Abstract
The elusive task of defining the character of γδ T cells has been an evolving process for immunologists since stumbling upon their existence during the molecular characterization of the α and β T cell receptor genes of their better understood brethren. Defying the categorical rules used to distinctly characterize lymphocytes as either innate or adaptive in nature, γδ T cells inhabit a hybrid world of their own. At opposing ends of the simplified spectrum of modes of antigen recognition used by lymphocytes, natural killer and αβ T cells are particularly well equipped to respond to the ‘missing self' and the ‘dangerous non-self', respectively. However, between these two reductive extremes, we are chronically faced with the challenge of making peace with the ‘safe non-self' and dealing with the inevitable ‘distressed self', and it is within this more complex realm γδ T cells excel thanks to their highly empathetic nature. This review gives an overview of the latest insights revealing the unfolding story of human γδ T cells, providing a biographical sketch of these unique lymphocytes in an attempt to capture the essence of their fundamental nature and events that influence their life trajectory. What hangs in their balance is their nuanced ability to differentiate the friends from the foe and the pathological from the benign to help us adapt swiftly and efficiently to life's many stresses.
Keywords: antigen recognition, danger-associated molecular patterns, gamma delta T cell subsets, immune homeostasis, innate immunity, stress response
Prologue
Since the time of their accidental discovery during the molecular characterization of the α and β T cell receptor (TCR) genes belonging to their relatively unambiguous brethren,1 ‘enigmatic' is still one of the most commonly used adjectives to describe γδ T cells. This can be attributed to the fact that these unconventional lymphocytes have thus far been remarkably successful in thwarting most attempts at definition. Much of what we know about T cell biology and function comes from what has been discerned through studies done on αβ T cells; however, one thing that is certain is that the majority of the rules governing the lives of αβ T cells are surprisingly irrelevant when it comes to understanding the elusive character of γδ T cells. Table 1 highlights some of the major differences between these two T cell subsets in humans.
Table 1. Major differences between human αβ and γδ T cells.
| Feature | αβ T cells | γδ T cells |
|---|---|---|
| Frequency in blood | • 65%–75% of PBMC | • <10% of PBMC (25%–60% gut) |
| MHC restriction | • CD4+: MHC class II | • No MHC restriction |
| • CD8+: MHC class I | • Possible roles of CD1 and MICA/B | |
| CD4/CD8 expression | • ∼60% CD4+; ∼30% CD8+; <1% double positive; | • Majority (>70) double negative; <1% CD4+; ∼30% CD8+αα (as IELs in gut) |
| • <1% double negative | ||
| Antigen recognition | • Processed peptide/MHC | • Unprocessed, not peptides |
| TCR V gene germ line repertoire | • Large | • Small |
| TCR diversity | • Very diverse | • Relatively restricted expression despite high potential for junctional diversity; expression variance dictated by tissue localization |
| Function | • Adaptive immunity | • Immune regulation, surveillance and homeostasis |
Abbreviations: IEL, intraepithelial lymphocyte; MHC, major histocompatibility complex; PBMC, peripheral blood mononuclear cell; TCR, T cell receptor.
Recognition by γδ T cells is not typically in the context of classical major histocompatibility complex (MHC) class I or II molecules and neither is antigen processing required.2,3 These observations are corroborated by the fact that the majority of γδ T cells are CD8 and CD4 negative. Crystal structure analysis of the γδ TCR determined that the length and conformation of γδ TCR resemble immunoglobulins (Ig) more than the αβ TCR, which was taken to suggest that antigen recognition by γδ T cells may be more similar to the binding of antibody to antigen rather than the MHC/peptide complex recognized by αβ T cells.4 The very basis of foreign antigen recognition by lymphocytes of the adaptive immune system is a contentious point for these unique T cells. Although γδ T cells appear to have antigen recall memory by their demonstrated rapid expansion and antimicrobial response upon reinfection with a particular pathogen, much like lymphocytes of adaptive immunity,5,6,7 clonally expanded γδ T cells with very restricted receptor repertoires are able to respond readily to a wide range of both infectious and non-infectious stressors. Natural killer (NK) cells share some characteristics with γδ T cells as both are usually considered constituents of innate immunity, recognize transformed cells, play a prominent role in antiviral protection and are cytolytic lymphocytes.8,9,10 Like NK cells, human γδ T cells express activating receptors, such as NKG2D that recognizes stress-inducible MHC class I-related MICA/MICB molecules and the UL16-binding proteins that are upregulated on malignant or stressed cells and induce cytolysis,11,12 and they are inhibited by the expression of killer Ig-like receptors, which bind certain MHC class I alleles expressed by non-malignant cells and trophoblasts.13,14,15 However, the versatility of γδ T cells is further extended by their demonstrated ability to assume the role and appearance of professional antigen presenting cells.16,17 This unusual functional plasticity has given the impression that these multitalented T cells are ‘Jacks of all-trades, but masters of none', and they are sometimes erroneously delegated to a niche occupied by vestigial features of modern anatomy by some who are left confounded as to their real purpose.
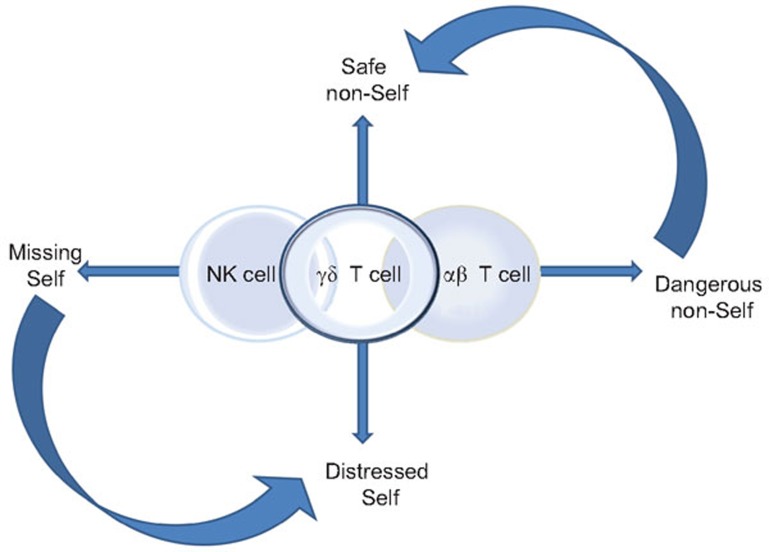
This review provides a biographical sketch of human γδ T cells in an attempt to convey the essence of their fundamental nature and what may influence their life trajectory. Figure 1 illustrates the highly simplified paradigm for NK cells, αβ T cells and the emerging view of where in the continuum that encompasses immune homeostasis and protection γδ T cells take the throne. At opposing ends, NK and αβ T cells are particularly well equipped to respond to the ‘missing self' and the ‘dangerous non-self', respectively, or as Klas Kärre had so aptly put it—‘to recognize a foreign submarine'.18 Between these two extremes, we are chronically faced with the challenge of making peace with the ‘safe non-self' and dealing with the inevitable ‘distressed self', and it is within this more complex realm γδ T cells excel thanks to their highly empathetic nature. What hangs in the balance is the ability to differentiate the friends from the foe and the pathological from the benign—and adapt swiftly and efficiently to life's many stresses.
Figure 1.
A simplified paradigm illustrating where in the continuum of immune protection and homeostasis γδ T cells fall in relation to innate NK cells and the adaptive αβ T cells. At the two extreme ends, NK and αβ T cells are particularly well equipped to respond to the ‘missing self' and the ‘dangerous non-self', respectively. Between these two extremes, we are chronically faced with the challenge of making peace with the ‘safe non-self' and dealing with the inevitable ‘distressed self', and it is within this more complex realm γδ T cells excel. It should be recognized that these different ‘selves' and the immune response(s) that they trigger exist in a continuum and are modulated by the context in which they are presented. Both NK and αβ T cells work with γδ T cells to fill in the gaps of this spectrum—with NK cells contributing to responding to the ‘distressed self' and αβ T cells having some regulatory training to temper the response to the ‘safe non-self'. NK, natural killer.
