Singlet oxygen acts through a histidine residue to delay HCN channel deactivation and enhance voltage-insensitive current.
Abstract
Singlet oxygen (1O2), which is generated through metabolic reactions and oxidizes numerous biological molecules, has been a useful tool in basic research and clinical practice. However, its role as a signaling factor, as well as a mechanistic understanding of the oxidation process, remains poorly understood. Here, we show that hyperpolarization-activated, cAMP-gated (HCN) channels–which conduct the hyperpolarization-activated current (Ih) and the voltage-insensitive instantaneous current (Iinst), and contribute to diverse physiological functions including learning and memory, cardiac pacemaking, and the sensation of pain–are subject to modification by 1O2. To increase the site specificity of 1O2 generation, we used fluorescein-conjugated cAMP, which specifically binds to HCN channels, or a chimeric channel in which an in-frame 1O2 generator (SOG) protein was fused to the HCN C terminus. Millisecond laser pulses reduced Ih current amplitude, slowed channel deactivation, and enhanced Iinst current. The modification of HCN channel function is a photodynamic process that involves 1O2, as supported by the dependence on dissolved oxygen in solutions, the inhibitory effect by a 1O2 scavenger, and the results with the HCN2-SOG fusion protein. Intriguingly, 1O2 modification of the HCN2 channel is state dependent: laser pulses applied to open channels mainly slow down deactivation and increase Iinst, whereas for the closed channels, 1O2 modification mainly reduced Ih amplitude. We identified a histidine residue (H434 in S6) near the activation gate in the pore critical for 1O2 modulation of HCN function. Alanine replacement of H434 abolished the delay in channel deactivation and the generation of Iinst induced by photodynamic modification. Our study provides new insights into the instantaneous current conducted by HCN channels, showing that modifications to the region close to the intracellular gate underlie the expression of Iinst, and establishes a well-defined model for studying 1O2 modifications at the molecular level.
INTRODUCTION
Molecular oxygen has three different electron configurations: the triplet ground state (3Σ), and the first and second singlet excited states (1Δ and 1Σ) (Kochevar, 2004; Ogilby, 2010). Singlet oxygen (1O2) mainly refers to the 1Δ state because of its longer lifetime compared with the 1Σ state. 1O2 is highly reactive and oxidizes a wide range of biomolecules including DNA, proteins, and unsaturated lipids. Among the 20 amino acids, 1O2 mainly reacts with histidine, cysteine, methionine, tyrosine, and tryptophan (Gracanin et al., 2009). Histidine is an effective quencher of 1O2 and the major target of 1O2 oxidation. Under physiological conditions, 1O2 can be generated through metabolic reactions and functions as a signaling factor, such as in plants and stimulated neutrophils and macrophages (Steinbeck et al., 1992; Triantaphylidès and Havaux, 2009). Native compounds including flavins and NADH/NADPH inside the cell can function as photosensitizers and produce 1O2 upon sunlight excitation, which has been linked to aging and cancer development in skin cells (Baier et al., 2006; Bäumler et al., 2012). Low levels of 1O2 play an instructive role in intracellular signaling, such as promoting gene expression and triggering apoptosis (Anthony et al., 2005; Guo et al., 2010; Nam et al., 2013). However, high levels of 1O2, especially the 1O2 produced in vitro through photosensitization processes, can lead to excessive oxidation of biomolecules and even cell necrosis. Photosensitization or photodynamic processes require three key elements: photosensitizer, oxygen, and light (Fig. S1) (DeRosa and Crutchley, 2002). Actually, many photo-excitable molecules used in basic research, such as FITC and fluorescent proteins, are effective photosensitizers and can produce 1O2 upon excitation.
Two unique features of 1O2 are its short lifetime (µs) and its short working distance (nm), which make it effective in eliminating the function of specific proteins and cells with high temporal and spatial precision. In chromophore-assisted light inactivation, a fluorophore-tagged antibody recognizes and forms a complex with the target protein. Upon light excitation, an excessive amount of 1O2 is generated and inactivates the function of the protein in close proximity (Liao et al., 1994; Tour et al., 2003). Similarly in photodynamic therapy (PDT), light is guided to the target tissue to excite the pre-administered drugs (photosensitizers) to produce 1O2, which eventually leads to the death of the target cells through a combination of apoptosis, necrosis, and acutely triggered local immune responses. The major advantage of PDT is the minimal side effects. PDT is a procedure approved by the US Food and Drug Administration for treating cancer and some other diseases (Agostinis et al., 2011).
However, in contrast to its application potential and physiological significance, little is known about 1O2-mediated modification of biomacromolecules at the molecular level. Previous studies on 1O2 mainly relied on cell and tissue preparations in the presence of complex intracellular signaling pathways. Numerous factors make 1O2 a challenging research target: the volatile chemical nature of 1O2; the wide range of target molecules; and the heterogeneous distributions of oxygen, photosensitizers, and quenchers (Schweitzer and Schmidt, 2003; Skovsen et al., 2005; Latch and McNeill, 2006; Pedersen et al., 2011). Isolated reports have addressed the effects of 1O2 modification of channel and transporter proteins (Valenzeno and Tarr, 1991; Eisenman et al., 2007, 2009). Most of these studies used whole-cell preparations and applied nonspecific photosensitizers, such as rose bengal, to generate 1O2. Thus, interactions between 1O2 and lipid membranes or other membrane-affiliated elements could not be completely ruled out. Notably, all those studies required a long duration of light exposure—up to minutes or even longer—to produce observable effects, in contrast to the short lifetime of 1O2 (µs). A well-defined and sensitive working model for studying 1O2 modification is needed.
Here we report that hyperpolarization-activated, cAMP-gated (HCN) channels are sensitive to modification by photochemically generated 1O2. HCN channels are involved in physiological functions including cardiac pacemaking, sensation of pain, learning, and memory (Robinson and Siegelbaum, 2003; Biel et al., 2009). HCN channels are tetrameric. Each subunit contains a transmembrane domain of six α helices (S1–S6), homologous to that of voltage-gated potassium channels, and the cyclic nucleotide–binding domain (CNBD) in the C terminus of the channel (Robinson and Siegelbaum, 2003; Biel et al., 2009). In the transmembrane domain, S4 contains multiple positively charged residues and functions as the voltage sensor for membrane hyperpolarization. Intracellular cAMP directly binds to the channel and increases current amplitude, shifts channel opening to less negative potentials, and slows down channel deactivation. cAMP-dependent gating in HCN channels contributes to the storage of working memory in the prefrontal cortex, the maintenance of heart rate at the resting level, and the sensation of pain (Nolan et al., 2003; Wang et al., 2007; Baruscotti et al., 2011). In addition to the hyperpolarization-activated current (Ih), HCN channels also conduct the voltage-insensitive instantaneous current (Iinst), although its molecular nature is not clear (Proenza et al., 2002; Mistrík et al., 2006; Proenza and Yellen, 2006). It was reported that Ih and Iinst could be conducted by two distinct populations of HCN channels that are not in rapid equilibrium (Proenza and Yellen, 2006).
The dual regulation of HCN channels by voltage and ligand provides a unique opportunity to study 1O2 modification of membrane proteins. In our previous studies, we have used fluorescent cAMP analogues as a reporter to address the dynamic interaction between cAMP and the whole channel (Wu et al., 2011, 2012). Using 8-NBD-cAMP as the marker, we discovered that channels in the open state have an increased binding affinity for cAMP, and residues near the activation gate in S6 remotely control ligand binding. During these studies we noticed that applying high concentrations of fluorescein-cAMP (8-fluo-cAMP or FITC-cAMP) resulted in interesting changes to HCN function. Following this lead, we identified 1O2 as the central player. Intriguingly, 1O2 modification of the HCN channel is state dependent and depends on a critical histidine residue near the activation gate.
