Abstract
Apoptotic cell transfer has been found to be able to facilitate engraftment of allograft. However, the underlying mechanisms remain to be fully understood. Here we demonstrate that intravenous administration of donor apoptotic splenocytes can promote pancreatic islet engraftment by inducing generation of tolerogenic dendritic cells (Tol-DCs) and expansion of CD4+Foxp3+ regulatory T cells (Tregs). In vivo clearance of either dendritic cells (DCs) or Tregs prevented the induction of immune tolerance by apoptotic cell administration. Transient elimination of Tregs using anti-CD25, monoclonal antibody (mAb) abrogated the generation of Tol-DCs after administration of apoptotic splenocytes. Reciprocally, depletion of DCs within CD11c-DTR mice using diphtheria toxin (DT) prevented the generation of Tregs in the recipients with administration of apoptotic splenocytes. Induction of Tregs by Tol-DCs required direct cell contact between the two cell types, and programmed death 1 ligand (PD-L1) played important role in the Tregs expansion. Apoptotic cell administration failed to induce Tol-DCs in IL-10-deficient and Smad3-deficient mice, suggesting that IL-10 and transforming growth factor-β (TGF-β) are needed to maintain DCs in the tolerogenic state. Therefore, we demonstrate that Tol-DCs promote the expansion of Tregs via PD-L1 on their surface and reciprocally Tregs facilitate Tol-DCs to maintain transplantation tolerance induced by apoptotic cells via secreting IL-10 and TGF-β.
Keywords: apoptotic cell, dendritic cell, regulatory T cell, PD-L1, transplantation tolerance
Introduction
Most of the immunosuppressive agents we use today are nonspecific and their immunosuppressive effects on chronic allograft rejection need to be further improved. Long-term administration of immunosuppressant may also lead to infection or cancer.1,2 So, induction of long-term donor-specific immune tolerance is the best way for prevention of graft rejection. Cells that undergo apoptosis rarely cause autoimmunity for the following reasons. First, apoptotic cells are captured quickly by phagocytes such as macrophages and dendritic cells (DCs).3 Secondly, apoptotic cells can promote the release of IL-10 and transforming growth factor-β (TGF-β) from monocytes and inhibit the release of tumor-necrosis factor-α (TNF-α), IL-1β and IL-12.4 Apoptotic cells may also release IL-10 and TGF-β themselves.5 Immunosuppressive function of apoptotic cells has been confirmed in many model systems.6,7,8 Within the context of transplantation, transfusion with donor apoptotic splenocytes prolongs allograft survival.9 Previous studies have revealed a critical role of inducible regulatory T cells (Tregs) in immune tolerance induced by apoptotic cells.8 However, the detailed mechanisms for the induction of Tregs or other kind of immunosuppressive cells by apoptotic cells remain poorly understood.
Tregs play a critical role in maintaining immunological unresponsiveness to self-antigens and in suppressing excessive immune responses detrimental to the host.10 Tregs were first identified as a suppressive T-cell subset expressing IL-2 receptor α chains (CD25).11 The transcriptional factor Foxp3 (forkhead box protein 3) is a dominant regulator of Treg development and function.12 Specifically, Foxp3 transduction in naive T cells upregulates the expression of CD25 and other Tregs associated cell-surface molecules, such as cytotoxic T cell-associated antigen-4 and glucocorticoid-induced TNF receptor family-related gene/protein, and represses the production of IL-2, IFN-γ and IL-4.13 Tregs could directly eliminate responder T cells via a granzyme/perforin dependent mechanism, or indirectly by inducing apoptosis through absorption of cytokines.14 Several studies have suggested that IL-10 and TGF-β secreting by Tregs may also contribute to their immunosuppressive activity.15,16 However, the mechanisms for the immunosuppressive effect of Tregs need to be further investigated.
DCs are professional antigen-presenting cells of multiple lineages and have the potential to induce both immunity and tolerance.17,18,19 Tolerogenic DCs (Tol-DCs) are immature, maturation-resistant or alternatively activated DCs that express low levels of surface MHC and costimulatory molecules. Many strategies have been used to expand Tol-DCs. For example, Tol-DCs can be derived by genetic manipulation that enhances the expression of T cell-associated antigen-4, indoleamine 2,3-dioxygenase, CD95L, IL-10 or TGF-β.20,21,22 We also show that soluble TNF-α receptor gene-modified immature DCs can prolong allograft survival more significantly than immature DCs used alone, indicating soluble TNF-α receptor gene-modified DCs exhibit more tolerogenicity.23 Bone marrow-derived DCs (BMDCs) could also be rendered tolerogenic in the presence of IL-10, TGF-β and vascular endothelia growth factor or immunosuppressive drugs.24,25,26 Tol-DCs can induce alloantigen specific T cell anergy and drive de novo differentiation of Tregs from naive T cells.27,28,29,30,31 Recent studies show that Tol-DCs can also induce anergy and regulatory properties in tolerance-resistant memory CD4+ T cell and dampen memory T-cell response.32 Repetitive intravenous administration of Tol-DCs has been shown to prolong cardiac allograft survival in mice.33 Tregs could aggregate around DCs,34 and compete with naïve T cells for interaction with DCs.35,36 Whether the reciprocal induction and functional interaction of Tol-DCs and Tregs contribute to the tolerance induction by apoptotic cells needs to be further explored.
In this study, we demonstrated that reciprocal interaction between Tol-DCs and Tregs is essential for the induction of immune tolerance by in vivo infusion with apoptotic cells, which contribute to promote pancreatic islet engraftment by apoptotic cell transfer. In the immune tolerance induced by apoptotic cell administration, Tol-DCs promote the expansion of Tregs via programmed death 1 ligand (PD-L1) on their surface, and Tregs facilitate Tol-DCs to sustain tolerogenic state via IL-10 and TGF-β.
Materials and methods
Mice and reagents
Female BALB/c and C57BL/6 mice (6–8 weeks) were purchased from SIPPER BK Experimental Animals Co. (Shanghai, China). CD11c-DTR mice, Smad3-deficient (Smad3−/−) mice and IL-10-deficient (IL-10−/−) mice were bred and maintained in a specific pathogen free facility.37,38 All animal experiments were undertaken in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Second Military Medical University, Shanghai, China. Collagenase V, streptozocin (STZ), dithizone, diphtheria toxin (DT), lipopolysaccharide (LPS; Escherichia coli, O26:B6), annexin V-FITC and propidium iodide (PI) were all purchased from Sigma-Aldrich (St Louis, MO, USA). Recombinant mouse granulocyte-macrophage colony-stimulating factor and IL-4 were from PeproTech (London, UK). Anti-CD3ε (145-2C11), anti-CD16/32, anti-PD-L1 (10F.9G2), antiPD-L2 (TY25) and isotype control antibodies were from BioLegend (San Diego, CA, USA). Anti-CD25 antibody (PC61) was purified in this laboratory. FITC-CD11c (N418), FITC-CD4 (L3T4), PE-CD25 (7D4), PE-CD80 (16-10A1), PE-CD86 (GL1), PE-CD40 (1c10), PE-Iad (39-10-8), PE-Iab (AF6-120.1) and PE-PD-L1 (10F.9G2) were from BD Pharmingen (San Diego, CA, USA). PE-Foxp3 was from eBioscience (San Diego, CA, USA). CD11c monoclonal antibody (mAb) microbeads and Treg isolation kit were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany).
Cell preparation
Signal cell suspensions were obtained by homogenizing spleens. Spleenocytes were resuspended in phosphate-buffered saline (PBS) after dissolving erythrocytes in ammonium chloride solution. Apoptotic splenocytes were obtained as previously described.39 Briefly, splenocytes (5×106) were suspended in 5 ml PBS in a 10-cm-diameter Petri plate and exposed to a 40 W UV (320 nm) source at a distance of 40 cm for 10 min. After irradiation, splenocytes were resuspended in RPMI-1640 (with 10% fetal bovine serum) and cultured for 4 h before infusion. Splenic CD11c+ DCs were labeled by CD11c mAb microbeads and isolated with MACS. Tregs were isolated using a CD4+CD25+ Regulatory T Cell Isolation Kit. Briefly, non-CD4+ T cells were removed. The remaining cells were labeled with anti-CD25 microbeads for magnetic separation. Purity of enriched CD4+CD25+T cell fraction was >90% as confirmed by FACS. Apoptotic donor splenocytes were induced by UVB irradiation and verified by annexin V-FITC and PI staining.40
Generation of bone marrow-derived DCs
BMDCs were generated as previously described.41 Briefly, bone marrow progenitors were cultured for 48 h in RPMI-1640 supplemented with 10% fetal bovine serum, 10 ng/ml murine granulocyte-macrophage colony-stimulating factor and 1 ng/ml IL-4. Nonadherent cells were gently removed; the remaining loosely adherent clusters were cultured for an additional 3 days prior to the harvest. Mature BMDCs were generated from immature BMDCs by stimulation with 10 ng/ml LPS for 1 day.
Islet isolation and transplantation
Islets were isolated from C57BL/6(H-2b) mice as previously described with some modifications (BALB/c mice were used as donors while CD11c-DTR mice act as recipients).42 Briefly, the pancreatic duct was distended with collagenase V and purified on an OptiPrep gradient (AXIS-SHIELD, Dundee, Scotland). Islets with diameters between 75 and 250 mm were hand-picked and transplanted under the renal capsule of STZ induced diabetic BALB/c (H-2d) mice (non-fasting blood glucose >20 mmol/l on 3 consecutive days). Each recipient received 200–250 islets. Apoptotic donor splenocytes (107 cells) were infused to recipient mice via tail vein 1 week prior to islet transplantation. Blood glucose <10 mmol/l after transplantation was considered engraftment, and >20 mmol/l was considered islet graft rejection. In some experiments, mice received intraperitoneal injection of DT (16 ng/g), PC61 (500 µg) or anti-PD-L1 antibody (100 µg) at 24 h prior to infusion with apoptotic cells.
Mixed-lymphocyte reaction and in vitro suppression assay
A total of 1×104 mature BMDCs from C57BL/6 donor mice or third party (C3H mice) were cultured with 1×105 freshly isolated CD4+CD25− T cells from BALB/c recipient mice for 3 days, together with 1×105 CD4+CD25+ Tregs from tolerant mice (grafts surviving >60 days) or age matched diabetic BALB/c mice. The responder CD4+CD25−T cells were labeled with CFSE for FACS analysis.43 Cytokines in the supernatant were assayed by enzyme-linked immunosorbent assay kit (R&D Systems, Minnesota, MN, USA).
In vitro conversion assay
For the Tregs conversion assay, CD4+CD25− T (5×104) cells isolated from BALB/c mice were cultured with splenic DCs (5×104) purified from tolerant mice or syngeneic BALB/c mice for 3 days in the presence of 100 ng/ml anti-CD3ε mAb. In some experiments, antibody against PD-L1 or PD-L2 (0.5 µg/ml for both) was included. Foxp3 expression was detected by FACS analysis. For the tolerogenic DCs conversion assay, imDCs (1×105) from BALB/c mice were cultured with Tregs or CD4+CD25− T cells (1×105) isolated from tolerant mice or a mixture of these two populations at 1∶1 ration for 3 days. The phenotype of DCs was analyzed by FACS Callibur. Cytokines in the supernatant were assayed by enzyme-linked immunosorbent assay.
Flow cytometry
The phenotypes of splenocytes, isolated/cultured DCs and T cells were analyzed by FACSCallibur with CELLQUEST software (BD Biosciences). For intracellular analysis of Foxp3 expression, cells were stained with PE-Foxp3 according to the manufacturer's protocol (eBioscience). All experiments were carried out with appropriate isotype control as described previously.23
Statistical analysis
Data are shown as means±s.d. The Wilcoxon rank sum test was conducted in the two group comparisons. Graft survival was compared among experimental groups using the Kaplan–Meier test. The statistical significance is satisfied at P<0.05 level.
Results
Infusion of apoptotic cells prolongs islets allograft survival
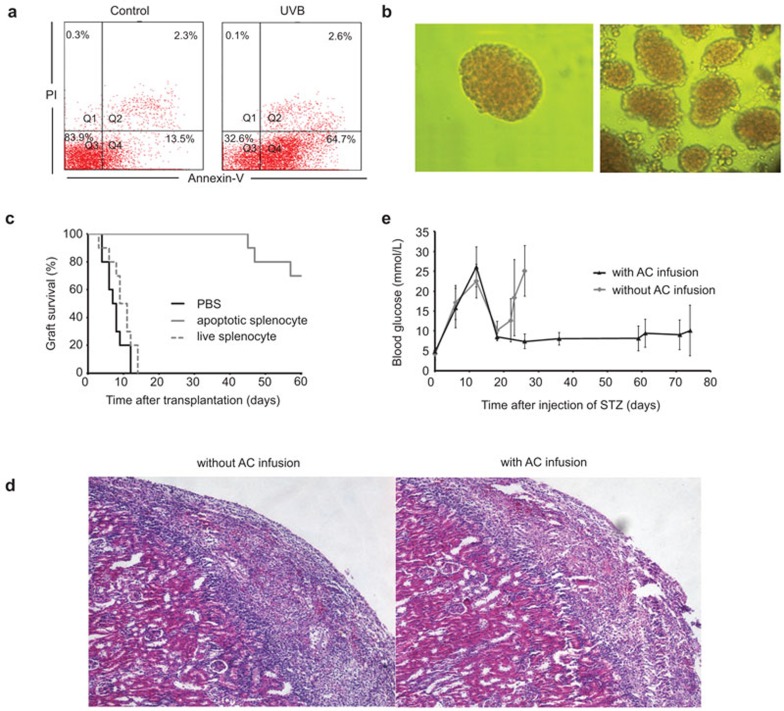
The apoptosis induction of splenocytes was detected by AnnexinV and PI stain. More than 60% of the splenocytes were rendered apoptotic by 4 h after irradiation (Figure 1a). Islets were purified from C57BL/6 mice and visualized by optical microscopy (Figure 1b), and then transplanted into the diabetic BALB/c mice which were prepared by i.p. injected of STZ. The graft survival (blood glucose <10 mmol/l) was enhanced significantly in the recipient mice with apoptotic cell pre-infusion than that in recipients without apoptotic cell pre-infusion (56.9±1.8 days versus 7.6±0.8 days, n=10, P<0.01) (Figure 1c). Pre-infusion with live splenocytes from donor mice could not enhance engraftments in recipients (Figure 1c). Grafts in PBS-treated recipients have heavier infiltration of lymphocytes than that in recipients with pre-infusion of apoptotic splenocytes treated as shown in histological analysis (Figure 1d). Transfusion with apoptotic cells derived from donor C57BL/6 mice prior to islet transplantation resulted in relatively low and stable blood glucose during the entire experiment period (Figure 1e). Also, we observed that infusion with apoptotic cells alone did not affect blood glucose level in the diabetic mice (data not shown). Therefore, pre-infusion with donor apoptotic cells can significantly promote the islets engraftment in recipient mice.
Figure 1.
In vivo infusion of apoptotic cells facilitates islets engraftment. (a) Splenocytes were exposed to UVB radiation for 10 min and incubated for 4 h in vitro. Treated (right panel) and untreated (left panel) cells were then stained with FITC-Annexin V and PI to detect apoptosis induction. Results are representative of three independent experiments. (b) Isolated pancreatic islets stained with DTZ (×100). (c) Graft survival of diabetic mice receiving transplantation with (gray solid line, MST=56.9±1.8 days) or without (black solid line, MST=7.6±0.8 days) donor apoptotic splenocytes pre-infusion. Graft survival of diabetic mice receiving live splenocyte pre-infusion is showed in gray dashed line (n=10, P<0.01). (d) Histological analysis of the transplanted islets. Tolerant recipients with apoptotic splenocytes pre-infusion had minor lymphocytes infiltration (right panel) compared to the rejecting recipients (left panel). (e) Blood glucose levels of the mice after STZ injection. The mice received apoptosis cells or PBS injection on day 7 and transplantation on day 14. Apoptotic splenocytes administrations facilitate the engraftment of islets and their function. DTZ, dithizone; MST, mean survival time; PBS, phosphate-buffered saline; PI, propidium iodide; STZ, streptozocin.
Induction of Tregs and tolerogenic DCs in vivo by apoptotic cell infusion
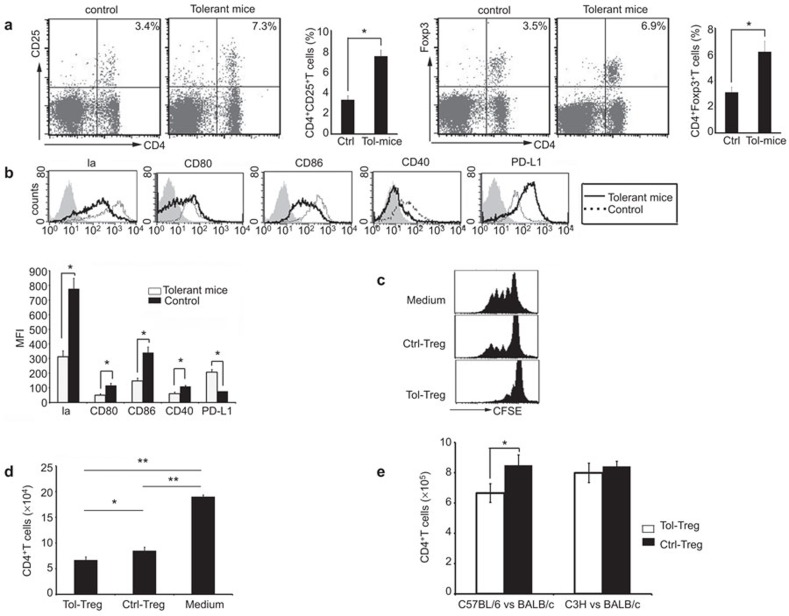
In comparison to the age-matched diabetic BALB/c mice receiving no treatment (neither apoptotic cell infusion nor transplantation), diabetic mice receiving apoptotic cell transfusion prior to islet transplantation and with the engraftment surviving >60 days (referred to as ‘tolerant mice' below) had increased CD4+Foxp3+ Tregs (6.2±0.8% versus 3.1±0.4%, P<0.05) and CD4+CD25+ T cells (7.5±0.6% versus 3.3±0.4%, P<0.05) in the splenocytes (Figure 2a). Surface expression of MHC class II molecule, CD80, CD86 and CD40 was downregulated, but the expression of PD-L1 was upregulated on splenic DCs in tolerant mice (Figure 2b). This indicated that DCs from tolerant mice are Tol-DCs. In comparison to the age-matched control diabetic mice receiving no treatment, Tregs isolated from tolerant mice had stronger inhibitory effect in a mixed-lymphocyte reaction (MLR) assay that included donor (C57BL/6) DCs as stimulators and recipient (BALB/c) T cells as responders (Figure 2c and d). Tregs from tolerant mice could not inhibit MLR between third party (C3H mice) stimulators and BALB/c responders (Figure 2e). These findings suggest that apoptotic cell transfusion could induce generation of Tol-DCs and donor-specific Tregs in the recipients.
Figure 2.
Induction of Tregs and Tol-DCs in diabetic mice by apoptotic cell administration. (a) Tregs in tolerant recipients (right panel) versus age-matched control diabetic mice (without apoptotic cell administration or transplantation) (left panel). Data are based on three independent experiments. (b) Phenotype analysis of splenic DCs (by gating CD11c+ cells) in tolerant (solid line) vs. control (dashed line) mice using staining profiles or MFI assay. Data are based on three independent experiments. *P<0.05. (c, d) Suppression of T-cell proliferation in MLR by Tregs. CD4+ T cells from recipients syngenic (BALB/c) mice were labeled with CFSE and cocultured with donor derived DCs in the presence of Tregs from tolerant mice (Tol-Treg) or control mice (described as previously before) (Ctrl-Treg). The divisions (c) and proliferate number (d) of CD4+ T cells were assessed by FACS. Data are based on five independent experiments. **P<0.01; *P<0.05. (e) Tregs (1×105) from tolerant recipients or control BALB/c mice were added to MLR as inhibitors. Mature BMDCs from C57BL/6 mice or third-party C3H mice (1×104) were used as stimulators and CD4+CD25− T cells from BALB/c mice (1×105) were used as responders. The proliferation of CD4+CD25− T cells was assessed by FACS. Data are representative of three independent experiments. *P<0.05. BMDC, bone marrow-derived dendritic cell; DC, dendritic cell; MFI, mean fluorescence intensity; MLR, mixed-lymphocyte reaction; Tol-DC, tolerogenic dendritic cell; Tregs, T regulatory cell.
Depletion of recipients DCs or Tregs in vivo abrogates tolerance induction by apoptotic cell infusion
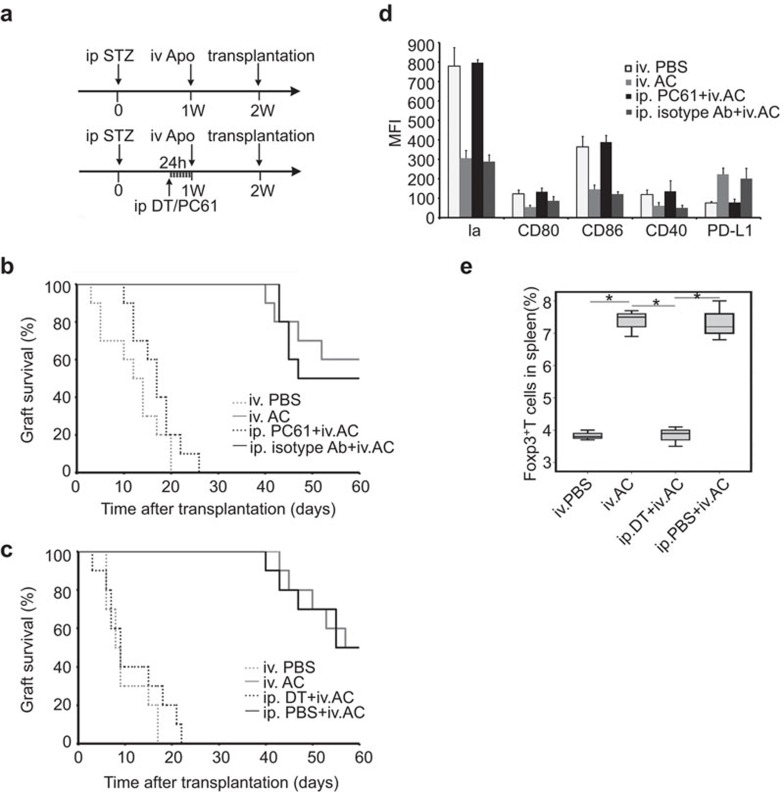
The experiments procedures are shown as indicated (Figure 3a). DT and PC61 administrations, which were done 24 h before apoptotic cell infusion, caused DCs and Tregs to be eliminated separately within 24 h and the depletion could sustain for 2–3 days as observed in our study and previous researches.44,45 The beneficial effect of apoptotic cell infusion prior to islet transplantation on grafts survival was abrogated by depletion of Tregs with PC61 in diabetic recipients or CD11c+ DCs with DT in diabetic CD11c-DTR recipients (Figure 3b and c). By mean fluorescence intensity (MFI) assay of the staining, apoptotic cell administration failed to induce downregulation of surface expression of MHC class II molecule, CD80, CD86 and CD40 on DCs in the absence of Tregs. This indicated that elimination of Tregs prevented the induction of Tol-DCs by apoptotic cell infusion (Figure 3d). Depletion of DCs also prevented Tregs expansion induced by apoptotic cell infusion (Figure 3e). The results suggest that recipient DCs and Tregs are important for the immune tolerance induced by apoptotic cell infusion.
Figure 3.
Depletion of DCs or Tregs prevents apoptotic cells-induced immune tolerance. (a) Schematic illustration of the experimental protocols. The upper arrow indicate a normal procedure of our experiment: apoptotic cells administration (Apo) was done one week after intraperitoneal injection of STZ, while islet transplantation was performed one week after apoptotic cell injection. When using DT to eliminate DCs or PC61 to ablate Tregs, DT or PC61 was given 24 h before apoptotic cell administration. (b) Grafts survival of diabetic mice receiving transplantation with apoptotic cell pre-infusion in the presence (gray solid line, MST=54.1±2.5 days) or absence of Tregs (black dashed line, MST=16.9±1.6 days, P<0.01), diabetic recipients with no apoptotic cell pre-infusion (gray dashed line, MST=12±1.9 days) or diabetic recipients received apoptotic cell pre-infusion in the presence of unrelated antibody (black solid line, MST=52.3±2.4 days) are used as control (n=10). (c) Grafts survival of diabetic CD11c-DTR mice receiving transplantation with apoptotic cell pre-infusion in the presence (gray solid line, MST=54.8±2.0 days) or absence of DCs (black dashed line, MST=11.7±2.1 days, P<0.01), diabetic CD11c-DTR with no apoptotic cell pre-infusion (gray dashed line, MST=10.1±1.4 days) or diabetic CD11c-DTR received apoptotic cell pre-infusion with intraperitoneal injection of PBS (black solid line, MST=54.0±2.3 days) are used as control (n=10). (d) Phenotype changes of splenic DCs in the BALB/c mice receiving different treatment were assessed by MFI two weeks after apoptotic cell administration. Phenotype changes induced by apoptotic cells were abrogated in absence of Tregs (n=3). (e) Tregs proportion in splenocytes of CD11c-DTR mice receiving different treatment was assessed by FACS 2 weeks after apoptotic cell administration (n=3, *P<0.05). DC, dendritic cell; DT, diphtheria toxin; MFI, mean fluorescence intensity; PBS, phosphate-buffered saline; