by γδ T cells, in comparison to CD4 T cells, is a high level of T-bet expression versus that of GATA-3, whereas the paucity of IL-4 synthesis in these cells is secondary to the low level of GATA-3 expression.17 However, while an increase in GATA-3 expression augments IL-4 levels in γδ T cells, it fails to concomitantly downregulate IFN-γ production, unlike that which was observed in CD4 T cells,17 and an uncoupling of the functional antagonism between GATA-3 and T-bet forms the molecular basis for the default production of IFN-γ by Vγ9Vδ2 T cells (our unpublished data).
Moreover, epigenetic and transcriptional programs both regulate IFN-γ production in γδ T cells,18 as evidenced by three findings: (i) the kinetics of IFN-γ transcription are increased in γδ T cells compared with that observed in CD4 and CD8 T cells, and γδ T cells produce significantly greater amounts of IFN-γ in a proliferation-independent manner when compared with other T-cell subsets; (ii) the intron 1 region of the Ifnγ locus is hypomethylated in γδ T cells relative to the same element in naive CD4 and CD8 T cells; and (iii) γδ T cells constitutively express eomesodermin (Eomes), a transcription factor important for IFN-γ production in CD8 T cells, and Eomes expression levels are enhanced upon activation, indicating a critical role for this transcription factor in mediating IFN-γ production by γδ T cells in a T-bet-independent manner.18 Our preliminary results indicate that epigenetic mechanisms also regulate IFN-γ production in human γδ T cells.
Expanding the γδ1–γδ2 paradigm. Part I: γδ17 and γδreg
Recent studies indicate that the binary paradigm of Th1 and Th2 cells represents an oversimplification. These studies indicate the existence of multiple pathways of effector T-cell differentiation and multiple layers of memory T cells, which provide tailored mechanisms of protection and immune surveillance against different pathogens in different tissues.
Based on seminal work on CD4 Th1 and Th2 cells, a T-cell lineage is defined as a cell population in which a change in cytokine production is promoted by polarizing signals and stably imprinted by a lineage-specifying transcription factor through epigenetic mechanisms.19 In healthy adults, 50%–80% of blood Vγ9Vδ2 T cells have a distinctive Th1 signature and produce IFN-γ and TNF-α, but fewer than 1% produce IL-17.5,20 Ribot et al.21 and the Chien group22 have demonstrated that murine γδ T cells acquire their capacity to produce IL-17 or IFN-γ in the thymus, and they have defined TCR and CD27 signals as key determinants of this thymic functional/developmental choice. Whether human Vγ9Vδ2 T cells follow similar rules of developmental pre-programming as their mouse counterparts remains to be answered.
Interestingly, human cord blood and neonatal peripheral blood Vγ9Vδ2 T cells promptly secrete IFN-γ. To emphasize that functional competence in this cell population is acquired in utero, IFN-γ is produced by Vγ9Vδ2 T cells from prematurely born infants, and although a 1-month post-partum environmental exposure invariably increased TNF-α production, it had no consistent effect on IFN-γ production.23
In human Vγ9Vδ2 T cells, polarization towards IL-17 production is efficiently induced by coordinated antigen stimulation of the specific TCR, a combination of the polarizing cytokines IL-1β, IL-6, transforming growth factor (TGF)-β and IL-23 and aryl hydrocarbon receptor (AHR) ligands.8 Differentiation to γδ17 T cells is due to high levels of RORC and AHR expression versus a low level of T-bet expression in these cells.8 γδ17 T cells exhibit a terminally differentiated phenotype, illustrated by the expression of CD45RA in the absence of CD27 and by the expression of CD161, TRAIL, FasL, granzyme B and the chemokine receptor CCR6.8 Thus, the selective expression of characteristic markers of the Th17 lineage (RORC, IL-17 and CCR6) on γδ17 T cells and the requirement for a medium that is rich in aromatic amino acids support the concept that there is a coordinated regulation of migratory capabilities and effector functions in differentiating γδ17 T cells. Notably, the CCR6 agonist CCL20, which is constitutively expressed in normal skin and mucosa-associated tissues, is upregulated by IL-1724 and mediates the recruitment of T cells and dendritic cells to sites of inflammation.25 In addition, γδ17 T cells rapidly induce IL-17-dependent production of β-defensin, another CCR6 agonist,26 by epithelial cells, and promote CXCL8-mediated recruitment and enhancement of neutrophil phagocytosis.27 A striking consequence of these findings is that TCR engagement is required in the differentiation of human γδ17 T cells, in contrast with mouse studies in which the role of the TCR in γδ17 T cells may be redundant, in concordance with their predetermined phenotype in the thymus, without positive or negative selection. Thus, deciphering the relative roles of cytokines and of TCR-dependent or TCR-independent activation of human γδ17 T cells and their role in protective immune response or in pathology is a great challenge for the potential use of these cells in clinical settings.
Th17 responses are important for the host defense against microorganisms, particularly extracellular bacteria.28 IL-17 that is produced by migrating Vγ9Vδ2 T cells may trigger a positive feedback loop that further attracts Th17 and Th1 cells, as well as dendritic cells and neutrophils, and amplifies host inflammatory responses. Accordingly, we found that in the blood and cerebrospinal fluid of children suffering from bacterial meningitis, 60%–70% of the Vγ9Vδ2 T cells were γδ17 T cells.8 Interestingly, this phenotype was reversed after successful antibacterial therapy.8 Of note, Vγ9Vδ2 T cells are not the only human T-cell subset capable of producing IL-17; Vδ1 T cells also can produce IL-17 specifically in the context of HIV-1 infection, in which they are markedly expanded.29
In our experimental system,8 antigen-stimulated γδ17 T cells produce neither IL-22 nor IFN-γ, in contrast with studies by Morita and colleagues,7 which show that γδ17 T cells also produce IL-22 and/or IFN-γ. This finding is consistent with the concept that although they retain the memory of the imprinted cytokine, polarized T cells may undergo further differentiation in response to polarizing cues. Alternatively, it is also possible that γδ17 T cells may be re-programmed to differentiate to IL-22- and/or IFN-γ-producing cells, as has been shown for cells that had been previously committed to Th1 or Th2 differentiation.30 The commitment and flexibility of effector T-cell populations are most likely controlled by the balanced expression of lineage-specifying transcription factors.31 It is plausible that under certain conditions of antigenic stimulation, cytokine microenvironment or both, γδ T cells may differentiate into multifunctional cells that are able to trigger additional responses in the periphery.
IL-22 was originally described in mice and humans as a cytokine that is characteristic of Th17 cells;32,33 however, further studies showed that a distinct subset of human skin-homing memory T cells produced IL-22 but neither IL-17 nor IFN-γ.34,35 Differentiation of IL-22 producing T cells can be promoted by the stimulation of naive T cells in the presence of IL-6 and TNF or by plasmacytoid “dendritic cells. This differentiation appears to be independent from RORC, but dependent on the AHR.34,35 Similar to our findings,8 studies of human CD4 T cells that were differentiated under IL-17-polarizing conditions have found production of IL-17 and expression of AHR in the absence of IL-22 production.36,37 A likely explanation for the dissociation between IL-17 and IL-22 production in the presence of AHR expression is that culture conditions may have a profound influence on the outcome of the response. For instance, high levels of TGF-β may give rise to Th17 cells but inhibit IL-22 production.38
Differences in the requirements for exogenous TGF-β may also apply to γδreg. γδreg are induced in vitro following antigen stimulation in the presence of low concentrations of TGF-β (typically five to sevenfold less than the concentrations required for γδ17 T cell differentiation) and IL-2 or IL-15.9 These cells express Foxp3 and similarly to thymus-derived natural or induced-CD4 T regulatory cells, suppress the proliferation of anti-CD3/anti-CD28 stimulated mononuclear cells.
Altogether, these findings are highly suggestive of a requirement for high concentrations of TGF-β (in combination with other cytokines) to promote human γδ17 differentiation,39,40 whereas low concentrations of TGF-β promote the differentiation of γδregs.
Expanding the γδ1–γδ2 paradigm. Part II: γδFH
There is increasing evidence that help to B cells for promoting antibody production is mediated by a dedicated lineage of follicular Th cells (TFH).41 TFH cells are defined by follicular localization and high expression of the specific markers CXCR5, which drives TFH cells to migrate into the B-cell follicles, PD-1 and ICOS, which interact with their corresponding ligands on B lymphocytes, and the signature cytokine IL-21, which predominantly acts as a paracrine factor for germinal center (GC) B lymphocytes, but has only limited autocrine function as a regulator of TFH lineage fate.41 The transcriptional repressor Bcl-6 is a crucial intrinsic regulator of TFH lineage commitment,41 but the differentiation pathway to TFH cells is still under study.
