Abstract
Toll-like receptors (TLRs) are sentinels of the host defense system, which recognize a large number of microbial pathogens. The host defense system may be inefficient or inflammatory diseases may develop if microbial recognition by TLRs and subsequent TLR-triggered cytokine production are deregulated. Activating transcription factor 4 (ATF4), a member of the ATF/CREB transcription factor family, is an important factor that participates in several pathophysiological processes. In this report, we found that ATF4 is also involved in the TLR-mediated innate immune response, which participates in TLR4 signal transduction and mediates the secretion of a variety of cytokines. We observed that ATF4 is activated and translocates to the nucleus following lipopolysaccharide (LPS) stimulation via the TLR4-MyD88-dependent pathway. Additionally, a cytokine array assay showed that some key inflammatory cytokines, such as IL-6, IL-8 and RANTES, are positively regulated by ATF4. We also demonstrate that c-Jun directly binds to ATF4, thereby promoting the secretion of inflammatory cytokines. Taken together, these results indicate that ATF4 acts as a positive regulator in TLR4-triggered cytokine production.
Keywords: ATF4, cytokine, MyD88, TLR4 signaling pathway, TRIF
Introduction
The innate immune system plays a critical role in host defense against microbial pathogen invasion. Unlike the adaptive immune system, the innate immune system recognizes pathogens using receptors without rearrangement, such as Toll-like receptors (TLRs). TLRs are a type of pattern recognition receptor that recognizes molecular patterns that are broadly shared among microbial pathogens but distinguishable from host molecules and that are collectively referred to as pathogen-associated molecular patterns.1 Upon pathogen-associated molecular pattern recognition, TLRs are activated and subsequently transduce signals from the cell membrane to the nucleus. Currently, two main branches of the TLR signal transduction pathway have been identified: the MyD88-dependent pathway and the TRIF-dependent pathway.2,3 Among the various TLR family members, TLR1, TLR2, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10 and TLR11 activate the MyD88-dependent pathway, TLR3 provokes the TRIF-dependent pathway and TLR4 is capable of activating both the MyD88- and TRIF-dependent pathways.4 The principal signaling pathways downstream of MyD88 are the TGF-beta-activated kinase (TAK)-nuclear factor (NF)-κB and TAK-mitogen-activated protein kinase (MAPK)-AP-1 pathways.5 The activation of NF-κB and AP-1 promotes the secretion of multiple inflammatory factors.
ATF4, a member of the ATF/CREB transcription factor family, forms homodimers and heterodimers with other transcription factors, including some members of the AP-1 family. ATF4 plays an important role in many physical processes, such as the stress response, bone resorption, medullary hematopoiesis and lens formation.6,7 It remains unclear whether ATF4 participates in TLR signaling. Two observations suggesting that ATF4 participates in the TLR4 signaling pathway have been described. First, an ATF4 homologous molecule, ATF3, is an important negative regulator of the TLR4 signaling pathway.8 Second, TLR ligand stimulation causes macrophages to secrete high levels of IL-8, while ATF4 positively regulates IL-8 secretion in the same type of cells.9,10 In this study, we demonstrated that ATF4 participates in the TLR4 signaling pathway and positively regulates TLR4-triggered cytokine production.
Materials and methods
Cells and reagents
The THP-1 and U937 cell lines were obtained from the American Type Culture Collection (ATCC No. TIB-202 and No. CRL-1593.2, respectively). The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1 U penicillin and 1 µg streptomycin. The 293T cell line was also obtained from the American Type Culture Collection (ATCC No. CRL-11268). The 293T cells were cultured in DMEM medium supplemented with 10% fetal bovine serum, 2% L-glutamate, 1% non-essential amino acid and 1% sodium pyruvate. All cell lines were maintained at 37 °C in a 5% CO2 incubator. The anti-TLR4-PE antibody (#12-9917-42) used for FACS analysis was obtained from eBioscience (San Diego, CA, USA). The Alexa Fluor 488-goat-anti-rabbit IgG (H+L) (#1008), Alexa Fluor 546-goat-anti-mouse IgG (H+L) (#11003), CD80-FITC (#8001) and CD86-PE (#8624) antibodies used for the FACS analysis were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). The ATF4 (#200), c-Jun (#44), phospho-c-Jun (#16312), AKT1/2/3 (#81434), phospho-AKT1/2/3 (#7985R), eIF2α (#133132), phospho-eIF2α (#101670), CHOP (#824), β-actin (#81178), CD44 (#53505), HRP-goat-anti-mouse (#2005) and HRP-goat-anti-rabbit (#sc-2004) antibodies used for western blot analysis were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The MyD88 (#3699) and TRIF (#4596) antibodies used for western blot analysis were purchased from Cell Signaling Technology (Beverly, MA, USA). The red anti-FLAG M2 affinity gel used for immunoprecipitation was purchased from Sigma-Aldrich (St Louis, MO, USA). LY294002, JNK inhibitors II 420119, PD98059 and SB202190 were purchased from Merck and Co (Darmstadt, Germany).
Plasmid construction
The lentivirus system containing three packaging vectors (pCMV-VSVG, pRRE and pRSV-REV) and the expression vectors (pLV-EF1α-IRES-bsd and pLV-H1-EF1α-puro) were obtained from Biosettia Company (San Diego, CA, USA). The ATF4, MyD88 and TRIF silence sites were screened on the Invitrogen web site using block-iT RNAi designer software. Short hairpin RNAs were obtained from Shenggong Company (Shanghai, China) and inserted into the pLv-EF1α-IRES-bsd plasmid. The target sequences of designed shRNAs are as follows: Leti-hATF4 ShRNA1: 5′-GCC TAG GTC TCT TAG ATG A-3′ Leti-hATF4 ShRNA2: 5′-CAG ATT GGA TGT TGG AGA A-3′ Leti-hMyD88 ShRNA1: 5′-GCC TGT CTC TGT TCT TGA A-3′ Leti-hMyD88 ShRNA4: 5′-GCA AGG AAT GTG ACT TCC A-3′ Leti-h-TRIF ShRNA2: 5′-GCC AGG ACA AGC TCT TGT A-3′ and the scramble control is: 5′-GCT ACA CTA TCG AGC AAT T-3′. Flag-tagged full-length ATF4 was cloned into the pLv-EF1α-IRES-bsd vector via insertion of the PCR-amplified fragments using THP-1 cDNA and pCMV-TAG2b (Addgene).