MATERIALS AND METHODS
Functional expression of HCN channels in Xenopus laevis oocytes
The DNA plasmid containing the sequence of WT mouse HCN2 (mHCN2) channel was provided by S. Siegelbaum (Columbia University, New York, NY). The HCN2-SOG and HCN2-EGFP constructs were made by inserting the DNA sequence encoding 1O2 generator (SOG) or EGFP at the C terminus of the CNBD through the BsmI cut site, flanked by the CNBD and the downstream sequence of the mHCN2 channel (Figs. S2 and S3). The H434A mutation was introduced by a two-step PCR method. DNA plasmids were linearized by SphI and purified by phenol-chloroform extraction. mMessage machine (Ambion) was used to make cRNA. 40–50 ng cRNA was injected into each oocyte at stage VI. Injected cells were cultured between 16 and 18°C for 2–4 d before experiments.
Electrophysiology and optical setup
We used symmetrical solutions during patch-clamp recording in the inside-out configuration. The solution contained 110 mM KCl, 1 mM EDTA, and 10 mM HEPES, pH 7.4 adjusted by KOH. To measure ion selectivity, K+ ions in the bath solution, which faced the intracellular side of the channel, were replaced by Na+. All experiments were performed at room temperature. Current traces were amplified by a patch-clamp amplifier (model 2400; A-M Systems) and digitized by a Digidata 1440 (Axon Instruments). Voltage-clamp and laser-pulse protocols are illustrated in the figures. Typically the membrane potential was held at −40 mV. Hyperpolarizing voltage steps were applied every 15 s.
We calculated the percentage of Iinst based on the following equation:
where Iinst is the instantaneous current measured just after the hyperpolarizing voltage step, i is the stimulation index (episode number), is the instantaneous current before laser treatment, and Imax is the peak current measured near the end of the hyperpolarizing voltage step before laser treatment and includes both Ih and Iinst. We used a single-exponential equation to fit the tail current measured at −40 mV. Leak current was not subtracted from the recordings. To determine the V1/2 values, a series of hyperpolarizing voltage steps was applied to activate the channel. Tail currents were measured at −40 mV, and the amplitudes were fitted with the Boltzmann equation.
The ratio of permeability for Na+ and K+ ions was calculated based on the value of the reversal potential and the Goldman–Hodgkin–Katz equation:
where EREV represents the reversal potential, and [X]o and [X]i represent the ion concentrations in the pipette and in the bath, respectively.
The setup was based upon a microscope (IX71; Olympus) equipped with a 100× oil-immersion objective (NA 1.25; Olympus Plan). A 473- or a 532-nm diode-pumped, solid-state laser was used as the excitation light source. The following filter set was used to collect the fluorescence signal from FITC, EGFP, and SOG: exciter, D480/30; dichroic mirror, DC505LP; emitter, D510LP. The intensity of the laser light applied to the membrane patch was estimated to be ∼350 mW/cm2. Optical signals were detected by an EMCCD camera (Cascade 1K; Photometrics). The laser light source, the CCD camera exposure time, and the amplifier for patch-clamp recording were synchronized by transistor–transistor logic signals. Fluorescence images were analyzed with ImageJ software (Schneider et al., 2012). The TurboReg program was used for image alignment. The fluorescent cAMP analogue, FITC-cAMP (8-fluo-cAMP), was obtained from Biolog. The stock solution of FITC-cAMP (1 mM, dissolved in ddH2O) was kept frozen at −20°C. TROLOX was from Sigma-Aldrich. ZD7288 was from Tocris Bioscience.
Data analysis
Summarized data are presented as mean ± SEM. The number of experiments is indicated by n. One-way ANOVA (or one-way repeated measures ANOVA for the shift in V1/2), followed by Tukey’s post-hoc test, was used to evaluate data groups. P-values of <0.05 were considered significant and are indicated by an asterisk in the figures.
Online supplemental material
Fig. S1 shows working models for 1O2 modification of HCN channel. Figs. S2 and S3 show the DNA and protein sequences of mHCN2-EGFP and mHCN2-SOG constructs. Fig. S4 shows decreases of Ih under different conditions. Fig. S5 shows the current trace at the moment when the laser pulse was applied. Fig. S6 shows that cAMP increases Iinst, and the negative control is shown in Fig. S7. Fig. S8 shows the results with mHCN2-EGFP as a negative control. Fig. S9 shows the results with rose bengal as the photosensitizer. Figs. S10 and S11 illustrate basic biophysical properties of the mHCN2/H434A mutant channel. Fig. S12 shows that the decrease of Ih amplitude in the mHCN2/H434A mutant channel was more dramatic than that in the WT channel. Fig. S13 shows the photodynamic transformation of the HCN channel with 2-ms laser pulses. The online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201311112/DC1.
RESULTS
Photodynamic transformation of the mHCN2 channel
We performed experiments on a setup that enables simultaneous electrical recording of channel activity and optical detection of associated fluorescence signals (Wu et al., 2011). To prevent the interference from intracellular signal transduction pathways, we used a cell-free system and studied the channels expressed on a membrane patch held within the patch-clamp recording pipette in the inside-out configuration. To increase the spatial specificity of the photodynamic transformation, we used FITC-cAMP that specifically binds to the HCN channel on the cell membrane as the photosensitizer. Using uninjected oocytes as a negative control, we confirmed the specific interaction between FITC-cAMP and HCN channels (Fig. 1 A).
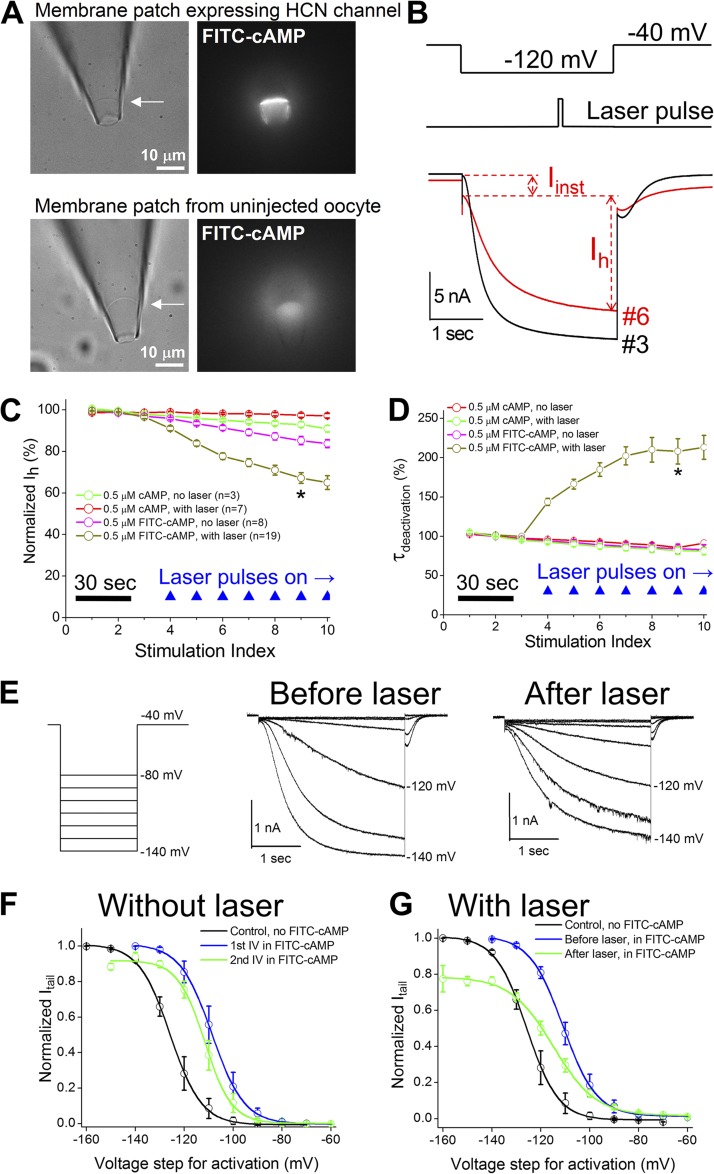
Figure 1.