Human Vγ9Vδ2 T cells that are cultured with antigen and IL-21 are polarized towards a TFH phenotype,42,43 which is dependent on high levels of Bcl-6 expression.43 The in vitro differentiated γδFH cells distinctively express CD40L, ICOS and CXCR5 (at low levels), and help tonsillar B cells to produce IgM, IgG and IgA, thus fully identifying this cell population as classical helper cells.42,43
The acquisition of TFH-associated markers by Vγ9Vδ2 T cells and their dependence on IL-21 was initially suggested by microarray studies.4 IL-21 turned out to have a similar capacity as the related cytokine IL-2 to support and sustain antigen-induced Vγ9Vδ2 T-cell proliferation, without promoting the supposedly signatory cytokines IFN-γ and TNF-α.4,42 While IL-21 may potentiate the cytolytic function of Vγ9Vδ2 T cells when combined with IL-2,44 IL-21 alone specifically costimulates expression of the chemokine receptor CXCR5, which enables TFH cells to migrate into the B-cell follicles, and costimulates expression of the CXCR5 ligand, CXCL13, which attracts further cells, such as naive B cells and early activated CD4 T cells.4,42,43 As CXCR5 and CXCL13 are uniquely expressed in B cell follicles but mostly absent from extrafollicular areas, including the T zones of lymph nodes, the spleen and Peyer's patches, this finding implicates a role for IL-21-stimulated Vγ9Vδ2 T cells in orchestrating immune cell trafficking to the GCs.
In contrast to CD4 TFH cells, γδFH cells do not produce IL-21, but display a Th2-type pattern of cytokine production, as they secrete IL-2, IL-4 and IL-10.43 Therefore, it was a surprising finding that γδFH cells lack expression of GATA-3 and IL-13 mRNAs,43 both of which are signatures of Th2 cells. A similarly dissociated expression of IL-4 from IL-13/GATA-3 has been found recently45 in a mouse study showing that IL-4, but not IL-13, is made by TFH cells, whereas Th2 cells produce both cytokines. In that study, IL-13 production by Th2 cells was associated with large amounts of GATA-3 expression, which was necessary for sustaining IL-13 production. Conversely, TFH cells produced only IL-4 and did not express GATA-3.45 It is likely that elevated levels of Bcl-6 expression in γδFH cells restrict GATA-3 expression to levels that are insufficient to activate expression of IL13.
The contribution of γδFH cells to antibody-mediated immune responses may occur early during microbial infections, before the full development of acquired responses that are mediated by CD4 T cells. In humans, Vγ9Vδ2 TCM cells reside in the paracortical areas of lymph nodes, where they may become stimulated by antigen and express IL-21R. Thus, these pre-activated cells may encounter IL-21 that is produced by CD4 T cells and, as a consequence, express a distinct set of molecules associated with relocation to the GCs and with the provision of B-cell help. The interaction between γδFH cells, IL-21 producing CD4 T cells and B cells in reactive secondary lymphoid tissues is likely to have an impact on the production of high-affinity antibodies against microbial pathogens.
Because αβ and γδ T cells recognize different types of antigens, the presence of a subset of each of these populations that is capable of inducing immunoglobulin secretion would provide a mechanism whereby humoral immune responses could be elicited against a diverse array of antigens, irrespective of the type of responding T cell. Thus, the presence of Vγ9Vδ2 T cells in GCs would broaden the repertoire of antibodies produced by responding B cells.
Chemokine and homing receptor expression further contribute to γδ T-cell heterogeneity
Effector T-cell function is dependent not only on the expression of cytokines but also on the capacity of these cells to migrate to sites of antigen encounter. T-cell migration is dependent on the expression of chemokine receptors and integrins that determine, in a combinatorial fashion, extravasation and positioning in different tissue microenvironments.46
Chemokine receptors are particularly useful for classifying T-cell subsets that have distinct migratory capacities and effector functions. Studies using chemokine receptors, mostly performed in CD4 T cells, can now be translated to γδ T cells. Similarly to CD4 T cells, Vγ9Vδ2 T cells comprise distinct memory populations distinguishable on the basis of surface markers, effector functions and trafficking properties. Central memory TCM cells express CCR7 and CD62 L, home to secondary lymphoid organs and are involved in recall responses having high proliferative and reconstituting capacity,2 whereas the so-called effector-memory (TEM) cells home to peripheral tissues where they display immediate effector functions such as cytokine production and cytotoxicity.2
Within Vγ9Vδ2 TEM, CXCR3 and CCR5 expression primarily define γδ1 and γδ2 cells and CCR6 defines γδ17 cells;8 however, no single chemokine receptor is distinctively expressed on γδ2. The differential expression of adhesion and chemokine receptors on Vγ9Vδ2 T cells and the constitutive expression of the corresponding ligands in different tissues allows for the specificity of T cells that provide surveillance in different organs.47
Related to this finding, a novel pro-inflammatory human skin-homing Vγ9Vδ2 T-cell subset has been identified, which is characterized by early migration to perturbed human skin in vivo, suggesting a role in tissue immunosurveillance.48 This subset is preferentially increased in psoriatic skin, indicating a potential clinical relevance in the pathogenesis of this major inflammatory skin disease. Interestingly, this population of Vγ9Vδ2 T cells expresses the adhesion molecule CLA,48 which interacts with vascular E-selectin and mediates entry into the skin. Surprisingly, these cells do not express either CCR4,48 which is required for the transition from the blood to the dermis, or CCR10,48 which is required for targeting T cells from the dermis to the epidermis, where its ligand, CCL27, is produced by keratinocytes.49 Thus, the skin-homing Vγ9Vδ2 T cells differ from CD4 T cells that migrate to the skin and express CCR4, CCR10 and CLA. Finally, the skin-homing Vγ9Vδ2 T cells produce IL-17 and IFN-γ, but whether these cytokines are produced by a single-cell subset or by different subsets is unknown.48
In summary, currently there is little doubt that the regulation of homing receptor expression is an integral part of the differentiation program of Vγ9Vδ2 T cells, although the exact mechanisms and precision of this regulation remain to be established.
Concluding remarks
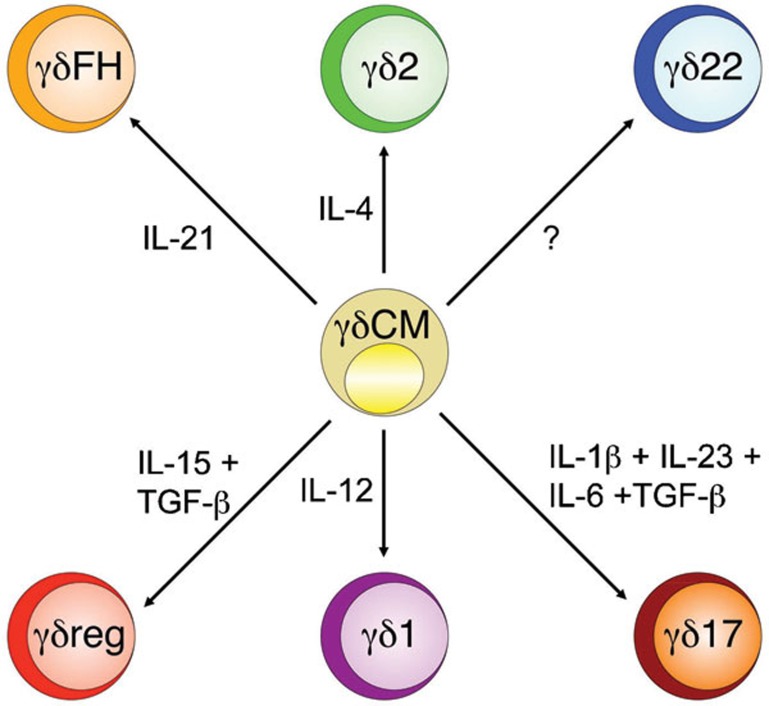
To understand the heterogeneity of Vγ9Vδ2 T cells, we propose a classification based on distinct functional phenotypes (Figure 1) that are each tailored to a particular class of immune responses against different types of pathogens (Table 1). The function of each phenotype requires a combination of molecular and cellular interactions that includes polarizing cues (usually a single cytokine or a cytokine combination), lineage-specifying transcription factors, homing receptors and effector molecules (usually cytokines). These components have been extensively validated for γδ1 T cells and, to a lesser extent, for γδ2 cells, which are involved in responses to intracellular microbes and extracellular parasites, respectively. A more recent addition to these functional phenotypes, namely, γδ17, is required for immunity to extracellular bacteria. For some phenotypes, there is a general consensus on all or most of the above aspects, but for others, some pieces of information are still missing. Compelling evidence for a γδFH phenotype comes from recent in vitro data that demonstrate the capability of this cell subset to help B cells to promote antibody production in B-cell follicles. However, as yet, there is no clear evidence on the nature of the polarizing cues and of the lineage-determining transcription factors of this phenotype. Similarly, while there is compelling evidence for a role of the γδreg phenotype in regulating immune response, there are still uncertainties as to the nature of the polarizing stimuli and the suppressor mechanisms.