RT-PCR and quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed with PrimeScript RT reagent (TaKaRa, Shiga, Japan). The human genes were amplified using the following primers: hTLR1 (5′-AGT TGT CAG CGA TGT TCG G-3′/5′-GAT CAA GTA CCT TGA TCC TGG G-3′); hTLR2 (5′-GGC TTC TCT GTC TTG TGA CC-3′/5′-GGG CTT GAA CCA GGA AGACG-3′); hTLR3 (5′-AGC CAC CTG AAG TTG ACT CAG G-3′/5′ CAG TCA AAT TCG TGC AGA AGG C-3′); hTLR4 (5′-GCA ACA CCT TCA GAT AAG CAG-3′/5′-AAG ATG ATT ACC AGC ACG ACG ACT G-3′); hTLR5 (5′-TTC TGG AAA TGG CTG GAC AG-3′/5′-TCG GAG ATC CAA GGT CTG TAA T-3′); hTLR6 (5′-CCT GGG AGG TAA ACA TCT GA-3′/5′-CCC TCA ACC ACA TAG AAA CGA-3′); hTLR7 (5′-GAT AAC AAT GTC ACA GCC GTC C-3′/5′-GTT CCT GGA GTT TGT TGA TGT TC-3′); hTLR8 (5′-CAG GGA CTT GCT TTC CAG GT-3′/5′-ACA CGG AAA CCC CTT TGA ATG-3′); hTLR9 (5′-CGC CAA CGC CCT CAA GA-3′/5′-GCA TCC TCA TCT CGC CCA CT-3′); hTLR10 (5′-AAG CCC ACA TTT ACG CCT ATC-3′/5′-CCG CGC TGG CGG AGG AGA TGG AC-3′); hMyD88 (5′-CTC CTC CAC ATC CTC CCT T-3′/5′-CAG TGG GGT CCG CTT GT-3′); hTRIF (5′-GGT ATC AAA CCC GGA ATA ATC-3′/5′-GTT GTG TCT TAT ACA CAG ACT CCT G-3′); and hGAPDH (5′-CTG ATG CCC CCA TGT TCG TC-3′/5′-CAC CCT GTT GCT GTA GCC AAA TTC-3′). SYBR Premix Ex Taq (TaKaRa) was used to amplify cDNA for real-time PCR. The primers used for real-time PCR are as follows: hATF4 (5′-GTC AGT CCC TCC AAC AAC A-3′/5′-GGT GTC TTC CTC CTT TAT GC-3′) and hβ-actin (5′-GGC ATC CAC GAA ACT ACC TT-3′/5′-CTC GTC ATA CTC CTG CTT GC-3′). Data analysis was performed using GelDoc XR (BioRad, Berkeley, CA, USA).
Lentiviral transduction and virus infection
The 293T cell line was transfected using the lentivirus system with Lipofectamine-2000 (Invitrogen) according to the manufacturer's instructions. Forty hours after the transfection, the supernatant was collected and centrifuged to harvest the lentivirus. The virus titers were measured, and the THP-1 cells were incubated with lentivirus (virus titer=107/ml; MOI=20) in a centrifuge at 1000g for 1 h at 37 °C. Then, complete medium was added to the THP-1 cells, which were incubated for an additional 36 h to allow for the expression of the target genes.
Flow cytometry
The cells were fixed with 4% fixation/penetration buffer (BD Bioscience, Franklin Lakes, NJ, USA) for 15 min at 25 °C and blocked with blocking buffer (phosphate-buffered saline with 1% bovine serum albumin) for 30 min on ice. The cells were incubated with either anti-ATF4 antibody or anti-TLR4-PE antibody for 30 min on ice, washed three times with permeabilization buffer (BD Bioscience) and then incubated with Alexa Fluor 488 goat anti-rabbit IgG (H+L) antibody for 30 min. Next, the cells were washed again and resuspended in 500 µl of phosphate-buffered saline, and the BD FACSCalibur and software CellQuest Pro were used to perform the FACS analysis.
Western blot and immunoprecipitation
Total cell protein was prepared by lysing the cells with radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin and 1 mM PMSF), and the protein concentration was determined using the bicinchoninic acid (BCA) protein assay reagent (Pierce, Rockford, IL, USA). Cytoplasmic protein and nucleoprotein were obtained using a Nuclear Cytosol Extraction Kit (KeyGEN BioTECH, Nanjing, China). For western blot analysis, 30 µg of each sample was fractionated via 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane. The membrane was visualized using ECL solution (Millipore, Billerica, MA, USA) after blocked with 5% bovine serum albumin and probed with primary and secondary antibodies.
Immunoprecipitation was performed as described in the red anti-FLAG affinity gel technical bulletin (#F2426; Sigma-Aldrich, Steinheim, Germany) with slight modifications. Briefly, THP-1 cells plated in 75-mm2 flasks were transfected with either the FLAG-ATF4 or control vectors. Forty-eight hours later, the cells were starved overnight, stimulated with 100 ng/ml lipopolysaccharide (LPS) for 30 min and lysed in 800 µl of cell lysis buffer (50 mM Tris HCl (pH 7.4), 150 mM NaCl, 1% EDTA, 1% NP-40, 100 mM NaF and 100 mM Na3VO4). After centrifugation at 13 800g for 15 min at 4 °C and quantification with a BCA protein assay kit (Thermo, Rockford, IL, USA), 500 µg of whole cell lysate was incubated with 30 µl of anti-FLAG M2 affinity gel at 4 °C overnight with gentle rotation. The beads were washed four times with cell lysis buffer, and the precipitates were eluted with 2× sodium dodecyl sulfate polyacrylamide gel electrophoresis sample buffer and analyzed via western blot using the anti-ATF4 and anti-p-c-Jun antibodies as described above. To monitor the protein expression levels, 30 µg of total cell lysate was loaded as a positive control.
Immunofluorescence
THP-1 cells were treated with 100 ng/ml LPS for 5, 15, 30 and 60 min and immediately centrifuged in 24-well plates, which had a coverslip in each well. The cells were fixed with 4% paraformaldehyde for 15 min at 25 °C and penetrated with 0.5% Triton X-100 for 15 min at 25 °C. Next, the cells were stained with fluorochrome-labeled antibodies and DAPI (Sigma-Aldrich, Steinheim, Germany). Images were acquired using a laser scanning confocal microscope (#TCS SP5; Leica, Heidelberg, Germany).
ELISA and human cytokine array
After treatment of the THP-1 cells with LPS (100 ng/ml) for 16 h, the supernatant was harvested. The concentration of IL-6, IL-8 and tumor-necrosis factor-α (TNF-α) in the cell supernatant was assessed on ELISA plates (#D8000C, #D6050 and #DTA00C, respectively; R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. A human cytokine array was performed using the Human Cytokine Array Panel A (#ARY005; R&D Systems). For this experiment, the cell density was 1×106 cells/ml. ATF4 knockdown cells and normal cells were treated for 16 h with 100 ng/ml LPS (Sigma-Aldrich, Steinheim, Germany). The supernatant derived from the cell culture was collected, and 500 µl of medium was used for each array.
Statistical analysis
The values are expressed as the mean±s.d. Statistical analysis was performed using the Student's t-test to compare the data for the experimental and control or vehicle-treated groups. The differences were considered significant at P<0.05.