Species specificity: the best laid plans of mice and men oft go awry
Among the many fascinating and important conundrums that have yet to be solved for γδ T cells is the large phenotypic difference between species when it comes to their relative numbers, location and antigen recognition. In both mice and primates, γδ T cells comprise a small population of peripheral T cells (averaging 5% of the total peripheral T cell pool in Caucasians living in Western Europe and North America), and they are poorly represented within conventional T cell zones found in lymphoid tissue.19 In contrast, sheep, cattle, rabbits and chickens have much higher levels of circulating γδ T cells.20 The reason behind this disparity in the constitution of γδ T cells between and within species is currently unknown, but the discrepancy seen between ‘low' and ‘high' γδ T cell species has been correlated with the total level of TCR variable genes, which is represented largely by the variable potential of the αβ TCR, in conjunction with the diversity present in the Ig variable genes. Thus, species having a high number of variable TCR and Ig genes tend to have low numbers of peripheral γδ T cells (Table 2).20 It can be speculated that the role of and exposure to microbes in the species' lifecycle may be associated with these immune parameters. For example, omnivorous rodents and primates may have less of a need to maintain peace with a diverse population of microbiota in comparison to cows, sheep, rabbits and chickens for which microbes play a more significant role in digestion and metabolism.21,22,23,24 Therefore, the immune cell repertoire that recognize more variable ‘non-self' as dangerous (i.e., the features that we depend on from the adaptive immune system) may be less advantageous when balancing the need to protect beneficial or non-threatening microbes that are needed for sustenance and/or to prevent chronic inflammation.
Table 2. Comparative diversity and percent composition of peripheral γδ T cells between species (adapted from Su et al., 199920).
| Percent peripheral γδ T cells | Diversity of αβ T cell receptor variable genes | Diversity of immunoglobulin variable genes | |
|---|---|---|---|
| Human | ∼5% (low) | High | High |
| Mouse | ∼5% (low) | High | High |
| Chicken | ∼20% (high) | Low | Low |
| Rabbit | ∼20% (high) | Low | Low |
| Sheep | ∼30% (high) | Low | Low |
| Cattle | ∼30% (high) | Low | Low |
As shown in Table 3, the numbers of Vγ and Vδ gene segments in both mice and men are greatly limited in comparison to αβ T cells, and despite their relative low levels in peripheral blood, γδ T cells are found to be enriched at mucosal interfaces, such as the intestinal tract, that are heavily populated by largely non-pathogenic and symbiotic organisms.19,25,26 In the murine system, distinct subsets of γδ T cells bearing specific pairs of the γ and δ TCR chains are targeted to particular epithelial locations during development such as the skin, lungs, uterus, vagina and tongue where they make up a significant portion of the local intraepithelial lymphocyte population and appear to respond to endogenous stress-induced antigens that have yet to be unambiguously identified.27,28,29,30,31 For some of these special niche localized γδ T cells, such as the invariant dendritic epithelial Vγ3Vδ1 T cells found in murine skin,28 no human equivalent has been found. However, it is interesting to note that the phenomenon observed at the specie level is extended to niches found within an organism and that an abundance of certain subsets of γδ T cells are found where there is a vital need to guard against inappropriate immune responses to ‘safe non-selves' at the same time as keeping in check the inevitable ‘distressed self'—a combinatorial function that neither αβ T cells nor NK cells are especially designed to deal with alone.
Table 3. Comparative diversity of the α, β, δ and γ TCR chains in mice and men32.
| Human | Mice | |
|---|---|---|
| Vγ | ∼6 (5 from same family and 1 distantly related) | ∼6 (2 from same family and 4 broadly diverged) |
| Vδ | ∼8 functional genes (only 3 commonly used—VD1, VD2 and VD3) | ∼16 (6 homologous and 10 distinct) |
| Vα | ∼42 | ∼75 |
| Vβ | ∼47 | ∼23 |
Abbreviation: TCR, T cell receptor.
‘Non-Vδ2 T cells': the high art of non-self acceptance
In adult humans, two subsets of γδ T cells, defined by the usage of either the Vδ2 or Vδ1 TCR, predominate. The majority of the tissue-associated γδ T cells bear the Vδ1 TCR with Vδ3 and Vδ5 making up minor populations.32,33 In the literature, these are often collectively called the ‘non-Vδ2' γδ T cells. In addition to their role in maintaining immune homeostasis in the local microenvironment,25 tissue-associated γδ T cells, in both mice and men, play an important function in wound healing, removing distressed or transformed epithelial cells and subduing excessive inflammation.19,26,34,35,36,37,38,39 For example, in the gastrointestinal tract γδ T cells play a non-redundant role in maintaining tolerance to food antigens as well as the intestinal flora.34,40 The Vγ chain pairing of tissue-associated Vδ1 T cells in humans is less stringent in comparison to the highly restricted Vγ9Vδ2 T cells found in peripheral blood—though they prefer pairing with Vγ4 and Vγ5 over Vγ9.32,41 This greater diversity in pairing and structure is taken to imply that there exists a broader range of ligands that are recognized—but the recognition is thought to be largely introspective as it is usually in response to the changing states of self. The actual antigen specificity and mechanism of recognition of these non-Vδ2 T cells is still in the unraveling. The stress-induced molecules, MICA and MICB, have been found to be tumor antigens recognized by cancer infiltrating Vδ1 T cells.42,43 The redundancy of this recognition is a point in the pondering since both Vδ1 and Vδ2 T cells express NKG2D, an activating C-type lectin that is the known receptor for MICA, MICB and related UL16-binding protein.44,45 Nevertheless, the crystal structure analysis showed that the binding of Vδ1 T cells to MICA was mutually exclusive to NKG2D, although the complementary determining region (CDR) of the TCR appeared to have an uncharacteristically flat potential binding surface.46 Extending this apparent tendency to respond to non-polymorphic MHC class I family-related proteins, CD1c, a molecule that is able to present both foreign and self lipid antigens,47 is another candidate ligand for Vδ1 T cells.48 It remains to be seen exactly how broadly based these recognition patterns are within the Vδ1 T cell family since many of these studies have relied on a limited number of γδ T cell clones or lines. Adding a new twist to this storyline, it was recently revealed by the working groups of Willcox and Dechanet-Merville that a ‘non-Vδ2' T cell clone expressing Vγ4Vδ5 TCR was activated by the recognition of endothelial protein C receptor.49 This interaction involved the CDR3 of Vγ4 and was similar in nature to the binding of antibody to antigen. Of particular note, recognition was highly dependent on the multimolecular stress signature that was induced by either infection by cytomegalovirus or malignant transformation.49 Endothelial protein C receptor is another member of the CD1/MHC class I superfamily that is expressed on endothelium and trophoblasts50 and can bind lipids similar to CD1 family members; however, binding of the Vγ4Vδ5 T cell clone was determined to be independent of the presence of any lipid antigen.49
In continuation of the theme of sometimes needing to accept the ‘safe non-self', the immunological state of pregnancy is an example of a time when this requirement is essential. It is still an enigma how the maternal immune system in mammals is not only able to prevent rejection of the fetal semiallograft, but also successfully support its growth for 9 months. Human Vδ1 T cells have been observed to be recruited to the maternal/fetal interface during pregnancy where their activation is believed to play a role in the induction of tolerance to paternal antigens.15,51,52,53,54 Furthermore, there is evidence suggesting that the early pregnancy human decidua is actually a site of extrathymic maturation of Vδ1 T cells.55,56 This enrichment of γδ T cells at the maternal/fetal interface and their mysterious role in pregnancy is not unique to humans as the same has been observed in sheep57 and mice58, suggesting a central conserved role of γδ T cells involved in pregnancy-related immune changes. In women with healthy pregnancies, the changes are not only found at the maternal/fetal interface since the number of Vδ1 T cells in circulation have also been reported to be increased in relation to the Vδ2 subset.59,60,61 Of note, some immune-related pregnancy complications where maternal immune tolerance is compromised and there is risk of premature pregnancy termination, a significant drop in the number of circulating Vδ1 T cells is observed.62 The cognate pregnancy-associated γδ T cells in the murine system were found to directly recognize an unknown antigen on trophoblasts via their TCR, and this recognition was MHC-unrestricted and, intriguingly, not species-specific.63 Although expression of inhibitory non-classical MHC class I molecules, such as HLA-E and HLA-G, on trophoblasts appear to play a role in suppressing Vδ2 T cells during pregnancy, they are unlikely to be the candidates recognized by the Vδ1 T cells actively on guard.15 Mammals require a reliable mechanism to maintain immune tolerance to ‘safe non-selves', as a failure to do so would be extinction. In this regard, Vδ1 T cells seem to have a conserved acumen for brokering peace for the benefit of generations to come.
Vδ2 T cells: to know the complex burden of being human
In the peripheral blood of most healthy adult humans and some other primates such as the rhesus monkey, the vast majority of γδ T cells bear the Vδ2 TCR—usually paired with the Vγ9 chain.64 These special canonical Vγ9Vδ2 T cells recognize non-peptidic phosphorylated antigens, such as isopentyl pyrophosphate (IPP), that are metabolites in the essential isoprenoid biosynthesis pathway present in virtually all living organisms.6 Endogenously, IPP and its stereoisomer dimethylallyl diphosphate are substrates produced in the mevalonate pathway for cholesterol metabolism; however, they can also be produced by the deoxyxylulose pathway commonly used by organisms, such as Escherichia coli and certain plant cells, that lack the critical HMG-CoA reductase enzyme of the mevalonate pathway.65,66 It was later determined that the stimulatory capacity of IPP and dimethylallyl diphosphate is actually fairly weak in comparison to some of the upstream intermediates of the alternative pathway of isoprenoid biosynthesis, such as (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate,67 and it is usually in the context of malignancy or distress that cellular endogenous levels are able to awaken the effector functions of Vγ9Vδ2 T cells.68 A class of drugs called aminobisphosphonates used for the prevention of bone fragility fractures and certain alkylamine compounds that are ubiquitously dispersed in everything from plants to amniotic fluid are also able to stimulate Vγ9Vδ2 T cells69,70 by virtue of their ability to induce cellular accumulation of IPP. Both these compounds modulate IPP levels by blocking the metabolic activity of farnesyl pyrophosphate synthase, a key enzyme in the mevalonate pathway.71,72
It is unclear whether these ‘phospho-antigens' (pAgs) require presentation via some cell-surface accessory molecule; however, the fact that contact with cells of human origin is necessary for their ability to fully activate Vγ9Vδ2 T cells seems to suggest that some kind of assistance is necessary.73 Recent developments in regards to alternative potential endogenous stress-induced ligands for Vγ9Vδ2 T cells provide new venues that may reveal how these cellular short-lived substrates can be potentially stabilized and presented extracellularly to the stress surveillance capabilities inherent in these lymphocytes. Scotet and colleagues74 first reported that tumor recognition by Vγ9Vδ2 T cells could be mediated by the ectopic expression of ATP synthase/F1-ATPase, which is normally expressed on the internal membrane of mitochondria, and this interaction was enhanced by the cobinding of apolipoprotein A-I that is usually present in serum. Literally along the same vein, it was later observed that shear stress experienced by endothelial cells also led to the translocation of the ATP synthase β chain to the cell surface which resulted in the binding and activation of Vγ9Vδ2 T cells.75 Reminiscent of the previous findings with F1-ATPase, the response of γδ T cells to endothelial cells expressing ATP synthase β was significantly potentiated by the coincident accumulation of cholesterol in the cell membrane, and this interaction led to the secretion of inflammatory cytokines by γδ T cells and upregulation of vascular cell adhesion molecules on endothelial cells.75 This phenomenon was taken to suggest that endothelial dysfunction, characterized by the disturbed flow created by shear stress, and hypercholesterolemia work synergistically to activate γδ T cells and the endothelium75—providing a novel mechanism contributing to cardiovascular pathology. In an attempt to reconcile the established reactivity of Vγ9Vδ2 T cells to non-peptidic pAgs and these new observations of alternative stress-induced ligands, experiments were carried out to demonstrate that ATP synthase can also potentially bind to pAgs, and it was concluded that it seemingly has the appropriate features to function as a possible pAg presenting molecule for Vγ9Vδ2 T cells.76 More recently, another putative Vγ9Vδ2 T cell tumor antigen, human MutS homologue 2 (MSH2), was identified using a peptide-based affinity screening system for the CDR3 of the δ chain.77 MSH2 is normally located in the nucleus where it functions as a DNA mismatch repair gene, but it is often mutated in a number of different types of epithelial cancers and can be ectopically expressed.78 Transformation of normal human B cells by Epstein–Barr virus or subjecting renal carcinoma cell lines to oxidative stress also led to an increased surface expression of MSH2 and rendered them susceptible to Vγ9Vδ2 T cell-mediated cell lysis that could be blocked by the use of anti-MSH2 antibodies or downregulation of its gene expression.78,79 These observations were collectively taken to suggest that MSH2 may be yet another damage-associated molecular pattern recognized by Vγ9Vδ2 T cells. An overlooked point worth considering is the fact that MSH2 also has an intrinsic ability to bind and hydrolyze ATP,80 which may be the property providing the common mechanism leading to the mobilization of human peripheral blood γδ T cells. Both ATP synthase/F1-ATPase and MSH2 are evolutionarily conserved molecules that serve essential stress-sensitive homeostatic functions in prokaryotes and eukaryotes and can bind nucleotide derivatives, and this may confer the potential to present low molecular weight pAgs to Vγ9Vδ2 T cells' clever immunosurveillance strategy. This is still in large part conjecture, and much of the story is still in the telling given that neither F1-ATPase nor MSH2 are absolutely indispensible for pAg recognition. Exogenously added pyrophosphate antigens readily stimulate Vγ9Vδ2 T cells in the presence of human accessory cells, yet both these molecules require some stress-induced event for their ectopic expression—which argues for there existing some other more widely expressed candidate(s) that would be more exclusive to the primate lineage. Harly and colleagues,81 from the working groups of Scotet and Bonneville, may have uncovered at least one of the missing pieces of the story in the form of a molecule known generically as CD277—or BTN3A. This unassuming molecule is a member of the butyrophilin/B7-like group of proteins belonging to the Ig superfamily that are found clustered at the extended end of the classical MHC I genes on human chromosome 6.82 In a series of elegant experiments involving specific activating and inhibitory antibodies to CD277 as well as domain-swapping and knockdown experiments, the Scotet–Bonneville group shows convincing evidence that this widely expressed molecule provides the necessary support that pAgs need to awaken Vγ9Vδ2 T cell effector functions.81 The fact that no functional orthologue of CD277 exists in rodents adds further credence to its purported role as the long-awaited missing link—or what may very well turn out to be a series of interconnected links.
Given the fact that these unique pAg-reactive Vγ9Vδ2 T cells have no known equivalent in either rodents or ruminants, it is perplexing why their effective stress surveillance tactic appears to be solely a primate advantage. It is of considerable interest to determine where along our common evolutionary lineage they first made their appearance. With the emergence of the peripheral Vγ9Vδ2 T cell subset, primates seem to have done away with the functional niches of a number of other various γδ T cell subsets and chiseled it down to mostly two highly specialized arsenals—the one in the periphery often found fanning the flames at the first site of trouble and the one entrenched in the tissues often putting out the fire before it starts.
Rites of passage
First there were γδ T cells
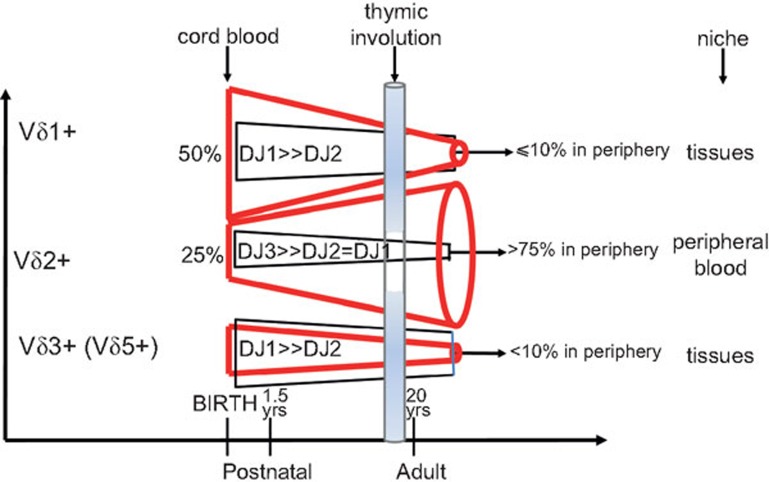
Befitting their deeper evolutionary roots, the ontogeny of γδ T cells precedes αβ T cells in expression. γδ TCR gene rearrangement can be detected by embryonic day 14 in the murine thymus83 and week 8 in humans,32 and canonical subsets can also be detected extrathymically during fetal development in both species.83,84 The most striking feature is the change from having fairly diverse pairs of γδ T cells (of which Vδ1+ serve as the majority in cord blood at birth) to increasingly restricted pairings, with Vγ9Vδ2 T cells becoming the major subset with very limited receptor diversity by adulthood (Figure 2).32 By 1 year of life, the majority of peripheral blood Vγ9Vδ2 T cells are non-naive, readily produce IFNγ upon stimulation and have cytolytic granules.85 It was concluded that this phenomenon was evidence of ‘the earliest immunological maturation of any lymphocyte compartment in humans.'85 In contrast, the Vδ1 T cells remaining in the periphery in early life were found to be still naive—similar to αβ T cells.85 This could be because the antigens that ‘non-Vδ2' T cells recognize are more abundantly expressed in the tissue microenvironment where they are targeted, and an analysis of Vδ1 T cells in their true niches, such as the intestine, in this early period may very well find that they too have a mature phenotype. The absolute number of γδ T cells in the periphery increases from birth to about 10 years of age, which is congruent with the expansion of the Vγ9Vδ2 T cell subset going from a minor population at birth to usually >75% of circulating γδ T cells.32 Although this clonal expansion has been seen as evidence of the vital role that these T cells play in responding to environmental challenges in early life, it has not been demonstrated that this phenomenon is in fact primarily in response to environmental challenges and not, at least in part, in response to endogenous stimuli as an extension of the adaptive changes taking place within the newborn as the immune system is being initiated.
Figure 2.
Schematic diagram of the changes in the diversity of γδ T cell repertoire through development in humans. At birth, γδ T cells show high junctional diversity, which is generated through the addition of ‘P' (palindromic) and ‘N' (non-templated) nucleotides while combining the V-D-J segments of their TCR. Despite the limited variable genes available for γδ T cells, their potential for junctional diversity rivals that of αβ T cells. However, with age, the diversity that is observed at birth gets noticeably restricted, especially for the main pool of circulating γδ T cells, the Vγ9Vδ2 T cell subset, which are a minor population in cord blood but become the predominant subset in circulation by 1 year of life. TCR, T cell receptor.32
Defined by experiences tainted by innate tendencies
It should be noted that what has been described for the early life and developmental trajectories of γδ T cells is based largely on studies conducted in North America and Western Europe, and it is now appreciated that there are great interindividual and regional differences in the make-up of one's γδ T cell repertoire. A small study looking at ethnic differences in peripheral γδ T cell numbers found Turkish and ‘non-Japanese Asians' (which comprised of a handful of people from Iran, India, Thailand and China) had significantly higher levels of γδ T cells (a median of 9.3% and 9.2%, respectively, of the total T cell pool) in comparison to Swedes (4.2%) and Japanese Asians (4.5%).86 A childhood history of tuberculosis in one Japanese man was associated with an unusually high percentage of γδ T cells (23.5%), which was similar to some of those sampled from Turkey and non-Japanese Asian countries. Thus, it was concluded that environmental factors, rather than genetic, are likely to be the driving force behind these ethnic and regional disparities in the proportion of γδ T cells found in peripheral blood. It is interesting to note the regional and interindividual differences in the distribution and number of γδ T cells is in line with the ‘hygiene hypothesis' which purports that the highly sterilized environment of industrialized or ‘Westernized' nations impacts the developmental trajectory of the immune system that is partly shaped by its reciprocal interaction with the microbial world at an early age.87 This is speculated to be a major contributive factor for the higher prevalence of immune and inflammatory disorders present in industrialized countries.87,88
Although a thorough study of potential genetic determinants is still lacking, its contribution to the observed phenotype of γδ T cells should not be ignored. An analysis of the repertoire of peripheral blood γδ T cells in the West African region of Ghana found that healthy people there had a five-fold higher level of circulating Vδ1 T cells in comparison to their European counterparts.89 This reversed ratio of Vδ1/Vδ2 T cells showed no age dependency nor did it appear to be an antigen-driven event.89 This region is highly endemic for Plasmodium falciparum, the causative agent of malaria, for which the Vδ1 T cell subset seems to have a special immunoregulatory function.90,91 It is therefore feasible that this change in γδ T cell distribution observed in never-infected individuals from this West African population stems from evolutionary selection based on an immune advantage by those having a high proportion of Vδ1 T cells—which would again align with the association of there being some adaptive influence of microbial exposure on the make-up of γδ T cells in an organism. Other infectious diseases that are associated with the recruitment and expansion of specifically the Vδ1 T cell subset in the periphery are cytomegalovirus,33,92 Epstein–Barr virus93,94 and HIV.95,96 In most of these cases, the specific activation and expansion appears to be driven by the presentation of endogenous molecules rather than virally derived antigens—keeping with the notion that these T cells are particularly well adapted to sensing the turmoil within rather than relying exclusively on external validation.
Tales told by battle scars and tree rings
A number of chronic inflammatory diseases are also associated with perturbed γδ T cell distributions.97,98,99,100,101 In some cases, such as systemic sclerosis, rheumatoid arthritis, Takayasu's arteritis and Wegner's granulomatosis, an inverse ratio of peripheral blood γδ T cells is seen again such that the Vδ1 T cell subset outnumber the Vδ2 T cells.100,101(and unpublished data) This occurrence is often associated with the concomitant loss of peripheral Vγ9Vδ2 T cells—possibly due to their tendency to undergo activation-induced cell death via FAS-mediated apoptosis which occurs following repeated stimulation of their TCR.102,103,104 Death by exhaustion appears to be a consequence for the highly empathetic in an environment subjected to chronic distress. An example of this is seen in people with Crohn's disease who were found to have a global deficit in the number of γδ T cells present.98 Evidence that this may be a result of activation-induced cell death as opposed to an inherent immune anomaly of the disease comes from the observation that treatment with infliximab, an anti-TNFα agent, resulted in clonal expansion of γδ T cells in Crohn's patients on treatment105, highlighting the potential in vivo role of TNFα signalling in γδ T cell self-modulation. However, in vitro studies looking at the functional effects of infliximab on γδ T cells derived from patients with Behçet's disease or rheumatoid arthritis, in contrast, found that the expansion and reactivity of these excitable T cells was notably impaired,106,107 underscoring again the often discrepant outcomes when attempting to extrapolate findings from manipulated artificial settings in vitro to what occurs in vivo. This has been a particularly challenging hurdle when trying to implement the remarkable anticancer arsenal of γδ T cells for cancer immunotherapy.10,108,109,110 Despite the ready expansion and cytolytic effector functions of γδ T cells demonstrated at the bench, attempts to harness this potential in the clinical setting have been disappointing due to the inevitable loss of these cells that is observed in many cancer patients.111,112 It is therefore of great interest to find ways to keep these cells from collapsing from the often overwhelming task of meeting the many needs of the distressed self.
Of course the cumulative stressors experienced over a lifetime also take their toll, and a gradual loss of peripheral γδ T cell pool is found to occur with age in the industrialized nations studied.113,114,115 The life events that affect γδ T cell function are not limited to infectious and physiological insults, but also include psychological stress that especially mobilize terminally differentiated ‘effector memory' cells characterized as being negative for cell surface marker CD27 but expressing CD45RA.116 This phenotype represents γδ T cells that have already tackled some hurdles in their life and have reduced proliferative capacity, but are still able to pull the trigger given their highly cytolytic potential.117 Thus, differences in γδ T cell numbers in relation to parameters such as gender and race that are inconsistent across the various populations studied89,113,114,115 may partly reflect the discrepant burdens that contribute to overall life stress experienced by members of these groups. For example, African Americans in Baltimore were found to have significantly lower peripheral blood γδ T cells in comparison to their Caucasian counterparts;113 however, Africans from Ghana have significantly more γδ T cells (of both the Vδ1 and Vδ2 T cell variety) in comparison to Europeans.89 This observation makes it highly suspect that the racial difference observed in the North American cohort is due to an intrinsic property of African Americans to have fewer γδ T cells, and it may reflect the different cumulative life stresses they face that take a toll on immune function.
‘While there is tea, there is hope.'
Fortunately, there is a good amount of evidence suggesting that the adaptogenic properties of γδ T cells can be protected and enhanced in those who need it most, namely those burdened with chronic stress. γδ T cells seem particularly responsive to certain nutrient properties found in various food and supplement products, especially those with high antioxidant values (Table 4).69,118,119,120,121,122,123,124,125,126,127 In a double-blind placebo-controlled randomized control trial, capsules containing fruit and vegetable concentrate (containing, among other compounds, standardized levels of β-carotene, vitamin C, vitamin E, folate and calcium) were given to law school students, taken as a representative of young adults living with modern day stresses, over a period of 11 weeks.128 γδ T cells were found to be specifically increased (by about 30% no change in circulating αβ T cells) in parallel to an increase in the antioxidant capacity of the study subjects, and in vitro analyses showed that this effect was associated with a more tempered inflammatory response upon phorbal myristate acetate stimulation.128 For some of these compounds with high antioxidant properties, their impact on γδ T cells may be partly derived from their direct effect on the distress levels of cells that γδ T cells are sensitive to. For example, treating renal carcinoma cells subjected to oxidative stress with the strong antioxidant, N-acetylcysteine, blunted the inflammatory response and activation of Vγ9Vδ2 T cells by downregulating the ectopic expression of the stress-induced molecule, MSH2.79 Therefore, some of these immunomodulatory bioactive compounds, which include diverse items like procyanidins from unripe apple peels129 and polysaccharide components of the Acai berry,119 their noted influence on γδ T cells may be through the modulation of the type of stress signals displayed by cells that mobilize their activity. In contrast, other compounds, such as the aforementioned alkylamines (e.g. ethylamine, propylamine, buylamine and amylamine),69 ℓ-theonine from tea69 and heat-treated mistletoe extracts,125 the effect may be more direct and specific to human peripheral blood Vγ9Vδ2 T cells through the modulation of isoprenoid synthesis and the generation of pAgs. Understanding the mechanism by which γδ T cell function is affected by various immunomodulatory compounds is central to formulating specific effective protocols for the targeted management of the various ailments these unique cells have the capacity to help treat and/or influence, including cancer, immunodeficiency and autoimmunity. Importantly, antioxidants could play an essential role in providing a buffer to prevent the untimely burn-out of these often over-worked but highly empathetic unconventional lymphocytes.
Table 4. Bioactive compounds that have been studied to enhance γδ T cell function.
Epilogue
In light of their story, it can be appreciated that the difficulty in accurately defining γδ T cells within existing prototypes stems from the fact that these T cells operate according to their own exclusive paradigm, as illustrated in Figure 1. In many ways they are the true ‘adaptive' T cells as they are able to sense changes within the self that have the potential to disturb equilibrium, and they help the ‘self' adapt as efficiently as possible to life's many challenges. However, unlike αβ T cells, their own multifaceted but sensitive character remains relatively invariant in the face of the diverse stressors to which they help the self acclimatize. One strategy that γδ T cells use to fulfill the complex nature of their task is to creatively merge the instincts of the innate with the diversity of the adaptive immune systems. Thus, some of the antigens that they recognize are evolutionarily shared by the self and non-self and their subsequent response is contextually guided by the level of distress that they sense from within—often in the context of the contingent presence of other damage-associated molecular patterns. Given that different organisms are subjected to different types of stressors that impact their livelihood, it is not surprising that the phenotypic repertoire and distribution of γδ T cells between species is quite diverse and tailored for the specific needs that they help meet. In fact, it was noted that substantial differences can be observed in the γδ T cell fingerprint born between individuals even of the same species depending on the nature and extent of their life stress exposures—somewhat like the story held within the rings of a tree trunk. It still remains to be seen how these interindividual differences in γδ T cell profiles are associated with health outcomes. For diseases such as cancer, a relationship between higher levels of circulating responsive γδ T cells and a more favorable outcome has been observed;108 however, there is still much to be learned about the potential contribution of γδ T cells in providing general resiliency to malaise. An important challenge is to determine how to best support these intuitive T cells with their burden—some food for thought while destressing over a cup of afternoon tea.
Acknowledgments
SK is supported by a fellowship from the Alexander von Humboldt Foundation of Germany. DK acknowledges grant support from the Deutsche Forschungsgemeinschaft (DFG WE 3559/2-1) and is also supported by the ‘Inflammation-at-Interfaces' Cluster of Excellence funded by the Deutsche Forschungsgemeinschaft (DFG).
The authors have no conflict of interest to disclose.
References
- Saito H, Kranz DM, Takagaki Y, Hayday AC, Eisen HN, Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984;312:36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, et al. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Tanaka Y, Harazaki M, Mikami B, Minato N. Recognition mechanism of non-peptide antigens by human gammadelta T cells. Int Immunol. 2003;15:1301–1307. doi: 10.1093/intimm/dxg129. [DOI] [PubMed] [Google Scholar]
- Li H, Lebedeva MI, Llera AS, Fields BA, Brenner MB, Mariuzza RA. Structure of the Vdelta domain of a human gammadelta T-cell antigen receptor. Nature. 1998;391:502–506. doi: 10.1038/35172. [DOI] [PubMed] [Google Scholar]
- Shao L, Huang D, Wei H, Wang RC, Chen CY, Shen L, et al. Expansion, reexpansion, and recall-like expansion of Vgamma2Vdelta2 T cells in smallpox vaccination and monkeypox virus infection. J Virol. 2009;83:11959–11965. doi: 10.1128/JVI.00689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Letvin NL. Adaptive immune response of Vgamma2Vdelta2 T cells: a new paradigm. Trends Immunol. 2003;24:213–219. doi: 10.1016/s1471-4906(03)00032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey J, Veuillen C, Vey N, Bouabdallah R, Olive D. Natural killer and gammadelta T cells in haematological malignancies: enhancing the immune effectors. Trends Mol Med. 2009;15:275–284. doi: 10.1016/j.molmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Shafi S, Vantourout P, Wallace G, Antoun A, Vaughan R, Stanford M, et al. An NKG2D-mediated human lymphoid stress surveillance response with high interindividual variation. Sci Transl Med. 2011;3:113–124. doi: 10.1126/scitranslmed.3002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetz S, Marischen L, Kabelitz D, Wesch D. Human gamma delta T cells: candidates for the development of immunotherapeutic strategies. Immunol Res. 2007;37:97–111. doi: 10.1007/BF02685893. [DOI] [PubMed] [Google Scholar]
- Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, et al. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol. 2007;66:320–328. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- Fisch P, Meuer E, Pende D, Rothenfusser S, Viale O, Kock S, et al. Control of B cell lymphoma recognition via natural killer inhibitory receptors implies a role for human Vgamma9/Vdelta2 T cells in tumor immunity. Eur J Immunol. 1997;27:3368–3379. doi: 10.1002/eji.1830271236. [DOI] [PubMed] [Google Scholar]
- Halary F, Peyrat MA, Champagne E, Lopez-Botet M, Moretta A, Moretta L, et al. Control of self-reactive cytotoxic T lymphocytes expressing gamma delta T cell receptors by natural killer inhibitory receptors. Eur J Immunol. 1997;27:2812–2821. doi: 10.1002/eji.1830271111. [DOI] [PubMed] [Google Scholar]
- Moris A, Rothenfusser S, Meuer E, Hangretinger R, Fisch P. Role of gammadelta T cells in tumor immunity and their control by NK receptors. Microbes Infect. 1999;1:227–234. doi: 10.1016/s1286-4579(99)80038-0. [DOI] [PubMed] [Google Scholar]
- Barakonyi A, Kovacs KT, Miko E, Szereday L, Varga P, Szekeres-Bartho J. Recognition of nonclassical HLA class I antigens by gamma delta T cells during pregnancy. J Immunol. 2002;168:2683–2688. doi: 10.4049/jimmunol.168.6.2683. [DOI] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Bioley G, Levy N, Eberl M, Luo M, et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci USA. 2009;106:2307–2312. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karre K. How to recognize a foreign submarine. Immunol Rev. 1997;155:5–9. doi: 10.1111/j.1600-065x.1997.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Marischen L, Oberg HH, Holtmeier W, Wesch D. Epithelial defence by gamma delta T cells. Int Arch Allergy Immunol. 2005;137:73–81. doi: 10.1159/000085107. [DOI] [PubMed] [Google Scholar]
- Su C, Jakobsen I, Gu X, Nei M. Diversity and evolution of T-cell receptor variable region genes in mammals and birds. Immunogenetics. 1999;50:301–308. doi: 10.1007/s002510050606. [DOI] [PubMed] [Google Scholar]
- Qu A, Brulc JM, Wilson MK, Law BF, Theoret JR, Joens LA, et al. Comparative metagenomics reveals host specific metavirulomes and horizontal gene transfer elements in the chicken cecum microbiome. PLoS One. 2008;3:e2945. doi: 10.1371/journal.pone.0002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WH, Miller TK. Rumen digestive physiology and microbial ecology. Vet Clin North Am Food Anim Pract. 1991;7:311–325. doi: 10.1016/s0749-0720(15)30801-x. [DOI] [PubMed] [Google Scholar]
- Ross JA, Scott A, Gardner IC. Bacterial localization in the caecum of the rabbit. Microbios. 1987;52:51–63. [PubMed] [Google Scholar]
- Stevens CE, Hume ID. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol Rev. 1998;78:393–427. doi: 10.1152/physrev.1998.78.2.393. [DOI] [PubMed] [Google Scholar]
- Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, et al. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci USA. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182:3047–3054. doi: 10.4049/jimmunol.0802705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal gammadelta TCRs. Nat Immunol. 2012;13:272–282. doi: 10.1038/ni.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J, Havran WL. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte-responsive gamma delta TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J Immunol. 2004;172:3573–3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- Boismenu R, Havran WL. Gammadelta T cells in host defense and epithelial cell biology. Clin Immunol Immunopathol. 1998;86:121–133. doi: 10.1006/clin.1997.4468. [DOI] [PubMed] [Google Scholar]
- Havran WL, Chen Y, Boismenu R. Innate functions of epithelial gamma delta T cells. Adv Exp Med Biol. 1998;452:29–35. [PubMed] [Google Scholar]
- McVay LD, Carding SR. Generation of human gammadelta T-cell repertoires. Crit Rev Immunol. 1999;19:431–460. [PubMed] [Google Scholar]
- Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, et al. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med. 2005;201:1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke NR, Stankovic S, Funda DP, Harrison LC. TCR gamma delta intraepithelial lymphocytes are required for self-tolerance. J Immunol. 2006;176:6553–6559. doi: 10.4049/jimmunol.176.11.6553. [DOI] [PubMed] [Google Scholar]
- Holtmeier W. Compartmentalization gamma/delta T cells and their putative role in mucosal immunity. Crit Rev Immunol. 2003;23:473–488. doi: 10.1615/critrevimmunol.v23.i56.60. [DOI] [PubMed] [Google Scholar]
- Hayday A, Gibbons D. Brokering the peace: the origin of intestinal T cells. Mucosal Immunol. 2008;1:172–174. doi: 10.1038/mi.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejdosiewicz LK. Intestinal intraepithelial lymphocytes and lymphoepithelial interactions in the human gastrointestinal mucosa. Immunol Lett. 1992;32:13–19. doi: 10.1016/0165-2478(92)90192-q. [DOI] [PubMed] [Google Scholar]
- Zocchi MR, Poggi A. Role of gammadelta T lymphocytes in tumor defense. Front Biosci. 2004;9:2588–2604. doi: 10.2741/1419. [DOI] [PubMed] [Google Scholar]
- Maeurer MJ, Martin D, Walter W, Liu K, Zitvogel L, Halusczcak K, et al. Human intestinal Vdelta1+ lymphocytes recognize tumor cells of epithelial origin. J Exp Med. 1996;183:1681–1696. doi: 10.1084/jem.183.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat G, Naiyer AJ, Shah JG, Harper J, Jabri B, Wang TC, et al. Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Invest. 2008;118:281–293. doi: 10.1172/JCI30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmeier W, Kabelitz D. gammadelta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151–183. doi: 10.1159/000086659. [DOI] [PubMed] [Google Scholar]
- Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition. Curr Opin Immunol. 2002;14:306–311. doi: 10.1016/s0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, et al. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci USA. 2011;108:2414–2419. doi: 10.1073/pnas.1015433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig NA, Guan Z, Li D, McMichael A, Raetz CR, Xu XN. Identification of self-lipids presented by CD1c and CD1d proteins. J Biol Chem. 2011;286:37692–37701. doi: 10.1074/jbc.M111.267948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, et al. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, et al. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat Immunol. 2012;13:872–879. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- Montes R, Puy C, Molina E, Hermida J. Is EPCR a multi-ligand receptor? Pros and cons. Thromb Haemost. 2012;107:815–826. doi: 10.1160/TH11-11-0766. [DOI] [PubMed] [Google Scholar]
- Mincheva-Nilsson L, Hammarstrom S, Hammarstrom ML. Human decidual leukocytes from early pregnancy contain high numbers of gamma delta+ cells and show selective down-regulation of alloreactivity. J Immunol. 1992;149:2203–2211. [PubMed] [Google Scholar]
- Bonney EA, Pudney J, Anderson DJ, Hill JA. Gamma-delta T cells in midgestation human placental villi. Gynecol Obstet Invest. 2000;50:153–157. doi: 10.1159/000010315. [DOI] [PubMed] [Google Scholar]
- Nagaeva O, Jonsson L, Mincheva-Nilsson L. Dominant IL-10 and TGF-beta mRNA expression in gamma delta T cells of human early pregnancy decidua suggests immunoregulatory potential. Am J Reprod Immunol. 2002;48:9–17. doi: 10.1034/j.1600-0897.2002.01131.x. [DOI] [PubMed] [Google Scholar]
- Fan DX, Duan J, Li MQ, Xu B, Li DJ, Jin LP. The decidual gamma-delta T cells up-regulate the biological functions of trophoblasts via IL-10 secretion in early human pregnancy. Clin Immunol. 2011;141:284–292. doi: 10.1016/j.clim.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hayakawa S, Shiraishi H, Saitoh S, Satoh K. Decidua as a site of extrathymic V gamma I T-cell differentiation. Am J Reprod Immunol. 1996;35:233–238. doi: 10.1111/j.1600-0897.1996.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Mincheva-Nilsson L, Kling M, Hammarstrom S, Nagaeva O, Sundqvist KG, Hammarstrom ML, et al. Gamma delta T cells of human early pregnancy decidua: evidence for local proliferation, phenotypic heterogeneity, and extrathymic differentiation. J Immunol. 1997;159:3266–3277. [PubMed] [Google Scholar]
- Meeusen E, Fox A, Brandon M, Lee CS. Activation of uterine intraepithelial gamma delta T cell receptor-positive lymphocytes during pregnancy. Eur J Immunol. 1993;23:1112–1117. doi: 10.1002/eji.1830230520. [DOI] [PubMed] [Google Scholar]
- Heyborne KD, Cranfill RL, Carding SR, Born WK, O'Brien RL. Characterization of gamma delta T lymphocytes at the maternal-fetal interface. J Immunol. 1992;149:2872–2878. [PubMed] [Google Scholar]
- Barakoryi A, Miko E, Varga P, Szekeres-Bartho J. V-chain preference of gamma/delta T-cell receptors in peripheral blood during term labor. Am J Reprod Immunol. 2008;59:201–205. doi: 10.1111/j.1600-0897.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- Polgar B, Barakonyi A, Xynos I, Szekeres-Bartho J. The role of gamma/delta T cell receptor positive cells in pregnancy. Am J Reprod Immunol. 1999;41:239–244. doi: 10.1111/j.1600-0897.1999.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Barakonyi A, Polgar B, Szekeres-Bartho J. The role of gamma/delta T-cell receptor-positive cells in pregnancy: part II. Am J Reprod Immunol. 1999;42:83–87. [PubMed] [Google Scholar]
- Szereday L, Barakonyi A, Miko E, Varga P, Szekeres-Bartho J. gamma/delta T-cell subsets, NKG2A expression and apoptosis of V delta 2+ T cells in pregnant women with or without risk of premature pregnancy termination. Am J Reprod Immunol. 2003;50:490–496. doi: 10.1046/j.8755-8920.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- Heyborne K, Fu YX, Nelson A, Farr A, O'Brien R, Born W. Recognition of trophoblasts by gamma delta T cells. J Immunol. 1994;153:2918–2926. [PubMed] [Google Scholar]
- Wang H, Lee HK, Bukowski JF, Li H, Mariuzza RA, Chen ZW, et al. Conservation of nonpeptide antigen recognition by rhesus monkey V gamma 2V delta 2 T cells. J Immunol. 2003;170:3696–3706. doi: 10.4049/jimmunol.170.7.3696. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Jomaa H, Feurle J, Luhs K, Kunzmann V, Tony HP, Herderich M, et al. Vgamma9/Vdelta2 T cell activation induced by bacterial low molecular mass compounds depends on the 1-deoxy-D-xylulose 5-phosphate pathway of isoprenoid biosynthesis. FEMS Immunol Med Microbiol. 1999;25:371–378. doi: 10.1111/j.1574-695X.1999.tb01362.x. [DOI] [PubMed] [Google Scholar]
- Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, de Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced gamma,delta-T-cell proliferation and activation in vitro. . J Bone Miner Res. 2004;19:278–288. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- Thompson K, Rojas-Navea J, Rogers MJ. Alkylamines cause Vgamma9Vdelta2 T-cell activation and proliferation by inhibiting the mevalonate pathway. Blood. 2006;107:651–654. doi: 10.1182/blood-2005-03-1025. [DOI] [PubMed] [Google Scholar]
- Green AE, Lissina A, Hutchinson SL, Hewitt RE, Temple B, James D, et al. Recognition of nonpeptide antigens by human V gamma 9V delta 2 T cells requires contact with cells of human origin. Clin Exp Immunol. 2004;136:472–482. doi: 10.1111/j.1365-2249.2004.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M, et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Fu Y, Hou Y, Fu C, Gu M, Li C, Kong W, et al. A novel mechanism of gamma/delta T-lymphocyte and endothelial activation by shear stress: the role of ecto-ATP synthase beta chain. Circ Res. 2011;108:410–417. doi: 10.1161/CIRCRESAHA.110.230151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookerjee-Basu J, Vantourout P, Martinez LO, Perret B, Collet X, Perigaud C, et al. F1-adenosine triphosphatase displays properties characteristic of an antigen presentation molecule for Vgamma9Vdelta2 T cells. J Immunol. 2010;184:6920–6928. doi: 10.4049/jimmunol.0904024. [DOI] [PubMed] [Google Scholar]
- Chen H, He X, Wang Z, Wu D, Zhang H, Xu C, et al. Identification of human T cell receptor gammadelta-recognized epitopes/proteins via CDR3delta peptide-based immunobiochemical strategy. J Biol Chem. 2008;283:12528–12537. doi: 10.1074/jbc.M708067200. [DOI] [PubMed] [Google Scholar]
- Dai Y, Chen H, Mo C, Cui L, He W. Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human gammadelta t cells to induce innate anti-tumor/virus immunity. J Biol Chem. 2012;287:16812–16819. doi: 10.1074/jbc.M111.327650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C, Dai Y, Kang N, Cui L, He W. Ectopic expression of human MutS homologue 2 on renal carcinoma cells is induced by oxidative stress with interleukin-18 promotion via p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) signaling pathways. J Biol Chem. 2012;287:19242–19254. doi: 10.1074/jbc.M112.349936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen CD, Cyr JL, Cook C, Punja N, Sakato M, Forties RA, et al. Human MSH2 (hMSH2) protein controls ATP processing by hMSH2-hMSH6. J Biol Chem. 2011;286:40287–40295. doi: 10.1074/jbc.M111.297523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harly C, Guillaume Y, Nedellec S, Peigne CM, Monkkonen H, Monkkonen J, et al. Key implication of CD277/Butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T cell subset. Blood. 2012;120:2269–2279. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DA, Stammers M, Malcherek G, Beck S, Trowsdale J. The cluster of BTN genes in the extended major histocompatibility complex. Genomics. 2001;71:351–362. doi: 10.1006/geno.2000.6406. [DOI] [PubMed] [Google Scholar]
- Carding SR, Kyes S, Jenkinson EJ, Kingston R, Bottomly K, Owen JJ, et al. Developmentally regulated fetal thymic and extrathymic T-cell receptor gamma delta gene expression. Genes Dev. 1990;4:1304–1315. doi: 10.1101/gad.4.8.1304. [DOI] [PubMed] [Google Scholar]
- McVay LD, Carding SR. Extrathymic origin of human gamma delta T cells during fetal development. J Immunol. 1996;157:2873–2882. [PubMed] [Google Scholar]
- de Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Herzenberg LA, et al. Ontogeny of gamma delta T cells in humans. J Immunol. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- Esin S, Shigematsu M, Nagai S, Eklund A, Wigzell H, Grunewald J. Different percentages of peripheral blood gamma delta+T cells in healthy individuals from different areas of the world. Scand J Immunol. 1996;43:593–596. doi: 10.1046/j.1365-3083.1996.d01-79.x. [DOI] [PubMed] [Google Scholar]
- Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both. Immunology. 2004;112:352–363. doi: 10.1111/j.1365-2567.2004.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garn H, Renz H. Epidemiological and immunological evidence for the hygiene hypothesis. Immunobiology. 2007;212:441–452. doi: 10.1016/j.imbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Hviid L, Akanmori BD, Loizon S, Kurtzhals JA, Ricke CH, Lim A, et al. High frequency of circulating gamma delta T cells with dominance of the v(delta)1 subset in a healthy population. Int Immunol. 2000;12:797–805. doi: 10.1093/intimm/12.6.797. [DOI] [PubMed] [Google Scholar]
- Hviid L, Kurtzhals JA, Adabayeri V, Loizon S, Kemp K, Goka BQ, et al. Perturbation and proinflammatory type activation of V delta 1+ gamma delta T cells in African children with Plasmodium falciparum malaria. Infect Immun. 2001;69:3190–3196. doi: 10.1128/IAI.69.5.3190-3196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M, Tongtawe P, Kriangkum J, Wimonwattrawatee T, Pattanapanyasat K, Bryant L, et al. Polyclonal expansion of peripheral gamma delta T cells in human Plasmodium falciparum malaria. Infect Immun. 1994;62:855–862. doi: 10.1128/iai.62.3.855-862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, van Rysselberge M, et al. Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J Exp Med. 2010;207:807–821. doi: 10.1084/jem.20090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini DL, Res PC, van Laar JM, Muller LM, Soprano AE, Kooy YM, et al. A subset of V delta 1+ T cells proliferates in response to Epstein–Barr virus-transformed B cell lines in vitro. . Scand J Immunol. 1993;38:335–340. doi: 10.1111/j.1365-3083.1993.tb01735.x. [DOI] [PubMed] [Google Scholar]
- Orsini DL, van Gils M, Kooy YM, Struyk L, Klein G, van den Elsen P, et al. Functional and molecular characterization of B cell-responsive V delta 1+ gamma delta T cells. Eur J Immunol. 1994;24:3199–3204. doi: 10.1002/eji.1830241243. [DOI] [PubMed] [Google Scholar]
- Zheng NN, McElrath MJ, Sow PS, Mesher A, Hawes SE, Stern J, et al. Association between peripheral gammadelta T-cell profile and disease progression in individuals infected with HIV-1 or HIV-2 in West Africa. J Acquir Immune Defic Syndr. 2011;57:92–100. doi: 10.1097/QAI.0b013e318215a877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol R, Dobmeyer JM, Dobmeyer TS, Klein SA, Rossol S, Wesch D, et al. Increase in Vdelta1+ gammadelta T cells in the peripheral blood and bone marrow as a selective feature of HIV-1 but not other virus infections. Br J Haematol. 1998;100:728–734. doi: 10.1046/j.1365-2141.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- Oswald E, Fisch P, Jakob T, Bruckner-Tuderman L, Martin SF, Rensing-Ehl A. Reduced numbers of circulating gammadelta T cells in patients with bullous pemphigoid. Exp Dermatol. 2009;18:991–993. doi: 10.1111/j.1600-0625.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- Andreu-Ballester JC, Amigo-Garcia V, Catalan-Serra I, Gil-Borras R, Ballester F, Almela-Quilis A, et al. Deficit of gammadelta T lymphocytes in the peripheral blood of patients with Crohn's disease. Dig Dis Sci. 2011;56:2613–2622. doi: 10.1007/s10620-011-1636-8. [DOI] [PubMed] [Google Scholar]
- Kiladjian JJ, Visentin G, Viey E, Chevret S, Eclache V, Stirnemann J, et al. Activation of cytotoxic T-cell receptor gammadelta T lymphocytes in response to specific stimulation in myelodysplastic syndromes. Haematologica. 2008;93:381–389. doi: 10.3324/haematol.11812. [DOI] [PubMed] [Google Scholar]
- Chauhan SK, Tripathy NK, Sinha N, Nityanand S. T-cell receptor repertoire of circulating gamma delta T-cells in Takayasu's arteritis. Clin Immunol. 2006;118:243–249. doi: 10.1016/j.clim.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Giacomelli R, Matucci-Cerinic M, Cipriani P, Ghersetich I, Lattanzio R, Pavan A, et al. Circulating Vdelta1+ T cells are activated and accumulate in the skin of systemic sclerosis patients. Arthritis Rheum. 1998;41:327–334. doi: 10.1002/1529-0131(199802)41:2<327::AID-ART17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Gan YH, Lui SS, Malkovsky M. Differential susceptibility of naive and activated human gammadelta T cells to activation-induced cell death by T-cell receptor cross-linking. Mol Med. 2001;7:636–643. [PMC free article] [PubMed] [Google Scholar]
- Gan YH, Wallace M, Malkovsky M. Fas-dependent, activation-induced cell death of gammadelta cells. J Biol Regul Homeost Agents. 2001;15:277–285. [PubMed] [Google Scholar]
- Kabelitz D. Effector functions and control of human gammadelta T-cell activation. Microbes Infect. 1999;1:255–261. doi: 10.1016/s1286-4579(99)80042-2. [DOI] [PubMed] [Google Scholar]
- Kelsen J, Dige A, Schwindt H, D'Amore F, Pedersen FS, Agnholt J, et al. Infliximab induces clonal expansion of gammadelta-T cells in Crohn's disease: a predictor of lymphoma risk. PLoS One. 2011;6:e17890. doi: 10.1371/journal.pone.0017890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina AR, Accardo-Palumbo A, Ciccia F, Ferrante A, Principato A, Impastato R, et al. Blocking TNF in vitro with infliximab determines the inhibition of expansion and interferon gamma production of Vgamma9/Vdelta2 T lymphocytes from patients with active rheumatoid arthritis. A role in the susceptibility to tuberculosis. Reumatismo. 2009;61:21–26. doi: 10.4081/reumatismo.2009.21. [DOI] [PubMed] [Google Scholar]
- Accardo-Palumbo A, Giardina AR, Ciccia F, Ferrante A, Principato A, Impastato R, et al. Phenotype and functional changes of Vgamma9/Vdelta2 T lymphocytes in Behcet's disease and the effect of infliximab on Vgamma9/Vdelta2 T cell expansion, activation and cytotoxicity. Arthritis Res Ther. 2010;12:R109. doi: 10.1186/ar3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyan S, Wesch D, Kabelitz D. Aminobisphosphonates and Toll-like receptor ligands: recruiting Vgamma9Vdelta2 T cells for the treatment of hematologic malignancy. Curr Med Chem. 2011;18:5206–5216. doi: 10.2174/092986711798184280. [DOI] [PubMed] [Google Scholar]
- Kabelitz D. Human gammadelta T lymphocytes for immunotherapeutic strategies against cancer. F1000 Med Rep. 2010;2:45. doi: 10.3410/M2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- Martinet L, Poupot R, Fournie JJ. Pitfalls on the roadmap to gammadelta T cell-based cancer immunotherapies. Immunol Lett. 2009;124:1–8. doi: 10.1016/j.imlet.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Lopez RD. Human gammadelta-T cells in adoptive immunotherapy of malignant and infectious diseases. Immunol Res. 2002;26:207–221. doi: 10.1385/IR:26:1-3:207. [DOI] [PubMed] [Google Scholar]
- Cairo C, Armstrong CL, Cummings JS, Deetz CO, Tan M, Lu C, et al. Impact of age, gender, and race on circulating gammadelta T cells. Hum Immunol. 2010;71:968–975. doi: 10.1016/j.humimm.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo N, Dieli F, Wesch D, Jomaa H, Eberl M. Sex-specific phenotypical and functional differences in peripheral human Vgamma9/Vdelta2 T cells. J Leukoc Biol. 2006;79:663–666. doi: 10.1189/jlb.1105640. [DOI] [PubMed] [Google Scholar]
- Michishita Y, Hirokawa M, Guo YM, Abe Y, Liu J, Ubukawa K, et al. Age-associated alteration of gammadelta T-cell repertoire and different profiles of activation-induced death of Vdelta1 and Vdelta2 T cells. Int J Hematol. 2011;94:230–240. doi: 10.1007/s12185-011-0907-7. [DOI] [PubMed] [Google Scholar]
- Anane LH, Edwards KM, Burns VE, Zanten JJ, Drayson MT, Bosch JA. Phenotypic characterization of gammadelta T cells mobilized in response to acute psychological stress. Brain Behav Immun. 2010;24:608–614. doi: 10.1016/j.bbi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, di Sano C, et al. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival SS, Bukowski JF, Milner J. Bioactive food components that enhance gammadelta T cell function may play a role in cancer prevention. J Nutr. 2008;138:1–4. doi: 10.1093/jn/138.1.1. [DOI] [PubMed] [Google Scholar]
- Holderness J, Schepetkin IA, Freedman B, Kirpotina LN, Quinn MT, Hedges JF, et al. Polysaccharides isolated from Acai fruit induce innate immune responses. PLoS One. 2011;6:e17301. doi: 10.1371/journal.pone.0017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JC, Kimmel EM, Freedman B, Schepetkin IA, Holderness J, Quinn MT, et al. Polysaccharides derived from Yamoa (Funtumia elastica) prime gammadelta T cells in vitro and enhance innate immune responses in vivo. . Int Immunopharmacol. 2009;9:1313–1322. doi: 10.1016/j.intimp.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderness J, Jackiw L, Kimmel E, Kerns H, Radke M, Hedges JF, et al. Select plant tannins induce IL-2Ralpha up-regulation and augment cell division in gammadelta T cells. J Immunol. 2007;179:6468–6478. doi: 10.4049/jimmunol.179.10.6468. [DOI] [PubMed] [Google Scholar]
- Daughenbaugh KF, Holderness J, Graff JC, Hedges JF, Freedman B, Graff JW, et al. Contribution of transcript stability to a conserved procyanidin-induced cytokine response in gammadelta T cells. Genes Immun. 2011;12:378–389. doi: 10.1038/gene.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CA, Nantz MP, Bukowski JF, Percival SS. Specific formulation of Camellia sinensis prevents cold and flu symptoms and enhances gamma,delta T cell function: a randomized, double-blind, placebo-controlled study. J Am Coll Nutr. 2007;26:445–452. doi: 10.1080/07315724.2007.10719634. [DOI] [PubMed] [Google Scholar]
- Nantz MP, Rowe CA, Muller CE, Creasy RA, Stanilka JM, Percival SS. Supplementation with aged garlic extract improves both NK and gammadelta-T cell function and reduces the severity of cold and flu symptoms: A randomized, double-blind, placebo-controlled nutrition intervention. Clin Nutr. 2012;31:337–344. doi: 10.1016/j.clnu.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Fischer S, Scheffler A, Kabelitz D. Activation of human gamma delta T-cells by heat-treated mistletoe plant extracts. Immunol Lett. 1996;52:69–72. doi: 10.1016/0165-2478(96)02584-9. [DOI] [PubMed] [Google Scholar]
- Rowe CA, Nantz MP, Nieves C, Jr, West RL, Percival SS. Regular consumption of concord grape juice benefits human immunity. J Med Food. 2011;14:69–78. doi: 10.1089/jmf.2010.0055. [DOI] [PubMed] [Google Scholar]
- Kamath AB, Wang L, Das H, Li L, Reinhold VN, Bukowski JF. Antigens in tea-beverage prime human Vgamma 2Vdelta 2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc Natl Acad Sci USA. 2003;100:6009–6014. doi: 10.1073/pnas.1035603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantz MP, Rowe CA, Nieves C, Jr, Percival SS. Immunity and antioxidant capacity in humans is enhanced by consumption of a dried, encapsulated fruit and vegetable juice concentrate. J Nutr. 2006;136:2606–2610. doi: 10.1093/jn/136.10.2606. [DOI] [PubMed] [Google Scholar]
- Holderness J, Hedges JF, Daughenbaugh K, Kimmel E, Graff J, Freedman B, et al. Response of gamma delta T cells to plant-derived tannins. Crit Rev Immunol. 2008;28:377–402. doi: 10.1615/critrevimmunol.v28.i5.20. [DOI] [PMC free article] [PubMed] [Google Scholar]