Figure 1.
Flexibility and plasticity of helper T cells. Initial studies arising from in vitro cultured mouse and human γδ T cells led to the idea that these subsets behaved as lineages, meaning that the skewed phenotypes were inflexible. Accordingly, γδ T cells expressed lineage-defining transcription factors that were sufficient to impart similarly selective cytokine production. Recent studies of γδ T cells have revealed more plasticity of γδ T-cell phenotypes than that which was predicted by earlier work. There are now circumstances in which γδ T cells can change their profile of cytokine production according to precise polarizing conditions.
Table 1. Phenotypical heterogeneity and functional package organization of human γδ T cells.
| Subset | γδ1 | γδ2 | γδ17 | γδ22 | γδFH | γδreg |
|---|---|---|---|---|---|---|
| Polarizing cytokine | IL-12 | IL-4 | IL-1-β, IL-6, IL-23, TGF-β | ? | IL-21 | IL-15, TGF-β |
| Transcription factor | T-bet, Eomes | GATA-3 | RORC, AHR | ? | Bcl-6 | Foxp3 ? |
| Homing receptors | CXCR3, CCR5 | ? | CCR6 | ? | CXCR5 | ? |
| Effector molecules | TNF-α, IFN-γ | IL-4 | IL-17 | IL-22 | IL-4, IL-10 | ? |
| Target cells | Macrophages, Dendritic cells | ? | Neutrophils, Epithelial cells | ? | B cells | T cells |
| Function | Intracellular bacteria | ? | Extracellular bacteria | ? | Antibody production | Regulation |
Abbreviations: IFN, interferon; TGF, transforming growth factor; TNF, tumor-necrosis factor.
Finally, and despite still fragmentary information, it is tempting to speculate that γδ22 cells may represent an additional phenotype that is possibly involved in epithelial homeostasis.
In conclusion, it appears that plasticity is an important part of the γδ T cell differentiation program, in that cells initially maintain a flexible lineage differentiation, while at later stages, they may become irreversibly committed to one lineage. Considering that in humans, the population of Vγ9Vδ2 TCM cells includes uncommitted cells and that most cells maintain cytokine flexibility, it is possible that the expression of opposing cytokines may be induced at different times, in different tissues, or enforced by antigen presentation together with appropriate polarizing signals. Given the complexity of the γδ T-cell differentiation process and the heterogeneity of effector γδ T cells, it is not surprising that the emerging picture is far from complete.
More generally, the prospect of lineage commitment versus flexible differentiation for γδ T cells has implications for disease pathogenesis and therapeutic interventions. A variety of autoimmune and allergic inflammatory disorders are associated with the presence of particular T-cell subsets, and these cells have a major influence on, and can even control, the pathophysiology of these disorders. If T-cell responses are plastic, one should be able to alter them therapeutically and thus interrupt the disorder. Hence, a better understanding of the molecular mechanisms that stabilize committed cytokine production may provide new therapeutic opportunities or revise our approaches for treating such diseases.
Acknowledgments
We thank Martin Lipp, Richard Kroczek and Vaclav Horejsi for providing us with reagents and Matthias Eberl, Marc Bonneville, Jean Jacques Fourniè and Emmanuel Scotet for sharing unpublished data. This work was supported by grants from the Ministry of University and Research (MIUR-PRIN 2008 to FD), the Ministry of Health ‘Ricerca Finalizzata 2007' (to FD) and the University of Palermo.
References
- Bonneville M, O'Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, et al. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser B. A rapid crosstalk of human γδ T cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathog. 2009;5:e1000308. doi: 10.1371/journal.ppat.1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermijlen D, Ellis P, Langford C, Klein A, Engel R, Williman K, et al. Distinct cytokine-driven responses of activated blood γδ T cells: insights into unconventional T cell pleiotropy. J Immunol. 2007;178:4304–4314. doi: 10.4049/jimmunol.178.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sireci G, Champagne E, Fournié JJ, Dieli F, Salerno A. Patterns of phosphoantigen stimulation of human Vγ9Vδ2 T cell clones include Th0 cytokines. Hum Immunol. 1997;58:70–82. doi: 10.1016/s0198-8859(97)00211-5. [DOI] [PubMed] [Google Scholar]
- Wesch D, Glatzel A, Kabelitz D. Differentiation of resting human peripheral blood γδ T cells toward Th1- or Th2-phenotype. Cell Immunol. 2001;212:110–117. doi: 10.1006/cimm.2001.1850. [DOI] [PubMed] [Google Scholar]
- Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vγ9Vδ2 T cells. J Immunol. 2010;184:7268–7280. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, et al. Differentiation, phenotype and function of interleukin-17-producing human Vγ9Vδ2 T cells. Blood. 2011;118:129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, et al. TGF-β1 and IL-15 Induce FOXP3+ γδ regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183:3574–3577. doi: 10.4049/jimmunol.0901334. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Devilder MC, Allain S, Dousset C, Bonneville M, Scotet E. Early triggering of exclusive IFN-γ responses of human Vγ9Vδ2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol. 2009;183:3625–3633. doi: 10.4049/jimmunol.0901571. [DOI] [PubMed] [Google Scholar]
- Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- Yin Z, Chen C, Szabo SJ, Glimcher LH, Ray A, Craft J. T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-γ by γδ T cells. J Immunol. 2002;168:1566–1571. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- Chen L, He W, Kim ST, Tao J, Gao Y, Chi H, et al. Epigenetic and transcriptional programs lead to default IFN-γ production by γδ T cells. J Immunol. 2007;178:2730–2736. doi: 10.4049/jimmunol.178.5.2730. [DOI] [PubMed] [Google Scholar]
- Reiner SL. Helper T cell differentiation, inside and out. Curr Opin Immunol. 2001;13:351–355. doi: 10.1016/s0952-7915(00)00226-0. [DOI] [PubMed] [Google Scholar]
- Sireci G, Espinosa E, Di Sano C, Dieli F, Fournié JJ, Salerno A. Differential activation of human γδ cells by nonpeptide phosphoantigens. Eur J Immunol. 2001;31:1628–1635. doi: 10.1002/1521-4141(200105)31:5<1628::AID-IMMU1628>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, et al. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons DL, Haque SF, Silberzahn T, Hamilton K, Langford C, Ellis P, et al. Neonates harbour highly active γδ T cells with selective impairments in preterm infants. Eur J Immunol. 2009;39:1794–1806. doi: 10.1002/eji.200939222. [DOI] [PubMed] [Google Scholar]
- Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, et al. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signalling pathway. J Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, et al. IL-17 markedly upregulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signalling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Fenoglio D, Poggi A, Catellani S, Battaglia F, Ferrera A, Setti M, et al. Vδ1 T lymphocytes producing IFN-γ and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood. 2009;113:6611–6618. doi: 10.1182/blood-2009-01-198028. [DOI] [PubMed] [Google Scholar]
- Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human TH1 and TH2 lymphocytes. Nat Immunol. 2002;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell–mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- O'Garra A, Stockinger B, Veldhoen M. Differentiation of human TH-17 cells does require TGFβ. Nat Immunol. 2008;9:588–590. doi: 10.1038/ni0608-588. [DOI] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Bansal RR, Mackay CR, Moser B, Eberl M. IL-21 enhances the potential of human γδ T cells to provide B-cell help. Eur J Immunol. 2012;42:110–119. doi: 10.1002/eji.201142017. [DOI] [PubMed] [Google Scholar]
- Caccamo N, Todaro M, La Manna MP, Sireci G, Stassi G, Dieli F. IL-21 regulates the differentiation of a human γδ T cell subset equipped with B cell helper activity. PLoS ONE. 2012;7:e41940. doi: 10.1371/journal.pone.0041940. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Thedrez A, Harly C, Morice A, Salot S, Bonneville M, Scotet E. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human Vγ9Vδ2 T cells for adoptive immunotherapy. J Immunol. 2009;182:3423–3431. doi: 10.4049/jimmunol.0803068. [DOI] [PubMed] [Google Scholar]
- Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187:2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, et al. CCL27–CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]