Results
ATF4 is activated via the TLR4 signaling pathway
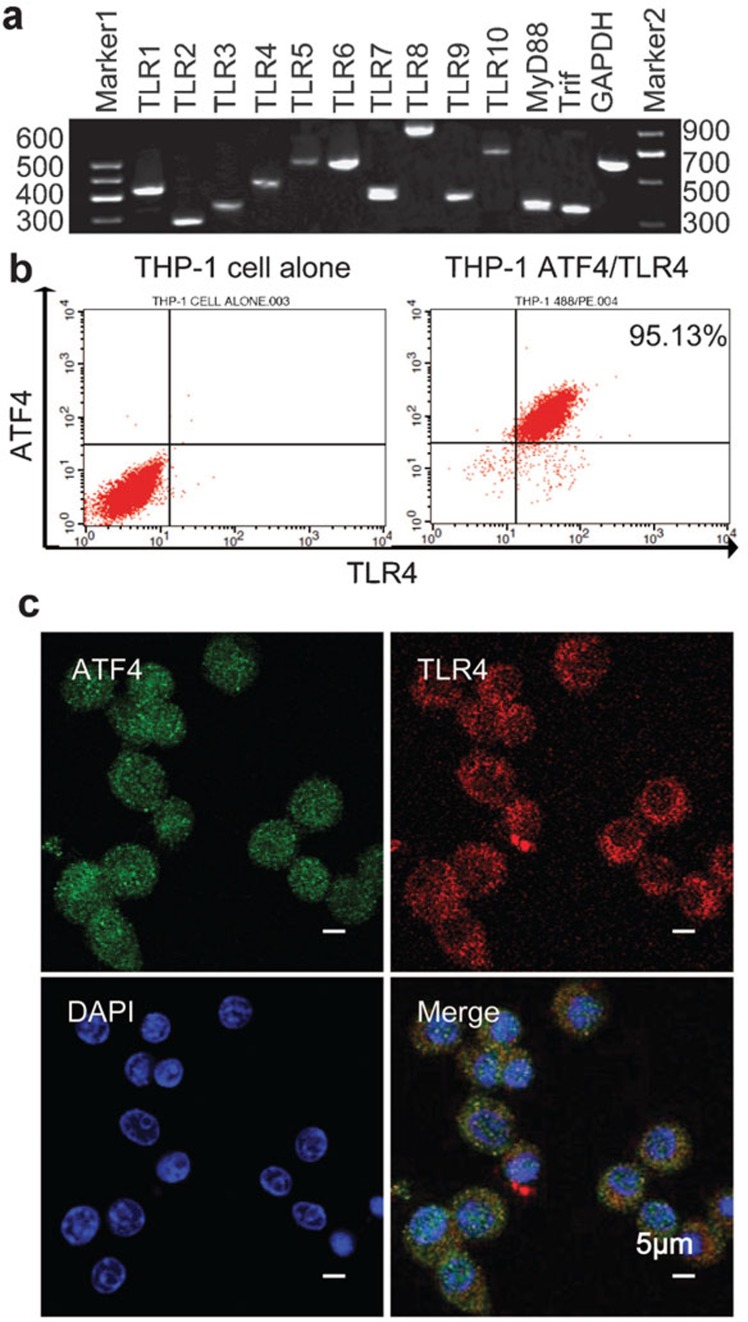
Previous work has demonstrated that THP-1, a human acute monocytic leukemia cell line, is sensitive to microbial recognition and secretes a mass of cytokines following activation.11 We found that TLR1–10, MyD88 and TRIF are transcriptionally expressed in THP-1 cells (Figure 1a), and we discovered via FACS analysis that almost all the cells simultaneously express ATF4 and TLR4 (Figure 1b). As shown in Figure 1c, TLR4 is localized to the plasma membrane, while ATF4 is uniformly distributed throughout the entire cell.
Figure 1.
The ATF4 and TLR family are expressed in the THP-1 cell line. (a) TLR family member mRNA expression levels were assessed in the THP-1 cell line via RT-PCR. (b) Flow cytometry analysis was performed to assess the expression levels of TLR4 and ATF4 in THP-1 cells. The left panel is a negative control, and the right panel shows the analysis of the THP-1 cells. (c) Confocal microscopy analysis of the location of TLR4 and ATF4 in THP-1 cells. The cells were immunostained with ATF4 antibody, which was labeled with Alexa 488 (green) and PE-TLR4 (red) antibodies. Cell nuclei were counterstained with DAPI (blue). The scale bar is equal to 5 µm. ATF4, activating transcription factor 4; DAPI, 4′,6-diamidino-2-phenylindole; TLR, Toll-like receptor.
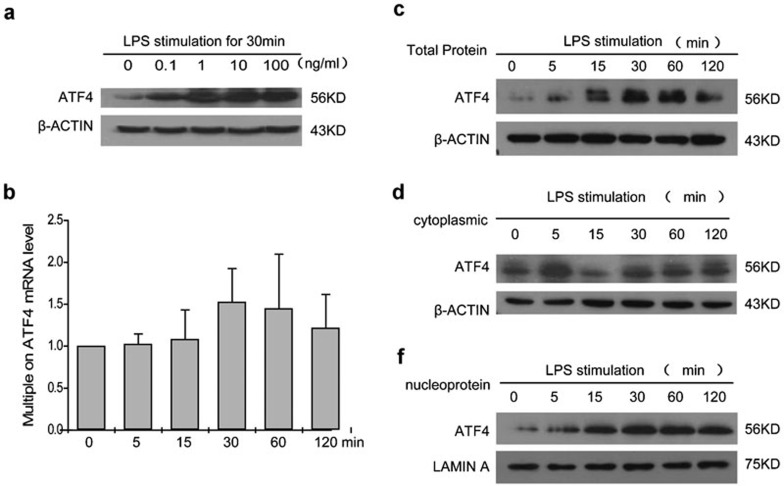
To further investigate whether the TLR signaling pathway activates ATF4, we treated THP-1 cells with a low-dose series of LPS, the specific ligand of TLR4. The results of the western blot analysis indicated that ATF4 levels increased at the translational level after stimulation, especially at 100 ng/ml LPS (Figure 2a), which is consistent with the observed pathophysiological stimulation in chronic inflammatory diseases and has been adopted as a surrogate stimulator in these conditions by many studies.12,13
Figure 2.
LPS stimulation activates ATF4 in THP-1 cells. (a) The expression of ATF4 following LPS stimulation at the indicated concentrations. (b) Real-time PCR assay of ATF4 mRNA expression within 2 h following treatment with 100 ng/ml LPS. The data are presented as the mean±s.d. from three experiments. (c–e) Western blot analysis of the expression levels of total ATF4 (c), cytoplasmic ATF4 (d) and nuclear ATF4 protein (e) in THP-1 cells after stimulation with 100 ng/ml LPS for 0, 5, 15, 30, 60 and 120 min. Similar results were obtained in three separate experiments. ATF4, activating transcription factor 4; LPS, lipopolysaccharide.
Activated ATF4 forms homogenic and heterogenic dimers and translocates into the cell nucleus for further regulation.6,7 To investigate the dynamics of LPS-induced ATF4 translocation, the expression levels of ATF4 in the cytoplasm and nucleus were assessed via western blot and compared with the total protein levels. The results displayed in Figure 2c–e show that both total ATF4 and nuclear ATF4 levels increased at 1 h after LPS stimulation. Quantitative real-time PCR analysis showed that there was no significant alteration in mRNA levels (Figure 2b). These findings suggest that both LPS induces ATF4 translational activity and ATF4 is involved in the TLR signaling pathway.
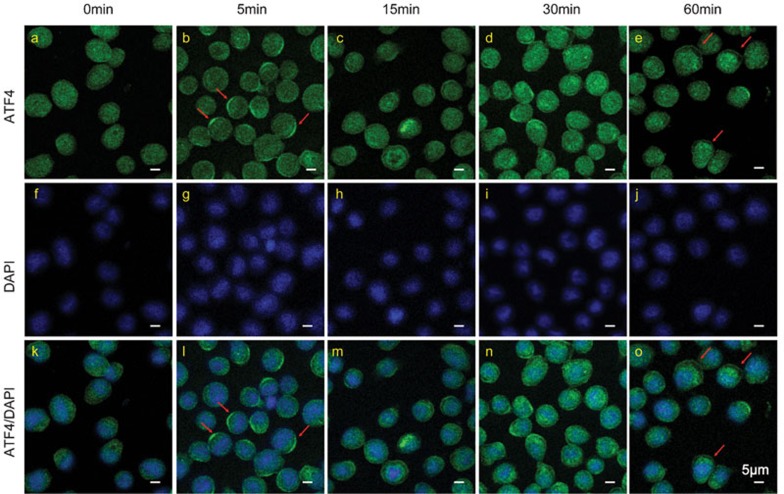
To further assess the translocation activity of ATF4, we imaged the translocation of ATF4 upon LPS stimulation at 5, 15, 30 and 60 min via confocal microscopy. ATF4 fluorescence had a diffused cellular distribution in both the cytoplasm and nucleus before stimulation (Figure 3a and k). However, as shown in Figure 3b and i, ATF4 translocated to the cytomembrane and formed a crescent-like structure at 5 min. At 15 min, ATF4 protein gradually moved into the cell nucleus. At the 60-min time point, ATF4 was completely localized at the nucleus. These experiments were repeated using the U937 cell line, a human leukemic monocyte lymphoma cell line, which yielded the same results (Supplementary Figure 1a–c). CD44 antigen, a cell-surface glycoprotein expressed in both THP-1 and U937 cells, was used to label the cellular membrane. These data strongly demonstrate that ATF4 is directly activated by TLR signaling, which functions not only by increasing the total protein levels but also by inducing nuclear translocation.14
Figure 3.
ATF4 translocates to cell nucleus after activation. THP-1 cells were stimulated with 100 ng/ml LPS for 0, 5, 15, 30 and 60 min and then collected for immunofluorescence analysis. The cells were immunostained with ATF4 antibody labeled by Alexa 488 (green). The cell nuclei were counterstained with DAPI (blue). At the 5 min time point, ATF4 translocated to the cytoplasmic membrane, which is indicated by red arrows. At 15 min, ATF4 began to move into the cell nucleus. At 60 min, ATF4 localized to the nucleus completely. The scale bar is equal to 5 µm. Similar results were obtained in three separate experiments. ATF4, activating transcription factor 4; DAPI, 4′,6-diamidino-2-phenylindole; LPS, lipopolysaccharide.
ATF4 positively regulates TLR4-trigged cytokine production
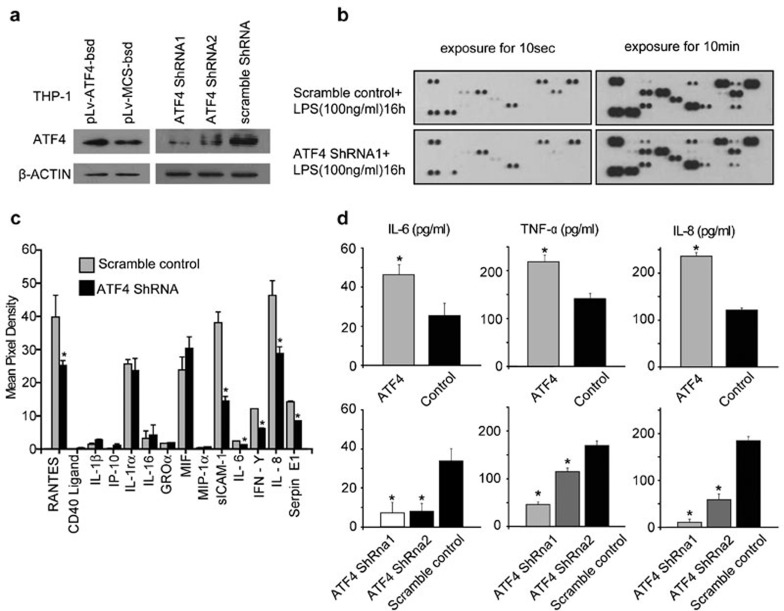
After stimulated with TLR ligands, monocytes secrete high levels of cytokines, such as IL-1β, IL-6, IL-8 and IFN-γ. To illuminate the function of ATF4 in TLR-triggered cytokine production, we infected THP-1 cells with lentiviruses expressing shRNA targeting ATF4 (Figure 4a). The infection rate of the THP-1 cells exceeded 80% (Supplementary Figure 2a) and seldom induced the MHC II phenotype, as observed with CD80 and CD86 (Supplementary Figure 2b). After the virus-infected cells were treated with 100 ng/ml LPS for 16 h, we compared the variations in cytokine secretion in the cell culture supernatants between the ATF4-deficient and wild-type cells using a human cytokine array assay. As shown in Figure 4b, the round spots represent different cytokines, which are thinner in the ATF4 knockdown group compared with the wild-type control. Based on the statistical analysis (Figure 4c), we found that following ATF4 knockdown, the cytokines, such as RANTES/CCL5 (chemokine ligand 5), sICAM-1 (Soluble Inter-cellular Adhesion Molecule-1), IL-6, IL-8, IFN-γ and Serpin E1, were reduced by approximately 36.67%, 62.02%, 43.58%, 49.22%, 37.79% and 40.44%, respectively. The concentrations and mRNA levels of IL-6, IL-8 and TNF-α in the different groups were further assessed via ELISA and quantitative real-time PCR (Supplementary Figure 3), respectively, and the results were consistent with the above findings (Figure 4d and Supplementary Figure 4). Taken together, these results indicate that ATF4 positively regulates TLR4-trigged cytokine production.
Figure 4.
ATF4 positively regulates TLR-trigged cytokine production. (a) ATF4 was either overexpressed or knocked down in THP-1 cells via lentivirus transfection for 48 h. The cells were then treated with LPS for 30 min, and total cellular protein was collected for western blot analysis. (b) The human cytokine array assay detects multiple analytes in cell culture supernatants. ATF4 knockdown and normal cells were treated with 100 ng/ml LPS for 16 h. Similar results were obtained in three separate experiments. (c) ImageJ analysis of the cytokine array. The data are presented as the mean±s.d. from three experiments, and the asterisks indicate statistically significant (P<0.05) differences between the mean values obtained with the ATF4 knockdown and scramble control experiments. (d) ELISA analysis revealed the concentrations of IL-6, IL-8 and TNF-α in the supernatant derived from the ATF4 overexpression and ATF4 knockdown THP-1 cells. The data shown represent the mean±s.d. of triplicate samples and are representative of three independent experiments performed. The asterisks indicate statistically significant (P<0.05) differences between the mean values obtained with the ATF4 knockdown and scramble control or the ATF4 overexpression and scramble control. ATF4, activating transcription factor 4; LPS, lipopolysaccharide; TLR, Toll-like receptor; TNF, tumor-necrosis factor.
TLR4 activates ATF4 via the MyD88-dependent pathway
Two main downstream branches of TLR signaling have been described, and TLR4 is the only receptor known to activate both the MyD88-dependent and the TRIF-dependent pathways, which mediate the production of proinflammatory cytokines and type 1 IFN, respectively.15,16 Currently, two reports have described the pathway regulation of ATF4 activation, although there is no consensus between them. Woo et al.17 found that the expression of CHOP and its activator, ATF4, which are induced by ER stress, was suppressed via prior engagement with TLR3 or TLR4 via a TRIF-dependent pathway. Bandow et al.18 reported that during osteoblast differentiation, LPS decreased the mRNA expression levels of ATF4 in wild-type cells but not in MyD88−/− osteoblasts. However, the cytokine array results suggest that ATF4 knockdown primarily interferes with the secretion of cytokines regulated by the MyD88-dependent pathway. Therefore, we designed and constructed four shRNAs targeting MyD88 and TRIF. After MyD88 or TRIF expression was silenced in THP-1 cells with the most effective shRNAs (MyD88 ShRNA4-1 and TRIF ShRNA2-3, as shown in the Supplementary Figure 5a and b), the activation of ATF4 was assessed after LPS stimulation. Figure 5a–d shows in MyD88 knockdown cells but not in TRIF knockdown cells, that the levels of ATF4 increased in the cytoplasm after LPS stimulation, while ATF4 was restrained to the cytoplasm and unable to translocate to the cell nucleus, which demonstrates that MyD88 plays an important role in the translocation of ATF4.
Figure 5.
TLR4 induces ATF4 via the MyD88-dependent pathway. (a–d) The knockdown of MyD88 (a, b) and TRIF (c, d) in THP-1 cells using lentivirus for 48 h, and the detection of the MyD88, TRIF and ATF4 expression levels in the cytoplasm and cell nucleus via western blot. (e) Confocal microscopy displays the expression and location of ATF4 in MyD88 knockdown and TRIF knockdown cells. The cells were immunostained with ATF4 antibody labeled with Alexa 488 (green) and cell nuclei were counterstained with DAPI (blue). ATF4 was blocked in the cytoplasm (red arrows in m, s) in MyD88 knockdown cells, while it transferred to nucleus (red arrows in p, v) in TRIF knockdown cells. The scale bar is equal to 5 µm. Similar results were obtained in three separate experiments. ATF4, activating transcription factor 4; DAPI, 4′,6-diamidino-2-phenylindole; TLR, Toll-like receptor; TRIF, TIR domain-containing adaptor-inducing interferon β.
To further confirm the above results, we imaged the translocation of ATF4 in the two knockdown groups and the scramble group using a confocal microscope. As shown in Figure 5e, during the first 5 min, ATF4 was recruited to the cytoplasm in both the MyD88-deficient and TRIF-deficient cells. However, the status then changed in the TRIF knockdown cells; ATF4 began to transfer to the cell nucleus at 15 min, which is consistent with the scramble control (data not shown), while in the MyD88 knockdown cells, ATF4 translocation to cell nucleus was hampered. Taken together, these results strongly suggest that both TLR4 induces ATF4 activation via the MyD88-dependent pathway and MyD88 is required for ATF4 to translocate to the cell nucleus where ATF4 further regulates the secretion of cytokines (Supplementary Figure 6a and b).
JNK is involved in the activation of ATF4 via TLR4 signaling
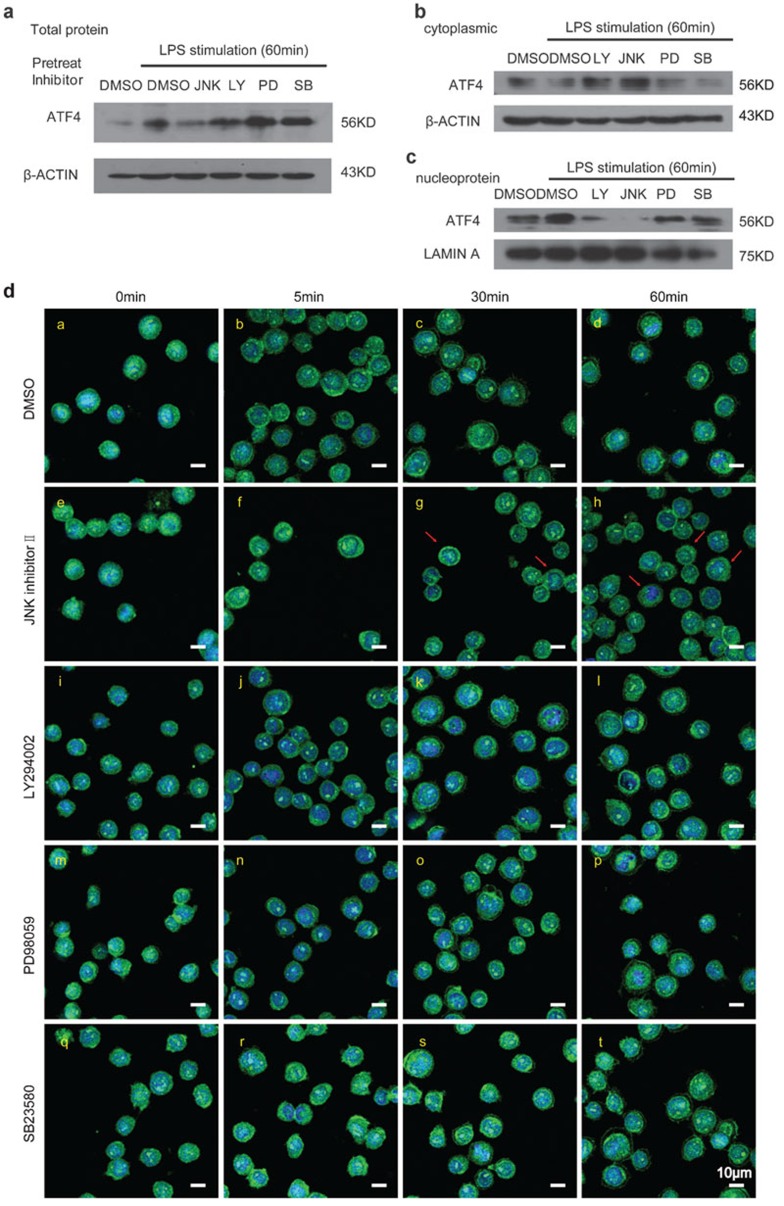
In the MyD88-dependent signaling pathway, MyD88 binds to TLRs via Toll/IL-1 receptor (TIR) domains. Upon stimulation, IRAK-4, IRAK-1 and TRAF6 are recruited to MyD88 and then activate the TAK1/TAB1/2 complex.19 Activated TAK1 phosphorylates the IKK complex (IKKα, IKKβ, IKKγ and NIK-IKKα) and then induces the activation of NF-κB.20 Activated TAK1 also phosphorylates MAPKs, including p38, ERK1/2 and JNK, to induce the activation of AP-1.21,22 ERK1/2 primarily regulates cell differentiation and proliferation, while p38 and JNK play an important role in inflammation, the stress response and cell survival/apoptosis. To determine whether MAPKs have an effect on the activation of ATF4 upon LPS stimulation, we performed experiments using four kinase inhibitors. THP-1 cells were pretreated with 40 nM JNK inhibitor II (JNK 420119), 10 µM PI3K inhibitor (LY294002), 20 µM MEK/MAPKK inhibitor (PD98059), 10 µM p38 MAP kinase inhibitor (SB203580) and dimethyl sulfoxide (DMSO) (as a negative control) for 30 min and subsequently treated with LPS for 60 min. The ATF4 protein levels were assessed via western blot. The results displayed in Figure 6a–c show that both JNK 420119 and LY294002 (especially the JNK inhibitor) blocked the accrescence of ATF4 for the total protein (Figure 6a) and the nucleoprotein (Figure 6c) but had no effect on cytoplasmic protein (Figure 6b). To ascertain whether toxicity caused by the drugs influenced the results, the WST-1 assay was used to test cell viability. The result revealed that DMSO and the inhibitors had, on average, little or no effect on the cells within 2 h (Supplementary Figure 7a).
Figure 6.
The effects of the JNK, PI3K, MEK/MAPKK and p38 MAP kinase inhibitors on ATF4 activation in THP-1 cells. (a–c) THP-1 cells were treated with DMSO, 40 nM JNK inhibitor II, 10 µM PI3K inhibitor (LY294002, LY), 20 µM MEK/MAPKK inhibitor (PD98059, PD) and 10 µM p38 MAP kinase inhibitor (SB203580, SB). After a 30-min incubation, the cells were stimulated with 100 ng/ml LPS for 60 min and then total protein (a), cytoplasmic protein (b) and nucleoprotein (c) was collected for western blot analysis. (d) THP-1 cells were cultured in 24-well plates and treated with DMSO, JNK inhibitor II, LY294002, PD98059 and SB203580 at the same concentration as described above. After a 30-min incubation, the cells were stimulated with 100 ng/ml LPS for 0, 5, 30 and 60 min and then harvested for immunofluorescence analysis. The cells were immunostained with ATF4 antibody labeled with Alexa 488 (green). The cell nuclei were counterstained with DAPI (blue). The red arrows indicate the block of ATF4 in the cytoplasm (as shown in g and h). The scale bar is equal to 10 µm. Similar results were obtained in three separate experiments. ATF4, activating transcription factor 4; DAPI, 4′,6-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide; LPS, lipopolysaccharide.
We also imaged the translocation of ATF4 in the cells and obtained the same results. As shown in Figure 6d, upon pre-treatment with JNK 420119 and LY294002, ATF4 was blocked in the cytoplasm and was unable to transfer to the cell nucleus, in contrast to the results observed for the DMSO control. However, the effect on ATF4 in response to PD98059 and SB203580 was similar to that of the DMSO control group. Therefore, in the TLR4-triggered MyD88-dependent pathway, JNK facilitates ATF4 activation by inducing its translocation into the cell nucleus. Therefore, JNK may be the key mediator in the interaction between TLR4 and ATF4.
ATF4 interacts with c-Jun and promotes gene transcription
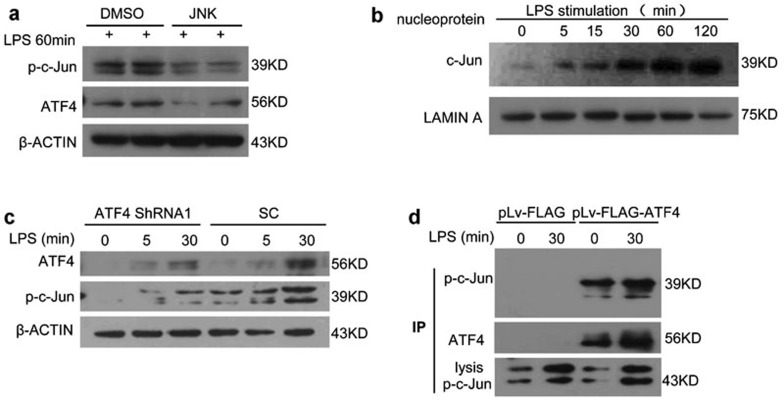
MAPK activity induces the activation of AP-1, which is a family of transcription factors (c-Jun and c-Fos) containing a leucine zipper domain.23 ATF4 forms heterodimers with c-Jun and c-Fos via its leucine zipper domain.24 Currently, JNK is the only kinase known to phosphorylate and activate c-Jun (Figure 7a).25,26 Therefore, we hypothesized that upon LPS stimulation in our experimental model, phosphorylated c-Jun forms a dimer with activated ATF4 and then translocates into the cell nucleus to bind to the target genes and regulate their transcription. Therefore, we examined p-c-Jun and p-AKT at 0, 5, 15, 30, 60 and 120 min after LPS stimulation and found that p-c-Jun levels but not p-AKT levels were increased at 120 min (Figure 7b and Supplementary Figure 8a and b), similar to the increase in ATF4 in the cell nucleus (Figure 2c). Furthermore, the expression of c-Jun in the nucleus decreased following ATF4 knockdown (Figure 7c). To further investigate the mechanism underlying the interaction between ATF4 and c-Jun, we performed an immunoprecipitation assay. A Flag tag was linked to ATF4, and the fusion protein was precipitated using a Flag antibody. Figure 7d shows that c-Jun binds to ATF4 and may then promote the gene transcription activity of ATF4 via this interaction.
Figure 7.
ATF4 interacts with c-Jun. (a) JNK inhibitor II repressed the phosphorylation of c-Jun and the activation of ATF4. (b) The expression of phosphorylated c-Jun increased following LPS stimulation for 0, 5, 15, 30, 60 and 120 min. (c) The effects of ATF4 knockdown on the activation of phosphor-c-Jun. (d) THP-1 cells were transfected with FLAG-tagged ATF4 vector and FLAG vector for 48 h and then treated with LPS for 30 min. The transfected cell lysates were immunoprecipitated with anti-Flag M2 affinity beads. The immunoprecipitation complexes and whole-cell lysates were analyzed via western blot with the antibodies as indicated. C-Jun expression was analyzed in whole cell lysates as a positive control. Similar results were obtained in three separate experiments. ATF4, activating transcription factor 4; LPS, lipopolysaccharide.
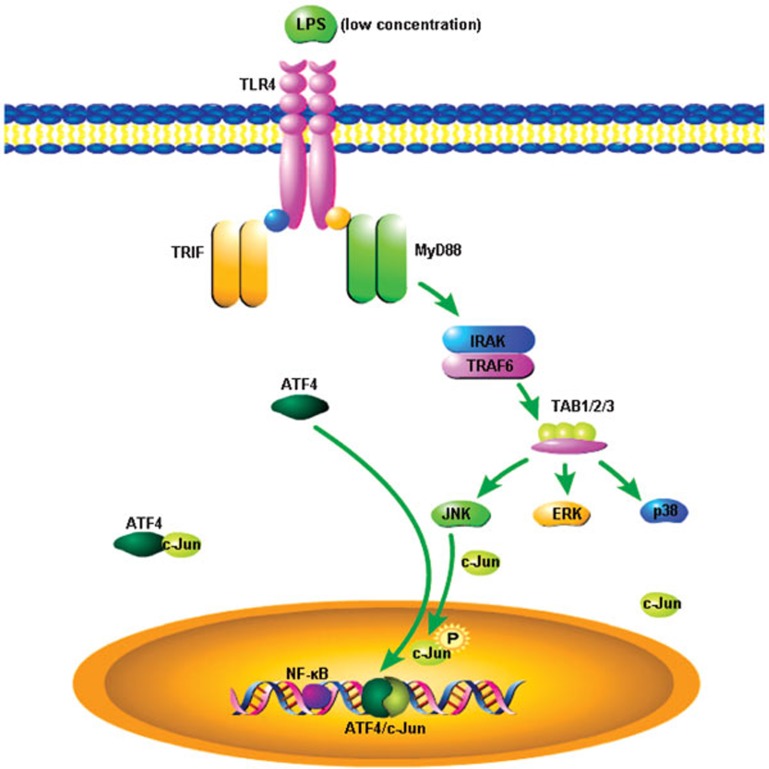
Consequently, these data prompted us to propose the following molecular mechanism (Figure 8). In the TLR4 signaling pathway, upon LPS stimulation, MyD88 is recruited to TLR4 via its TIR domain, and then MyD88 recruits IRAK and TRAF6, which activate the TAK1 and TAB1/2/3 complex. The activated complex phosphorylates JNK and then phosphorylates c-Jun, which forms heterodimers with ATF4 via the leucine zipper. This dimer binds to DNA and promotes the transcription of certain genes, thereby resulting in the secretion of relevant inflammatory cytokines.
Figure 8.
The summary of the molecular mechanism of ATF4 involvement in TLR4-triggered cytokine production as described in the text. ATF4, activating transcription factor 4; TLR, Toll-like receptor.
Discussion
The innate immune response is the first defense system of the immune system following activation by microbial pathogens. The types of secreted cytokines and activated innate immune cells determine the type and strength of the adaptive immunity response.27 TLR signaling represents one of the key mechanisms involved in these processes.28 Although ATF3, an ATF4 homologue, has been reported to participate in the TLR4 signaling pathway8,29 and recent reports have indicated that ATF4 is capable of regulating cytokine secretion,9 it remains unclear whether ATF4 plays a role in TLR signaling. In this study, we defined a novel mechanism in which ATF4 is recruited by TLR4 signaling via the MyD88-dependent pathway, translocates to the nucleus and stimulates TLR-triggered cytokine production to further promote an inflammatory reaction.
It has been established that ATF4 is primarily induced via metabolic stress (such as glucose and amino acid deprivation), oxidative stress and ER stress. A growing body of evidence has suggested that ATF4 may also interact with the TLR signaling pathway. Shimasaki et al.30 have shown that ER stress increases TLR2 expression levels in human epithelial cells and ER stress enhances TLR2-dependent proinflammatory cytokine production. Woo et al.17 have demonstrated that TLR signaling is a negative regulator of the ATF4-CHOP pathway. In this study, we provided evidence that ATF4 is directly induced by TLR4 signaling instead of via ER stress (Supplementary Figure 9c and 10). Many viruses, such as human cytomegalovirus,31 herpesvirus,32 HCV33 and HBV,34 have also been shown to stimulate ATF4 production via the ER stress response. The lentivirus infection system that we used in the experiment is only sustained for 1 h, and a scramble control was included, which have been used in many other studies.35,36
ATF4 acts as a transcription activator and inhibitor.35,37,38,39,40 The results of our cytokine array assay revealed that ATF4 positively regulated the secretion of RANTES, sICAM-1, IL-6, IL-8 and IFN-γ, all of which are proinflammatory cytokines. RANTES, a representative chemotactic factor, mediates T cell differentiation and cytokine release, and it is also a chemoattractant for blood monocytes, memory T-helper cells, mast leukocyte and eosinophils.41 sICAM-1 mediates adhesion between leukocytes and endotheliocytes and participates in inflammation and immunity processes.42 IL-8, IL-6, IFN-γ and TNF-α are critical cytokines that have been implicated in a variety of inflammatory conditions.43,44 In the present study, we used THP-1 cells, a human monocyte–macrophage cell line, as monocytes and macrophages have been shown to be critically involved in the pathogenesis of inflammatory diseases. Monocytes migrate from the bloodstream to inflamed tissues, including the joint and bowel, and differentiate into macrophages, thereby developing into the primary source of proinflammatory cytokines.43,45 Our data demonstrate that ATF4 may promote inflammation by upregulating these pro-inflammatory cytokines and chemokines after activation.
According to current studies, ATF4 activity is primarily regulated at the post-transcriptionally level, especially the translation of ATF4 mRNA.46,47 ATF4 protein degrades quickly, and its half-life is only 30–60 min.48,49 The confocal microscopy images showed that as early as 5 min after LPS stimulation, ATF4 translocated to the cytoplasmic membrane, and 15 min after stimulation, we observed the nuclear translocation of ATF4. Moreover, we treated the cells with 1 µg/ml tunicamycin and 100 ng/ml LPS for 12 h and detected the expression of phosphorylated eIF2a and CHOP. Supplementary Figure 9c shows that treatment with 100 ng/ml LPS fails to induce ER stress and significant phosphorylation of eIF2a. Thus, the increase in ATF4 levels after LPS stimulation may partly be due to the enhanced ATF4 stability.
The TLR signaling pathway consists of the MyD88-dependent pathway, which is common to most TLRs, and the TRIF-dependent pathway that distinctly involves the TLR3 and TLR4 signaling pathways.2 Studies regarding the ATF4 subpathway are very limited, and the opinions are contradictory. Woo et al.17 have found that the expression of ER stress-induced CHOP and ATF4 was suppressed by prior engagement with TLR3 or TLR4 via the TRIF-dependent pathway. Nonetheless, he also claimed that low-dose LPS (1–100 ng/ml) treatment prevents cellular ER stress.17 Endo et al.50 have used LPS (160 mg/kg of body weight) in a mouse model, and Gotoh et al.51 have used LPS (150 µg/ml) plus IFN-γ (100 units/ml) to induce ER stress. In our study, we found that low-dose LPS treatment activated ATF4. Our results reveal that ATF4 positively regulates TLR-triggered cytokine production via the MyD88-dependent subpathway. In MyD88 knockdown cells, ATF4 was blocked in the cytoplasm following LPS stimulation and failed to translocate to the cell nucleus to exert the transcription-promoting function, while TRIF knockdown had minor suppressive effects on ATF4 function.
ATF4 is thought to form heterodimers with the C/EBPs and AP-1 transcription factors.52,53 In the present study, we investigated c-Jun as the possible downstream mediator of ATF4 in the TLR4-trigged signaling pathway, which was prompted by previous reports showing that heterodimerization of ATF4 and c-Jun might occur.36,54 The study by An et al.26 has indicated that there is an AP-1 response element within the IL-6 promoter. The RANTES/CCL5 promoter displays binding activity with AP-1, C/EBP and NF-κB,55 while the IL-8 promoter contains NF-κB, AP-1, OCT-1 and C/EBP-binding sites.56,57,58 The studies by Hadad et al.59 and Clarke et al.60 have revealed that there is a CREB-binding site within the sICAM-1 and IFN-γ promoter. Promoter analysis by Yeligar et al.61 has revealed that c-Jun, c-Jun/c-Fos and JunD, but not JunB, bind to the AP-1 site of the promoters. The results of our IP experiment indicated that upon LPS stimulation, ATF4 forms dimers with c-Jun via the AP-1 and CREB binding sites, which may promote the secretion of various proinflammatory cytokines.
Taken together, the data reveal a novel mechanism in which ATF4 is both involved in an inflammatory reaction trigged by the TLR4 signaling pathway and positively regulates the production of some cytokines via interaction with c-Jun. Whether ATF4 represents a target for the regulation of cytokine secretion to further control inflammatory diseases will be addressed in future studies.
Acknowledgments
We thank Dr Feifei Liu and Xiaoyue Tan for kindly reading the manuscript and providing valuable advice. This work was supported by Tianjin Municipal Science and Technology Commission, Grant No. 08ZCGYSH04700.
Supplementary Information
References
- Belvin MP, Anderson KV. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Horng T, Medzhitov R. Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc Natl Acad Sci USA. 2001;98:12654–12658. doi: 10.1073/pnas.231471798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res Ther. 2005;7:12–19. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. All roads lead to ATF4. Dev Cell. 2003;4:442–444. doi: 10.1016/s1534-5807(03)00100-x. [DOI] [PubMed] [Google Scholar]
- Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40:14–21. doi: 10.1016/j.biocel.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441:173–178. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- Gargalovic PS, Imura M, Zhang B, Gharavi NM, Clark MJ, Pagnon J, et al. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc Natl Acad Sci USA. 2006;103:12741–12746. doi: 10.1073/pnas.0605457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Yadav R, Misra R, Naik S. In vitro effect of gold sodium thiomalate and methotrexate on tumor necrosis factor production in normal healthy individuals and patients with rheumatoid arthritis. Int J Immunopharmacol. 1997;19:111–114. doi: 10.1016/s0192-0561(97)00014-3. [DOI] [PubMed] [Google Scholar]
- Dabelic S, Novak R, Goreta SS, Dumic J. Galectin-3 expression in response to LPS, immunomodulatory drugs and exogenously added galectin-3 in monocyte-like THP-1 cells. In Vitro Cell Dev Biol Anim. 2012;48:518–527. doi: 10.1007/s11626-012-9540-x. [DOI] [PubMed] [Google Scholar]
- Takahata Y, Hinoi E, Takarada T, Nakamura Y, Ogawa S, Yoneda Y. Positive regulation by GABAB receptor subunit-1 of chondrogenesis through acceleration of nuclear translocation of activating transcription factor-4. J Biol Chem. 2012;287:33293–33303. doi: 10.1074/jbc.M112.344051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11:1473–1480. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandow K, Maeda A, Kakimoto K, Kusuyama J, Shamoto M, Ohnishi T, et al. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem Biophys Res Commun. 2010;402:755–761. doi: 10.1016/j.bbrc.2010.10.103. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Chun J, Choi RJ, Khan S, Lee DS, Kim YC, Nam YJ, et al. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-kappaB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol. 2012;14:375–383. doi: 10.1016/j.intimp.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Nie YC, Wu H, Li PB, Xie LM, Luo YL, Shen JG, et al. Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IkappaB-NF-kappaB signaling pathways. Eur J Pharmacol. 2012;690:207–213. doi: 10.1016/j.ejphar.2012.06.040. [DOI] [PubMed] [Google Scholar]
- Vesely PW, Staber PB, Hoefler G, Kenner L. Translational regulation mechanisms of AP-1 proteins. Mutat Res. 2009;682:7–12. doi: 10.1016/j.mrrev.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Clarke M, Pentz R, Bobyn J, Hayley S. Stressor-like effects of c-Jun N-terminal kinase (JNK) inhibition. PLoS One. 2012;7:e44073. doi: 10.1371/journal.pone.0044073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Sun Y, Rettig MB. Transcriptional coactivation of c-Jun by the KSHV-encoded LANA. Blood. 2004;103:222–228. doi: 10.1182/blood-2003-05-1538. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Sata T. Perioperative innate immunity and its modulation. J UOEH. 2011;33:123–137. doi: 10.7888/juoeh.33.123. [DOI] [PubMed] [Google Scholar]
- Manavalan B, Basith S, Choi S. Similar structures but different roles—an updated perspective on TLR structures. Front Physiol. 2011;2:41. doi: 10.3389/fphys.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg F, Haseeb A, Ahnfelt M, Ponten F, Westermark B, El-Obeid A. Herbal melanin activates TLR4/NF-kappaB signaling pathway. Phytomedicine. 2009;16:477–484. doi: 10.1016/j.phymed.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Koga T, Shuto T, Suico MA, Sato T, Watanabe K, et al. Endoplasmic reticulum stress increases the expression and function of toll-like receptor-2 in epithelial cells. Biochem Biophys Res Commun. 2010;402:235–240. doi: 10.1016/j.bbrc.2010.09.132. [DOI] [PubMed] [Google Scholar]
- Xuan B, Qian Z, Torigoi E, Yu D. Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J Virol. 2009;83:3463–3474. doi: 10.1128/JVI.02307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli E, Benedetti S, Grigolato J, Caruso A, di Luca D. Activating transcription factor 4 (ATF4) is upregulated by human herpesvirus 8 infection, increases virus replication and promotes proangiogenic properties. Arch Virol. 2012;157:63–74. doi: 10.1007/s00705-011-1144-3. [DOI] [PubMed] [Google Scholar]
- Merquiol E, Uzi D, Mueller T, Goldenberg D, Nahmias Y, Xavier RJ, et al. HCV causes chronic endoplasmic reticulum stress leading to adaptation and interference with the unfolded protein response. PLoS One. 2011;6:e24660. doi: 10.1371/journal.pone.0024660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HK, Cheong KJ, Kim HY, Cheong J. Endoplasmic reticulum stress induced by hepatitis B virus X protein enhances cyclo-oxygenase 2 expression via activating transcription factor 4. Biochem J. 2011;435:431–439. doi: 10.1042/BJ20102071. [DOI] [PubMed] [Google Scholar]
- Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, Townes TM, et al. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279:14844–14852. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- Fung H, Liu P, Demple B. ATF4-dependent oxidative induction of the DNA repair enzyme Ape1 counteracts arsenite cytotoxicity and suppresses arsenite-mediated mutagenesis. Mol Cell Biol. 2007;27:8834–8847. doi: 10.1128/MCB.00974-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, et al. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119:2807–2817. doi: 10.1172/JCI39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski BA, Morle GD, Huggenvik J, Uhler MD, Leiden JM. Molecular cloning of human CREB-2: an ATF/CREB transcription factor that can negatively regulate transcription from the cAMP response element. Proc Natl Acad Sci USA. 1992;89:4820–4824. doi: 10.1073/pnas.89.11.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A, Angelosanto JM, Nadwodny KL, Blackburn SD, Wherry EJ. A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection. PLoS Pathog. 2011;7:e1002098. doi: 10.1371/journal.ppat.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- Shin GT, Lee HJ, Kim H. GADD45gamma regulates TNF-alpha and IL-6 synthesis in THP-1 cells. Inflamm Res. 2012;61:1195–1202. doi: 10.1007/s00011-012-0515-x. [DOI] [PubMed] [Google Scholar]
- Freytes DO, Kang JW, Marcos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CL, Ge X, Xie Z, Zhou Y, Tsai LH. Control of activating transcription factor 4 (ATF4) persistence by multisite phosphorylation impacts cell cycle progression and neurogenesis. J Biol Chem. 2010;285:33324–33337. doi: 10.1074/jbc.M110.140699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameri K, Lewis CE, Raida M, Sowter H, Hai T, Harris AL. Anoxic induction of ATF-4 through HIF-1-independent pathways of protein stabilization in human cancer cells. Blood. 2004;103:1876–1882. doi: 10.1182/blood-2003-06-1859. [DOI] [PubMed] [Google Scholar]
- Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem. 2005;138:501–507. doi: 10.1093/jb/mvi143. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Oyadomari S, Mori K, Mori M. Nitric oxide-induced apoptosis in RAW 264.7 macrophages is mediated by endoplasmic reticulum stress pathway involving ATF6 and CHOP. J Biol Chem. 2002;277:12343–12350. doi: 10.1074/jbc.M107988200. [DOI] [PubMed] [Google Scholar]
- Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20:436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmuller L, Cibelli G, Moll JR, Vinson C, Thiel G. Regulation and composition of activator protein 1 (AP-1) transcription factors controlling collagenase and c-Jun promoter activities. Biochem J. 2001;360:599–607. doi: 10.1042/0264-6021:3600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Koike Y, Tomizawa K, Ogawa S, Hosaka K, Tanaka S, et al. Presence of activating transcription factor 4 (ATF4) in the porcine anterior pituitary. Mol Cell Endocrinol. 1999;154:151–159. doi: 10.1016/s0303-7207(99)00078-7. [DOI] [PubMed] [Google Scholar]
- Pocock J, Gomez-Guerrero C, Harendza S, Ayoub M, Hernandez-Vargas P, Zahner G, et al. Differential activation of NF-kappa B, AP-1, and C/EBP in endotoxin-tolerant rats: mechanisms for in vivo regulation of glomerular RANTES/CCL5 expression. J Immunol. 2003;170:6280–6291. doi: 10.4049/jimmunol.170.12.6280. [DOI] [PubMed] [Google Scholar]
- Boylan AM, Hebert CA, Sadick M, Wong WL, Chuntharapai A, Hoeffel JM, et al. Interleukin-8 is a major component of pleural liquid chemotactic activity in a rabbit model of endotoxin pleurisy. Am J Physiol. 1994;267:L137–L144. doi: 10.1152/ajplung.1994.267.2.L137. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Wang GQ, Yang XY, Jia YT, Xia ZF. Tec kinase mediating IL-8 transcription in monocytes stimulated with LPS. Inflammation. 2009;32:265–269. doi: 10.1007/s10753-009-9129-z. [DOI] [PubMed] [Google Scholar]
- Hadad N, Tuval L, Elgazar-Carmom V, Levy R. Endothelial ICAM-1 protein induction is regulated by cytosolic phospholipase A2alpha via both NF-kappaB and CREB transcription factors. J Immunol. 2011;186:1816–1827. doi: 10.4049/jimmunol.1000193. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Clifford RL, Jindarat S, Proud D, Pang L, Belvisi MG, et al. TNF{alpha} and IFN{gamma} synergistically enhance transcriptional activation of CXCL10 in human airway smooth muscle cells via STAT-1, NF-{kappa}B and the transcriptional coactivator CREB-binding protein. J Biol Chem. 2010. [DOI] [PMC free article] [PubMed]
- Yeligar SM, Machida K, Tsukamoto H, Kalra VK. Ethanol augments RANTES/CCL5 expression in rat liver sinusoidal endothelial cells and human endothelial cells via activation of NF-kappa B, HIF-1 alpha, and AP-1. J Immunol. 2009;183:5964–5976. doi: 10.4049/jimmunol.0901564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.