Commentary
Innate immune responses are essential for the host to fight against invading microbes. Innate immune receptors or pattern recognition receptors recognize conserved microbial molecules and trigger innate immune responses. Pattern recognition receptors such as Toll-like receptors primarily signal through the adaptor MyD88 to activate NF-κB, while other Toll-like receptors signal via TRIF to activate IRF-3 resulting in the expression of type I interferons (IFNs).1 Type I IFNs play a crucial role in antiviral immune responses but are also important for the host response against bacterial infection.2 Paradoxically, the production of type I IFNs leads to protection against some intracellular pathogens such as Mycobacterium tuberculosis,3 while it enhances the susceptibility to other intracellular pathogens such as Listeria monocytogens.4,5 The mechanisms whereby type I IFN signaling during intracellular bacterial pathogen infection causes differential outcomes remain to be established. In a recent study reported in Nature Immunology, Robinson et al. describe a pathway in which Salmonella enterica serovar Typhimurium (S. Typhimurium) exploits the host type I IFN signaling to kill macrophages through the induction of necroptosis, a specialized pathway of programmed necrosis.6
To evaluate the role of type I IFNs in infection, Robinson et al. challenged mice deficient in the receptor for IFN-α and IFN-β (IFNAR-deficient (Ifnar−/−)) with S. Typhimurium. Improved survival as well as lower bacterial burden in the spleen and liver was observed in Ifnar−/− mice relative to that of the wild-type mice. Furthermore, better protection against S. Typhimurium infection was also obtained via adoptive transfer of Ifnar−/− bone marrow into wild-type or Ifnar−/− hosts, compared with the bone marrow from wild-type mice, indicating that hematopoietic cell-derived type I IFNs play an prominent role in the susceptibility to infection in Ifnar−/− mice.
The improved early control of S. Typhimurium in Ifnar−/− mice was observed before T-cell activation, suggesting a dominant role of the modulation of the innate immune response. The researchers then assessed different innate immune cell types from the spleens of 5d-infected mice. Significantly, more macrophages were observed in Ifnar−/− mice than wild-type mice. To further determine whether these innate immune cells were sufficient for the enhanced protection against S. Typhimurium in Ifnar−/− mice, Ifnar−/− macrophages were transferred into naïve wild-type hosts. Indeed, improved protection was noted. However, there was no difference between bacterial burdens of S. Typhimurium-infected, bone marrow-derived macrophages from Ifnar−/− and wild-type mice, indicating that the improved control of S. Typhimurium was because of increased number of macrophages in Ifnar−/− mice. Considering that the induction of macrophage death is a key virulence strategy used by S. Typhimurium,7 it was reasonable to rationalize that type I IFN signaling may be related to cell death of macrophages through infection. Indeed, macrophages from Ifnar−/− mice survived from S. Typhimurium infection, while massive death was observed in those form wild-type mice.
To discern if the death of macrophages was caused by apoptosis or necrosis, the workers examined the cleavage of poly(ADP-ribose) polymerase 1. Poly(ADP-ribose) polymerase 1 is cleaved to fragments of 89 and 24 kDa in size during apoptosis, while in necrosis, it is excised into fragments of 72 and 50 kDa, respectively.8 The resultant data show a cleavage pattern consistent with necrotic death, demonstrating that S. Typhimurium infection exploited type I IFN signaling to induce macrophage death via necrosis.
The resistance of Ifnar−/− macrophages to death induction through S. Typhimurium infection could be via either cytokine signaling or inflammasome activation. However, little difference in cytokine expression was observed in Ifnar−/− macrophages vs. wild-type cells. Although the secretion of IL-1β in Ifnar−/− macrophages was elevated, there was no difference in caspase-1 activation between Ifnar−/− and wild-type macrophages. Furthermore, neutralization of IL-1β in vivo did not influence the bacterial burden in Ifnar−/− mice. These results indicate that there is no inflammasome activation or cytokine expression difference between Ifnar−/− and wild-type mice during S. Typhimurium infection.
Robinson et al. further assessed several mechanisms associated with cell death. TNF and nitrate ions are known to induce the death of macrophages. Neither genetic deficiency in TNF receptors nor neutralization of TNF-α could protect the death of macrophages during S. Typhimurium infection. Similar results were also retained when the production of nitrate ions was blocked. However, neutralization of IFN-β blocked the death of macrophages after infection. These results indicate that type I IFN signaling plays a commanding role in the necrotic death induced by S. Typhimurium infection.
Apoptosis is considered a form of programmed cell death during development, homeostasis and disease, whereas necrosis is regarded as an unregulated and uncontrolled process. However, a recent study has revealed the existence of programmed necrosis termed necroptosis.9 Necroptosis requires the kinase activity of receptor interacting protein 1 (RIP1)10 and RIP3.11,12,13 To determine whether the S. Typhimurium infection-induced macrophage death was via necroptosis, these researchers treated infected macrophages with necrostatin, which inhibit the activity of RIP1. As predicted, necrostatin protected macrophages from death induction. Furthermore, RIP3 deficiency also inhibited the death of infected macrophages. These data suggest that S. Typhimurium infection invokes the mechanism of necroptosis to abolish macrophages.
Since the RIP1 and RIP3 interaction protein complex is required for necroptosis, the authors hypothesized that infection of S. Typhimurium would manipulate the phosphorylation level of these two proteins. Compared with wild-type macrophages, S. Typhimurium-infected Ifnar−/− macrophages had less phosphorylated forms of RIP1 and RIP3, especially in the late infection period. When IC-21 macrophages were infected with S. Typhimurium or when macrophages were incubated with type I IFNs, phosphorylated RIP1 was induced. Furthermore, S. Typhimurium infection of macrophages induced the expression of RIP3, and immunoprecipitated RIP1 showed a stronger association of RIP3 with RIP1 in wild-type macrophages compared with the Ifnar−/− cells. These results indicate that S. Typhimurium infection activated RIP1 and RIP3 in a type I IFN signaling-dependent manner. Caspase-8 is an inhibitor of necroptosis, and blockade of caspase-8 induces necroptosis.14 There was less caspase-8 in macrophages after infection with S. Typhimurium. Consistent with this result, blocking caspase-8 activation and the engagement of type I INF signaling resulted in necroptosis, resulting in macrophage death. To further confirm the role of necroptosis in S. Typhimurium-infected macrophage death, in vivo experiments were performed, which showed that there were less phosphorylated RIP1 in the Ifnar−/− splenic macrophages after S. Typhimurium infection compared with the wild-type. These outcomes raise the point that IFNAR-mediated RIP1 signaling may be a new pathway leading to macrophage death.
The direct evidence between type I IFN signaling and necroptosis comes from the observation of a physical interaction of RIP1 with the IFNAR. Robinson et al. infected wild-type macrophages with S. Typhimurium and found that RIP1 was co-immunoprecipitated with the IFNAR and the interaction was enhanced after infection. Furthermore, treating macrophages with type I IFN increased the association of RIP1 with IFNAR. Staining S. Typhimurium-infected macrophages also revealed colocalization of RIP1 and IFNAR. These results demonstrate that type I IFN signaling promotes the formation of a RIP1–RIP3 complex and leads to necroptosis.
To further confirm the role of necroptosis in S. Typhimurium-induced macrophage death, Rip3−/− macrophages were transferred into wild-type host, and enhanced control of S. Typhimurium infection was observed compared to the transfer from wild-type macrophages, in a manner similar to the adoptive transfer of Ifnar−/− macrophages. In the animal infection assay, mice deficient in Ciap1, which is known to inhibit necroptosis through inhibiting RIP1, showed a higher burden of S. Typhimurium during infection than that of the wild-type mice. These results indicate that necroptosis is dependent on type I IFN signaling during S. Typhimurium-induced macrophage death.
It is well known that S. Typhimurium induces the expression of type I IFNs by macrophages15 and the induction of macrophage death is a crucial pathogenic strategy used by S. Typhimurium.7 The Robinson study uncovers a novel mechanism by which S. Typhimurium exploits the host type I IFN signaling to destruct macrophages through the induction of RIP-dependent cell death, hereby boosts its own invasion and persistence (Figure 1). This mechanism is not only limited in S. Typhimurium infection since inhibition of necroptosis also protects macrophages from death in infection with another intracellular bacterium, L. monocytogenes.16 Therefore, pathogens that manipulate host response to express type I IFNs and induce the death of macrophages may use this mechanism as a common strategy to facilitate their survival. Elucidation of this new pathway may lead to the development of innovative strategies to fight against chronic infections by interfering with the induced necroptosis of macrophages.
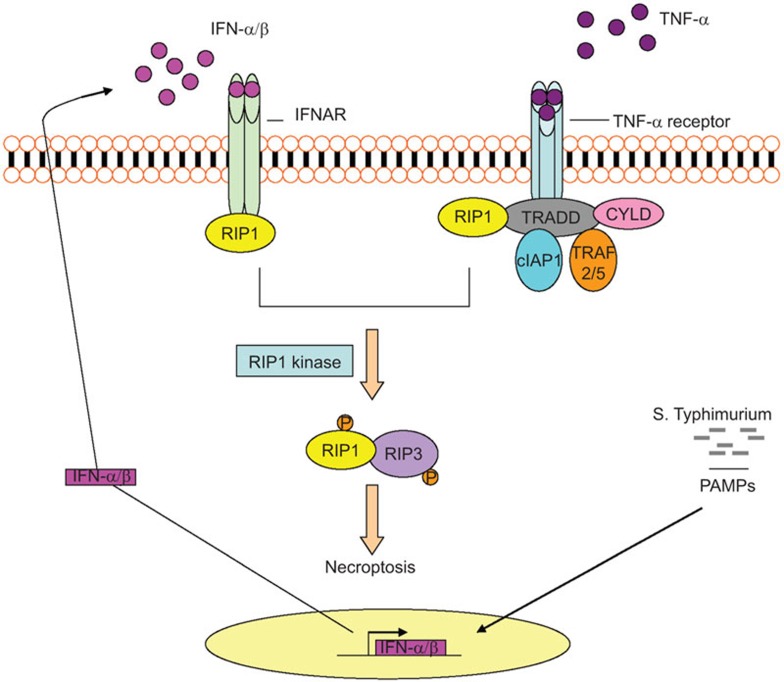
Figure 1.
Type I IFN-mediated necroptosis in macrophages during infection with S. Typhimurimum. After S. Typhimurium infection, type I IFN signaling is activated through the production of type I IFNs. This signal induces the interaction of IFNAR and RIP1, promoting the formation of RIP1–RIP3 (also known as RIPK1–RIPK3) complex, leading to necroptosis of macrophages, invasion and persistence of the bacteria. CYLD, cylindromatosis; IAP, inhibitor of apoptosis protein; IFN, interferon; PAMP, pathogen-associated molecular pattern; RIP, receptor interacting protein; TRADD, TNF receptor-associated death domain; TRAF2/5, TNF receptor-associated factor 2/5.
Acknowledgments
The authors (QD and JX) were supported by the grants from China Scholarship Council (File No. 2011699033), New Century Excellent Talents in Universities (NCET-11-0703), National Natural Science Foundation of China (81071316, 81271882) and Southwest University (XDJK2009A003, XDJK2011D006, kb2010017).
References
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;5633:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;9:675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- Desvignes L, Wolf AJ, Ernst JD. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J Immunol. 2012;12:6205–6215. doi: 10.4049/jimmunol.1200255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;4:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;4:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren SW, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;9:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil S, Boucher CC, Nadeau D, Poirier GG. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ. 2001;6:588–594. doi: 10.1038/sj.cdd.4400851. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;7:1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;7:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;6:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;6:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;5938:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Declercq W, van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;115:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- Sing A, Merlin T, Knopf HP, Nielsen PJ, Loppnow H, Galanos C, et al. Bacterial induction of beta interferon in mice is a function of the lipopolysaccharide component. Infect Immun. 2000;3:1600–1607. doi: 10.1128/iai.68.3.1600-1607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb S, Cheung HH, Korneluk RG, Wang S, Krishnan L, Sad S.cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ 2012. in press. [DOI] [PMC free article] [PubMed]