Effects on Ih current by photodynamic transformation. (A) Bright field (left) and fluorescence (right) images of a membrane patch expressing mHCN2 channels. Bottom pictures show a membrane patch from an uninjected cell. 0.5 µM FITC-cAMP was applied to the bath. (B; top) The voltage protocol for channel activation and the timing of 100-msec laser pulse. (Bottom) Representative current traces before (no. 3 in C) and after the application of laser pulses (nos. 6 and 9 in C). (C) Normalized Ih amplitude versus stimulation index. Voltage step was delivered every 15 s. (D) Normalized time constant of deactivation. *, P < 0.05 in C and D. (E; left) The voltage protocol for collecting I-V curve. (Middle) Current traces before the laser treatment. (Right) Current traces after the laser treatment. (F) I-V curves without laser treatment. The V1/2 values are −126.7 ± 3.2 mV (n = 8; control without cAMP), −109.4 ± 2.6 mV (first I-V in 0.5 µM FITC-cAMP), and −112.5 ± 2.4 mV (second I-V in 0.5 µM FITC-cAMP). The averaged shift in V1/2 is −3.1 ± 1.7 mV (n = 7) and not significant (one-way repeated measures ANOVA). (G) I-V curve with laser treatment. The V1/2 values are −114.6 ± 1.6 mV (I-V in 0.5 µM FITC-cAMP before laser) and −120.4 ± 1.2 mV (I-V in 0.5 µM FITC-cAMP after laser treatments). The averaged shift in V1/2 is −5.8 ± 0.8 mV (n = 6) and significant.
In the presence of 0.5 µM FITC-cAMP, a series of laser pulses applied during channel activation (473 nm, 100 msec, 1 pulse/episode) introduced significant functional changes in the mHCN2 channel (Fig. 1 B). Laser pulses significantly reduced the amplitude of the Ih current, slowed down channel deactivation, and enhanced the voltage-insensitive component (Iinst). First, we asked whether the changes in Ih after laser treatment are related to Ih rundown, which is a known phenomenon and reflected in both the reduction in current amplitude and the negative shift in the channel activation (I-V) curve. As a negative control, the same concentration of cAMP was applied to the patch. During the 3-min recording period, the reduction in Ih was minimal and less than ∼10% with laser pulses (Fig. 1, C and D, green trace). In contrast, Ih was reduced by ∼40% in the presence of FITC-cAMP and with laser pulses. Without laser treatment, applying FITC-cAMP led to a reduction in Ih, which was probably caused by the excitation of the fluorophore by ambient light. Further control experiments involving two other constructs were performed under different conditions (Fig. S4). Collectively, these results suggest that the photodynamic transformation of HCN channels is an active process and distinct from their rundown. Moreover, we studied hyperpolarization-dependent channel activation by measuring the I-V curve (Fig. 1 E). After adding FITC-cAMP to the bath, we collected an I-V curve as the first control and then applied two to four laser pulses before collecting the second I-V curve. Compared with the I-V curves collected without laser treatment, the photodynamic process decreased the maximal current amplitude and slightly shifted the I-V curve toward negative potentials (Fig. 1, F and G). Notably, for an HCN channel with a slow gating kinetics, it is known that the duration of voltage command affects the I-V curve and slight slowdown in activation could lead to a negative shift in V1/2.
To quantify the changes in the amplitude of Iinst and the kinetics of channel deactivation, we calculated the percentage of Iinst in the total current and measured the time constant of channel deactivation (Fig. 2). The slowdown in channel deactivation occurred immediately after the delivery of the first laser pulse and reached a plateau after four to six pulses, whereas Iinst did not reach a plateau (Fig. S5). The “use-dependent” increase in Iinst could be related to the fact that after laser treatment the channel closed so slowly that a substantial amount of channels remained open, even until the next voltage stimulation step.
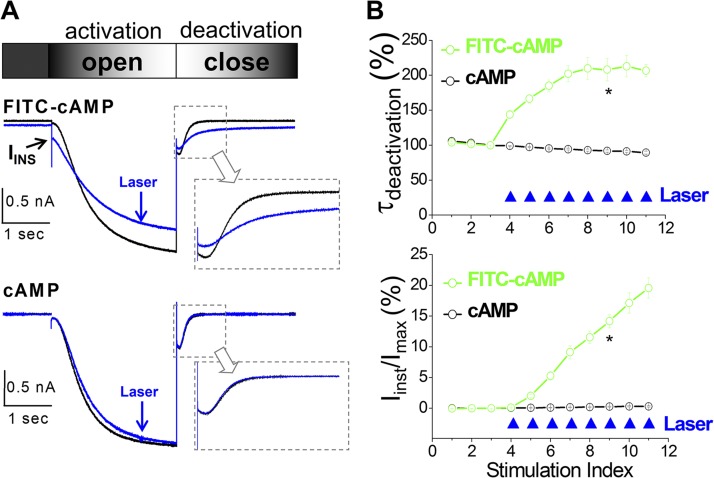
Figure 2.
Photodynamic transformation slows down channel deactivation and enhances voltage-insensitive Iinst. (A) Current traces before (black; no. 3 in B) and after (blue; no. 7 in B) laser treatments. Arrow indicates Iinst. Gray arrows point out close-up view over the current traces during channel deactivation. (B) Percentage of Iinst (top) or time constant of channel deactivation (bottom) versus episode number. Laser pulses (filled blue triangle) were given every 15 s after the third episode. FITC-cAMP, n = 19; cAMP, n = 7. *, P < 0.05.
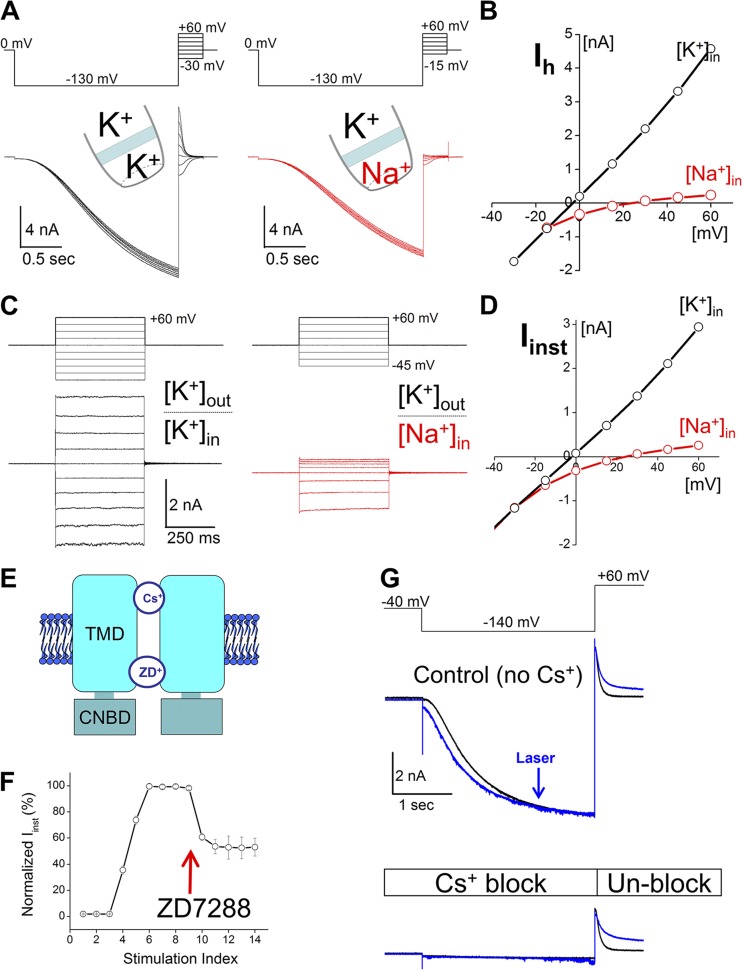
To confirm that the Iinst after laser treatment was carried by HCN channels but not related to nonspecific damage to the membrane patch, we characterized the biophysical properties of Iinst and compared them with those of the Ih current (Fig. 3). We started from ion selectivity because HCN channels have a distinctive mixed conductivity for K+ and Na+ ions. The ratio of permeability to K and Na is ∼3:1. We first measured the relative permeability of the Ih current as a positive control. We separately applied solutions containing either K+ or Na+ to the intracellular side (Fig. 3 A). Based on the reversal potential, we determined PK/PNa of the Ih conductance to be 3.61 ± 0.21 (n = 3; Fig. 3 B). Next, we applied the photodynamic procedure to transform the channel and then determined PK/PNa to be 3.03 ± 0.22 (n = 7), which is not significantly different from that of Ih (one-way ANOVA) (Fig. 3, C and D). To further address the biophysical nature of Iinst, we asked whether Iinst could be blocked by known HCN channel blockers including ZD7288 and Cs+ (Proenza and Yellen, 2006). Because two compounds block the channel from either side of the membrane, we separately tested them by applying 60 µM ZD7288 to the bath solution or 2 mM Cs+ to the pipette solution. Iinst was significantly reduced by ZD7288 and completely blocked by Cs+ (Fig. 3, E and F). Finally, we tested whether Iinst can be regulated by intracellular cAMP. After washing FITC-cAMP from the system, we applied 3 µM cAMP to the bath solution and observed a reversible increase in current amplitude (Figs. S6 and S7). These biophysical and pharmacological characterizations confirmed that the Iinst generated by the photochemical process carried key signatures of the HCN current.
Figure 3.
Photochemically generated Iinst has key biophysical features of canonical HCN channel. (A) Voltage protocol (top) and current traces for measuring pK+/pNa+ of Ih current. (B) I-V curves of Ih current. (C) Voltage protocols (top) and current traces of Iinst. (D) I-V curves of Iinst. (E) ZD7288 and Cs+ interact with different regions in the HCN pore. (F) Iinst was generated by applying laser pulses (nos. 3–5). 60 µM ZD7288 was added to the bath solution after number 9. (G) 2 mM Cs+ was added to the pipette solution. A voltage step to +60 mV released the Cs+ block. Current traces: black, control; blue, after three laser pulses. Current traces in A–C were measured in the absence of ligands (cAMP or FITC-cAMP). 0.5 µM FITC-cAMP was present in F and G.
1O2 is involved in the photochemical transformation of the mHCN2 channel
Next, we investigated the molecular basis of the photo-transformation process. To further exclude nonspecific effects on the HCN channel by blue excitation light or green fluorescent emission, we used the HCN-EGFP fusion channel because EGFP and FITC share similar excitation and emission spectra (Fig. S8). Laser treatment had little effect on the channel. To test any potential effects by the green fluorescent light, we replaced the 473-nm laser with a green laser (532 nm) and directly illuminated the membrane patch. None of these manipulations produced any noticeable change in channel function. Collectively, it appears that the transformation of the HCN channel is through a photochemical process that involves the excitation of FITC.
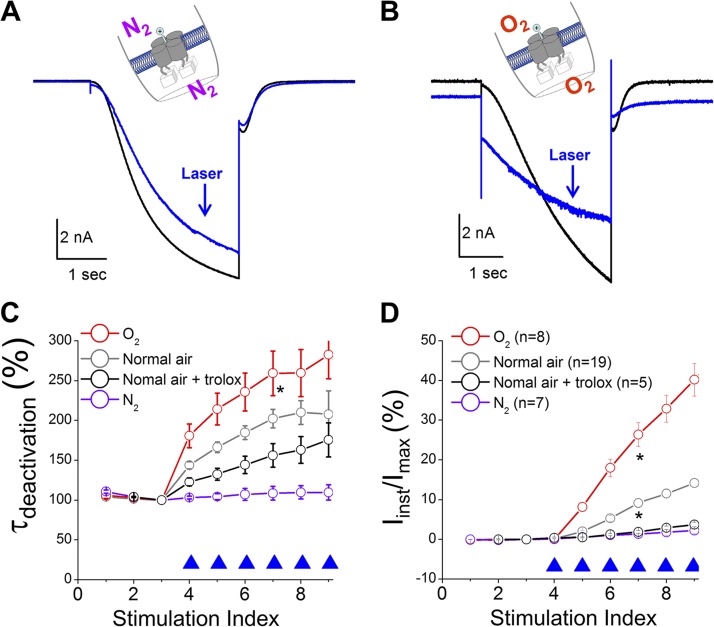
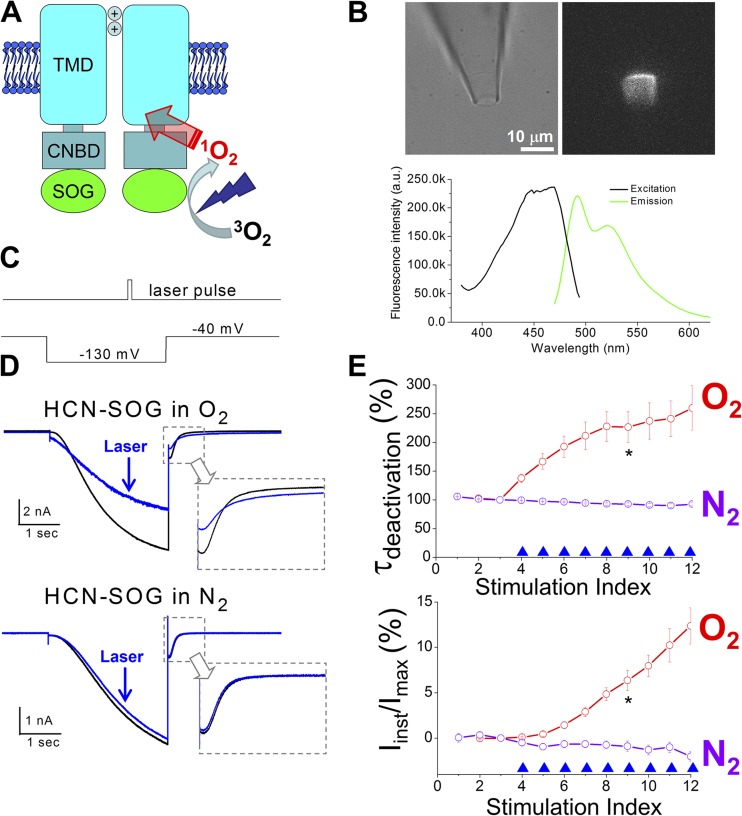
Why does exciting FITC, but not EGFP, produce such dramatic effects on channel function? Compared with EGFP, FITC is known to have a higher quantum yield for generating 1O2 (3 vs. 0.4%) (DeRosa and Crutchley, 2002; Jiménez-Banzo et al., 2008). To test the involvement of 1O2, we applied 0.1 µM rose bengal, a widely used photosensitizer (Fig. S9), or 10 mM of free FITC to the intracellular side of the patch and indeed obtained similar observations. Then we asked whether the photodynamic process was sensitive to oxygen. We degassed the solutions used in the recordings and separately purged them with pure nitrogen or pure oxygen (Fig. 4, A and B). Impressively, removing oxygen from the solution effectively abolished the photochemical transformation of HCN function whereas, in contrast, the solutions purged with oxygen exaggerated the transformation effects. We also tested Trolox C, a known 1O2 quencher (Ohara et al., 2009). As expected, 0.5 mM Trolox C (under normal air) suppressed the increases in τdeactivation and Iinst (Fig. 4, C and D).
Figure 4.
Photochemical transformation of the HCN channel is oxygen dependent. Solutions used in the experiments were degassed and then purged with pure N2 or O2. (A and B) Current traces before (black; no. 3 in C and D) and after laser pulses (blue; no. 8 in C and D). *, the group of O2 is significantly different from the data groups of Normal air + trolox and N2 (one-way ANOVA). (C and D) τdeactivation or percentage of Iinst versus episode number. 0.5 µM FITC-cAMP was applied to the bath solution. *, the groups of O2 and normal air are significantly different from the other two data groups.
To further study the involvement of 1O2, we engineered the recently developed mini-SOG protein to the C terminus of the HCN channel (Fig. 5, A and B). Mini-SOG was developed from the light, oxygen, voltage (LOV) domain of a plant protein. Mutations were introduced to increase the quantum yield of 1O2 (up to 47%, ∼20 times higher than that of ReAsh, a previously developed genetically targetable SOG) (Shu et al., 2011; Qi et al., 2012). In the absence of exogenous photosensitizers, light illumination of the HCN2-SOG channel reproduced the observations on the HCN2 channel in complex with FITC-cAMP (Fig. 5, C–E). Similarly, the photochemical transformation of the mHCN2-SOG channel was sensitive to oxygen.
Figure 5.
1O2 mediates photochemical transformation of the mHCN2 channel. (A) Construction of the HCN2-SOG fusion channel. (B; top) Bright field and fluorescence images of a membrane patch expressing HCN2-SOG channels. (Bottom) Excitation and emission spectra of purified mini-SOG protein. (C) Timing of the laser pulse and the voltage protocol. (D) Current traces before (black; no. 3 in E) and after (blue; no. 7 in E) laser treatments. (E) τdeactivation or percentage of Iinst versus episode number. Neither cAMP nor FITC-cAMP was applied. *, P < 0.05.
State dependency in 1O2 transformation of the mHCN2 channel and a critical histidine residue in the channel pore
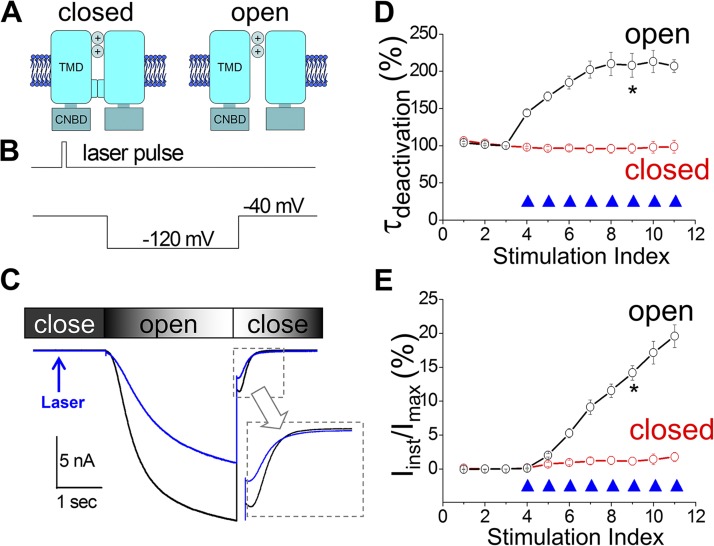
The short duration of laser pulses (ms) used in our study enabled us to probe whether the timing of the 1O2 modification, corresponding to different functional states of the channel, was a critical factor. We applied a light pulse preceding the hyperpolarizing voltage step, when most of the channels remained closed (Fig. 6, A and B). To our surprise, laser treatments of the closed channel reduced the Ih current amplitude but did not affect τdeactivation or increase Iinst (Fig. 6, C–E). This result suggested that some effects of the transformation process, including increases in τdeactivation and Iinst, are state dependent and require the channel to be in the open state when 1O2 is released.
Figure 6.
1O2 modification of the mHCN2 channel is state dependent. (A) Schematic drawings of closed and open channels. (B) Laser pulses were delivered preceding the hyperpolarization step. (C) Current traces before (black; no. 3 in D and E) and after (blue; no. 7 in D and E) laser treatments. (D and E) Normalized τdeactivation or percentage of Iinst versus episode number. 0.5 µM FITC-cAMP was applied to the bath. *, P < 0.05.
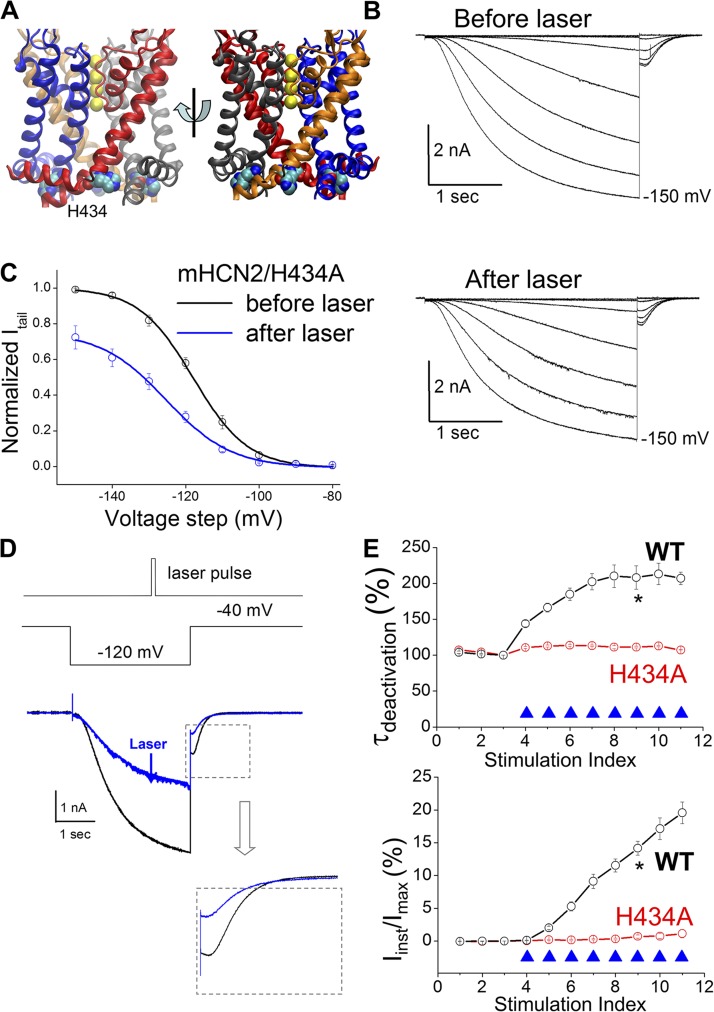
The above observation suggests that certain residue(s) might become more accessible to 1O2 when the channel is in the open state. Following this insight, we tried to locate the residue(s) that underlies the 1O2 modification effects. We focused on the regions that go through significant conformational changes during channel opening, such as the intracellular end of S6 where the activation gate was presumably located (del Camino et al., 2000; Shin et al., 2004; Prole and Yellen, 2006; Kwan et al., 2012; Wu et al., 2012). We discovered H434 to be important (Fig. 7 A). In the presence of FITC-cAMP, applying laser pulses negatively shifted the I-V curve of the mHCN2/H434A mutant channel and decreased the Ih amplitude (Figs. 7 B, S10, and S11). The negative shift in V1/2 was about the same as the control (without laser pulses between two I-Vs), indicating a contribution by current rundown (Fig. 7 C). The decrease in the Ih amplitude of mHCN2/H434A is more pronounced than that of the WT channel (Fig. S12).
Figure 7.
H434 is critical for 1O2 modification of the mHCN2 channel. (A) Structure model of the mHCN2 pore (based on Kv1.2-2.1 chimeric structure) (Long et al., 2007). (B) Current traces of mHCN2/H434A mutant channel in the presence of 0.5 µM FITC-cAMP. A series of hyperpolarizing voltage steps in a −10-mV interval was used to activate Ih. (C) I-V curves of mHCN2/H434A before (black) and after (blue) laser treatment. Current amplitudes were normalized to the maximal level before laser treatment. V1/2 values (with laser treatment) are −121.4 ± 1.7 mV (before laser in FITC-cAMP) and −129.7 ± 1.3 mV (after laser). The corresponding ΔV1/2 is −8.3 ± 2.0 mV (n = 7) and significant (one-way repeated measures ANOVA). As a control, the V1/2 values in the presence of FITC-cAMP but without laser treatment are −119.6 ± 3.3 mV (first I-V) and −128.2 ± 4.1 mV (second I-V). The ΔV1/2 is −8.5 ± 1.6 mV (n = 8; significant). (D; top) Laser pulse. (Middle) Voltage protocol. (Bottom) Current traces before (black; no. 3 in D and E) and after (blue; no. 7 in D and E) laser treatments. (E) Normalized τdeactivation or percentage of Iinst versus episode number. WT, n = 19; mHCN2/H434A, n = 13. *, P < 0.05.
Importantly, alanine replacement of H434 effectively abolished the increases in τdeactivation and Iinst upon light stimulation (Fig. 7, D and E). These results support the working model that for channels in the open state, H434 becomes more accessible to 1O2; subsequent modification of H434 leads to a delay in channel deactivation and the expression of the instantaneous HCN current (Fig. S1). In contrast, in the H434A mutant channel, 1O2 becomes more abundant and modifications of other parts of the channel by 1O2 might underlie the significant reduction in Ih current amplitude.
DISCUSSION
Here we report that HCN channels are very sensitive to photodynamic modification by 1O2. FITC-cAMP or in-frame inserted SOG greatly increases the spatial specificity of 1O2 production. In contrast with earlier studies that used second-to-minute long light illuminations, our study relied on millisecond light pulses, which exhibited significant changes in HCN channel function. Taking advantage of the short lifetime of 1O2 and the slow gating kinetics of the HCN channel, we discovered that 1O2 modification of HCN channels is state dependent and has distinct effects on channels in the open state compared with channels in the closed state. Following this lead, we identified a critical histidine residue at a critical region in S6. Impressively, replacing H434 abolished the effects of photodynamic transformation. Our study provides new insights into the instantaneous current conducted by the HCN channel and paves the way for further exploration of 1O2 as an effective photonics tool.
HCN channels are known to conduct Iinst, but the physiological significance and the molecular basis have been unclear. The Iinst carried by HCN channels has been recorded from native cells, including neurons and cardiomyocytes, and heterologous expression systems (McCormick and Pape, 1990; Hagiwara et al., 1992; Irisawa et al., 1993; Maccaferri and McBain, 1996; Graf et al., 2001; Anderson et al., 2011). The Iinst conducted by HCN channels might have significant physiological consequences. On the one hand, it is known that nonphysiological negative potentials are required to activate the canonical Ih current, especially the currents carried by HCN2 and HCN4 channels. On the other hand, any compromise to HCN channel function, through either genetic or pharmacological approaches, consistently leads to a hyperpolarization shift of the resting membrane potential and an increase in the input resistance. Iinst might provide a hint for the resolution of this dilemma and some answers for exactly how HCN channels contribute to various physiological functions.
A molecular understanding of Iinst has been lacking. The most recent study reported that Iinst is conducted by a group of HCN channels that are distinct from the Ih-conducting channels (Proenza and Yellen, 2006). That observation is consistent with our current study in that the photodynamic transformation is an irreversible process, and the generated Iinst stayed at the same level during the recording period. Moreover, we observed similar results showing that Iinst can be up-regulated by cAMP and responds much faster to open channel blockers than Ih does. What is the molecular basis of Iinst? Our study suggests that modifications made to residues near the activation gate, such as H434, could slow down channel deactivation and produce Iinst. It is possible that under physiological conditions, certain residues including H434 are modified so that the channel’s gate is decoupled from the gating machinery and remains open to produce Iinst. This is consistent with previous reports that the coupling between voltage sensor and gate in HCN channels is intrinsically weak compared with that in Kv channels (Bruening-Wright et al., 2007; Ryu and Yellen, 2012).
Our study revealed two major changes after photodynamic transformation: slowdown in channel deactivation and enhancement in Iinst. The two phenomena occurred concurrently and might even share the same molecular basis, but the connection between them requires further investigation. Delayed or incomplete deactivation should be able to directly contribute to the expression of Iinst by HCN channels, which had been seen in previous studies of a mutation in the S4–S5 linker that disrupts channel closing (Macri and Accili, 2004) and on the regulation of Iinst by intracellular chloride ions (Mistrík et al., 2006). As shown in Figs. 2 B and S5, it appears that the deactivation was slowed down after the first laser pulse (delivered in episode 4), but only in the next episode (5) could significant Iinst be detected. We kept the start-to-start interval between two episodes at 15 s, which is much longer than the cycle of membrane potential changes under physiological conditions when delayed deactivation will enhance the expression of Iinst upon repeated stimulations. Furthermore, the intrinsic connection between delayed deactivation and Iinst is supported by the observation that both were abolished when laser pulses were applied to the channels in the closed state or to the H434 mutant channel. However, we recognize the possibility that delayed deactivation and the generation of Iinst are not related. After photodynamic transformation, Iinst still exists even after a minute-long waiting period before the next hyperpolarizing stimulation. It is possible that modifications to H434 of different chemical natures are separately responsible for delayed or incomplete deactivation and the generation of Iinst. In the literature on Iinst, many studies do not report any changes in channel deactivation, although this could be caused by the fact that the Ih with delayed deactivation (and Iinst) only occupies a small fraction of the total current, so that the kinetics will not be affected macroscopically.
Our study only provided a peek into 1O2 modification of proteins, which understandably is complicated. State-dependent photodynamic modification, most likely through H434, suggests that the movement of 1O2 and the modification of protein residues are not random processes. H434 is probably more accessible to 1O2 when the channel is in the open state. This actually fits with a previous cysteine accessibility study reporting that the equivalent histidine in the spHCN channel (H462) is located approximately one to two α-helical turns above the critical region where four S6 α helices cross to form the channel gate (Rothberg et al., 2003). Because 1O2 does not carry any extra electrostatic charges, the van der Waals force or steric clashes might be the dominant factor influencing the diffusion of 1O2 through proteins. Moreover, we found that photochemical modification of the HCN channel reduced the Ih current amplitude and slightly shifted the channel activation curve toward negative potentials. The fact that Ih was reduced more in the mHCN2/434A mutant channel suggests that in the WT channel, H434 is dominant in the competition for photochemically generated 1O2, but residues other than H434 might also be targeted by 1O2, and the modification of those residues could underlie the decrease in Ih. Potential candidates include residues located in regions such as the S4–S5 linker or the intracellular ends of S5 and S6, which are known to be critical for channel function. In contrast to the decrease in Ih, the negative shift in V1/2 upon photodynamic transformation was less significant for either WT or mHCN2/H434A mutant channels. Notably, because of the slow activation (seconds), the measurement of V1/2 for HCN channels can be complicated by subtle changes in gating kinetics and the duration of voltage step applied. The effects on channel activation and the related shift in V1/2 by photodynamic transformation require further investigation. FITC-cAMP also binds to other cyclic nucleotide–binding proteins including PKA and exchange proteins activated by cAMP, which should not be a major concern, as our experiments only involve isolated membrane patches in the inside-out configuration (Fig. 1 A). Nonspecific modifications could be largely excluded, as supported by the clear state dependency of 1O2 modification of HCN channels and the results with the mHCN2/H434A mutant channel and the mHCN2-SOG channel.
The significance of the LOV domain in many different types of protein and the biological meaning of the sensitivity to 1O2 remain to be clarified. The SOG protein used in this study was derived from the LOV domain of phototropin 2, a blue light photoreceptor from Arabidopsis thaliana (Shu et al., 2011). This domain was also found in the N terminus of the eag family (KCNH) of Kv channels (Morais Cabral et al., 1998; Haitin et al., 2013). Interestingly, both the LOV and the CNBD domains in KCNH channels lose the ability to interact with their corresponding cofactors, flavin mononucleotide and cyclic nucleotides, respectively, but they interact with each other to regulate channel function. The LOV domain has also been discovered in transcription factors that regulate circadian rhythms (Sassone-Corsi, 1998), some of which play a role in regulating 1O2 signaling pathways (Metz et al., 2012). HCN channels share a similar topology with KCNH channels and do not contain the N-terminal LOV domain, but interestingly, they are very sensitive to regulation by 1O2 generated by a mutated LOV domain. We anticipate that with a more powerful laser source and an ample supply of oxygen in the local microenvironment, it will be possible to push the time limit further to submilliseconds, a resolution that is essential for probing protein dynamics (Fig. S13). Further investigation of 1O2 modification at the molecular level will provide insights into the role of 1O2 as a signaling molecule and help establish 1O2 as an effective photonics tool for biomedical research.
Supplementary Material
Acknowledgments
We thank Drs. Leon Avery and Roland Pittman for their suggestions and comments on this manuscript.
Q. Liu and L. Zhou are funded by startup funds from Virginia Commonwealth University and by the American Heart Association (grant 11BGIA7850004).
The authors declare no competing financial interests.
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- 1O2
- singlet oxygen
- CNBD
- cyclic nucleotide–binding domain
- HCN
- hyperpolarization-activated, cAMP-gated
- Ih
- hyperpolarization-activated current
- Iinst
- voltage-insensitive instantaneous current
- LOV
- light, oxygen, voltage
- mHCN2
- mouse HCN2
- SOG
- 1O2 generator
References
- Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W., Gollnick S.O., Hahn S.M., Hamblin M.R., Juzeniene A., Kessel D., et al. 2011. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 61:250–281 10.3322/caac.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W.D., Galván E.J., Mauna J.C., Thiels E., Barrionuevo G. 2011. Properties and functional implications of Ih in hippocampal area CA3 interneurons. Pflugers Arch. 462:895–912 10.1007/s00424-011-1025-3 [DOI] [PubMed] [Google Scholar]
- Anthony J.R., Warczak K.L., Donohue T.J. 2005. A transcriptional response to singlet oxygen, a toxic byproduct of photosynthesis. Proc. Natl. Acad. Sci. USA. 102:6502–6507 10.1073/pnas.0502225102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier J., Maisch T., Maier M., Engel E., Landthaler M., Bäumler W. 2006. Singlet oxygen generation by UVA light exposure of endogenous photosensitizers. Biophys. J. 91:1452–1459 10.1529/biophysj.106.082388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruscotti M., Bucchi A., Viscomi C., Mandelli G., Consalez G., Gnecchi-Rusconi T., Montano N., Casali K.R., Micheloni S., Barbuti A., DiFrancesco D. 2011. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4. Proc. Natl. Acad. Sci. USA. 108:1705–1710 10.1073/pnas.1010122108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler W., Regensburger J., Knak A., Felgenträger A., Maisch T. 2012. UVA and endogenous photosensitizers—the detection of singlet oxygen by its luminescence. Photochem. Photobiol. Sci. 11:107–117 10.1039/c1pp05142c [DOI] [PubMed] [Google Scholar]
- Biel M., Wahl-Schott C., Michalakis S., Zong X. 2009. Hyperpolarization-activated cation channels: From genes to function. Physiol. Rev. 89:847–885 10.1152/physrev.00029.2008 [DOI] [PubMed] [Google Scholar]
- Bruening-Wright A., Elinder F., Larsson H.P. 2007. Kinetic relationship between the voltage sensor and the activation gate in spHCN channels. J. Gen. Physiol. 130:71–81 10.1085/jgp.200709769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Camino D., Holmgren M., Liu Y., Yellen G. 2000. Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature. 403:321–325 10.1038/35002099 [DOI] [PubMed] [Google Scholar]
- DeRosa M.C., Crutchley R.J. 2002. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 233–234:351–371 10.1016/S0010-8545(02)00034-6 [DOI] [Google Scholar]
- Eisenman L.N., Shu H.J., Akk G., Wang C., Manion B.D., Kress G.J., Evers A.S., Steinbach J.H., Covey D.F., Zorumski C.F., Mennerick S. 2007. Anticonvulsant and anesthetic effects of a fluorescent neurosteroid analog activated by visible light. Nat. Neurosci. 10:523–530 [DOI] [PubMed] [Google Scholar]
- Eisenman L.N., Shu H.J., Wang C., Aizenman E., Covey D.F., Zorumski C.F., Mennerick S. 2009. NMDA potentiation by visible light in the presence of a fluorescent neurosteroid analogue. J. Physiol. 587:2937–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracanin M., Hawkins C.L., Pattison D.I., Davies M.J. 2009. Singlet-oxygen-mediated amino acid and protein oxidation: Formation of tryptophan peroxides and decomposition products. Free Radic. Biol. Med. 47:92–102 10.1016/j.freeradbiomed.2009.04.015 [DOI] [PubMed] [Google Scholar]
- Graf E.M., Heubach J.F., Ravens U. 2001. The hyperpolarization-activated current If in ventricular myocytes of non-transgenic and β2-adrenoceptor overexpressing mice. Naunyn Schmiedebergs Arch. Pharmacol. 364:131–139 10.1007/s002100100431 [DOI] [PubMed] [Google Scholar]
- Guo H.C., Qian H.S., Idris N.M., Zhang Y. 2010. Singlet oxygen-induced apoptosis of cancer cells using upconversion fluorescent nanoparticles as a carrier of photosensitizer. Nanomedicine. 6:486–495 10.1016/j.nano.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kasanuki H., Hosoda S. 1992. Background current in sino-atrial node cells of the rabbit heart. J. Physiol. 448:53–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haitin Y., Carlson A.E., Zagotta W.N. 2013. The structural mechanism of KCNH-channel regulation by the eag domain. Nature. 501:444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H., Brown H.F., Giles W. 1993. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 73:197–227 [DOI] [PubMed] [Google Scholar]
- Jiménez-Banzo A., Nonell S., Hofkens J., Flors C. 2008. Singlet oxygen photosensitization by EGFP and its chromophore HBDI. Biophys. J. 94:168–172 10.1529/biophysj.107.107128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochevar I.E. 2004. Singlet oxygen signaling: From intimate to global. Sci. STKE. 2004:pe7. [DOI] [PubMed] [Google Scholar]
- Kwan D.C., Prole D.L., Yellen G. 2012. Structural changes during HCN channel gating defined by high affinity metal bridges. J. Gen. Physiol. 140:279–291 10.1085/jgp.201210838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latch D.E., McNeill K. 2006. Microheterogeneity of singlet oxygen distributions in irradiated humic acid solutions. Science. 311:1743–1747 10.1126/science.1121636 [DOI] [PubMed] [Google Scholar]
- Liao J.C., Roider J., Jay D.G. 1994. Chromophore-assisted laser inactivation of proteins is mediated by the photogeneration of free radicals. Proc. Natl. Acad. Sci. USA. 91:2659–2663 10.1073/pnas.91.7.2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.B., Tao X., Campbell E.B., MacKinnon R. 2007. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 450:376–382 10.1038/nature06265 [DOI] [PubMed] [Google Scholar]
- Maccaferri G., McBain C.J. 1996. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J. Physiol. 497:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri V., Accili E.A. 2004. Structural elements of instantaneous and slow gating in hyperpolarization-activated cyclic nucleotide-gated channels. J. Biol. Chem. 279:16832–16846 10.1074/jbc.M400518200 [DOI] [PubMed] [Google Scholar]
- McCormick D.A., Pape H.C. 1990. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J. Physiol. 431:291–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz S., Jäger A., Klug G. 2012. Role of a short light, oxygen, voltage (LOV) domain protein in blue light- and singlet oxygen-dependent gene regulation in Rhodobacter sphaeroides. Microbiology. 158:368–379 10.1099/mic.0.054700-0 [DOI] [PubMed] [Google Scholar]
- Mistrík P., Pfeifer A., Biel M. 2006. The enhancement of HCN channel instantaneous current facilitated by slow deactivation is regulated by intracellular chloride concentration. Pflugers Arch. 452:718–727 10.1007/s00424-006-0095-0 [DOI] [PubMed] [Google Scholar]
- Morais Cabral J.H., Lee A., Cohen S.L., Chait B.T., Li M., Mackinnon R. 1998. Crystal structure and functional analysis of the HERG potassium channel N terminus: A eukaryotic PAS domain. Cell. 95:649–655 10.1016/S0092-8674(00)81635-9 [DOI] [PubMed] [Google Scholar]
- Nam T.W., Ziegelhoffer E.C., Lemke R.A., Donohue T.J. 2013. Proteins needed to activate a transcriptional response to the reactive oxygen species singlet oxygen. MBio. 4:e00541-12 10.1128/mBio.00541-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan M.F., Malleret G., Lee K.H., Gibbs E., Dudman J.T., Santoro B., Yin D., Thompson R.F., Siegelbaum S.A., Kandel E.R., Morozov A. 2003. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 115:551–564 10.1016/S0092-8674(03)00884-5 [DOI] [PubMed] [Google Scholar]
- Ogilby P.R. 2010. Singlet oxygen: there is indeed something new under the sun. Chem. Soc. Rev. 39:3181–3209 10.1039/b926014p [DOI] [PubMed] [Google Scholar]
- Ohara K., Kikuchi K., Origuchi T., Nagaoka S. 2009. Singlet oxygen quenching by trolox C in aqueous micelle solutions. J. Photochem. Photobiol. B. 97:132–137 10.1016/j.jphotobiol.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Pedersen B.W., Sinks L.E., Breitenbach T., Schack N.B., Vinogradov S.A., Ogilby P.R. 2011. Single cell responses to spatially controlled photosensitized production of extracellular singlet oxygen. Photochem. Photobiol. 87:1077–1091 10.1111/j.1751-1097.2011.00951.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proenza C., Yellen G. 2006. Distinct populations of HCN pacemaker channels produce voltage-dependent and voltage-independent currents. J. Gen. Physiol. 127:183–190 10.1085/jgp.200509389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proenza C., Angoli D., Agranovich E., Macri V., Accili E.A. 2002. Pacemaker channels produce an instantaneous current. J. Biol. Chem. 277:5101–5109 10.1074/jbc.M106974200 [DOI] [PubMed] [Google Scholar]
- Prole D.L., Yellen G. 2006. Reversal of HCN channel voltage dependence via bridging of the S4–S5 linker and Post-S6. J. Gen. Physiol. 128:273–282 10.1085/jgp.200609590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y.B., Garren E.J., Shu X., Tsien R.Y., Jin Y. 2012. Photo-inducible cell ablation in Caenorhabditis elegans using the genetically encoded singlet oxygen generating protein miniSOG. Proc. Natl. Acad. Sci. USA. 109:7499–7504 10.1073/pnas.1204096109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R.B., Siegelbaum S.A. 2003. Hyperpolarization-activated cation currents: From molecules to physiological function. Annu. Rev. Physiol. 65:453–480 10.1146/annurev.physiol.65.092101.142734 [DOI] [PubMed] [Google Scholar]
- Rothberg B.S., Shin K.S., Yellen G. 2003. Movements near the gate of a hyperpolarization-activated cation channel. J. Gen. Physiol. 122:501–510 10.1085/jgp.200308928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S., Yellen G. 2012. Charge movement in gating-locked HCN channels reveals weak coupling of voltage sensors and gate. J. Gen. Physiol. 140:469–479 10.1085/jgp.201210850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P. 1998. Molecular clocks: mastering time by gene regulation. Nature. 392:871–874 10.1038/31821 [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 9:671–675 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer C., Schmidt R. 2003. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 103:1685–1758 10.1021/cr010371d [DOI] [PubMed] [Google Scholar]
- Shin K.S., Maertens C., Proenza C., Rothberg B.S., Yellen G. 2004. Inactivation in HCN channels results from reclosure of the activation gate: Desensitization to voltage. Neuron. 41:737–744 10.1016/S0896-6273(04)00083-2 [DOI] [PubMed] [Google Scholar]
- Shu X., Lev-Ram V., Deerinck T.J., Qi Y., Ramko E.B., Davidson M.W., Jin Y., Ellisman M.H., Tsien R.Y. 2011. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 9:e1001041 10.1371/journal.pbio.1001041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovsen E., Snyder J.W., Lambert J.D., Ogilby P.R. 2005. Lifetime and diffusion of singlet oxygen in a cell. J. Phys. Chem. B. 109:8570–8573 10.1021/jp051163i [DOI] [PubMed] [Google Scholar]
- Steinbeck M.J., Khan A.U., Karnovsky M.J. 1992. Intracellular singlet oxygen generation by phagocytosing neutrophils in response to particles coated with a chemical trap. J. Biol. Chem. 267:13425–13433 [PubMed] [Google Scholar]
- Tour O., Meijer R.M., Zacharias D.A., Adams S.R., Tsien R.Y. 2003. Genetically targeted chromophore-assisted light inactivation. Nat. Biotechnol. 21:1505–1508 10.1038/nbt914 [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C., Havaux M. 2009. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 14:219–228 10.1016/j.tplants.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Valenzeno D.P., Tarr M. 1991. Membrane photomodification of cardiac myocytes: Potassium and leakage currents. Photochem. Photobiol. 53:195–201 10.1111/j.1751-1097.1991.tb03923.x [DOI] [PubMed] [Google Scholar]
- Wang M., Ramos B.P., Paspalas C.D., Shu Y., Simen A., Duque A., Vijayraghavan S., Brennan A., Dudley A., Nou E., et al. 2007. α2A-Adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 129:397–410 10.1016/j.cell.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Wu S., Vysotskaya Z.V., Xu X., Xie C., Liu Q., Zhou L. 2011. State-dependent cAMP binding to functioning HCN channels studied by patch-clamp fluorometry. Biophys. J. 100:1226–1232 10.1016/j.bpj.2011.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Gao W., Xie C., Xu X., Vorvis C., Marni F., Hackett A.R., Liu Q., Zhou L. 2012. Inner activation gate in S6 contributes to the state-dependent binding of cAMP in full-length HCN2 channel. J. Gen. Physiol. 140:29–39 10.1085/jgp.201110749 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.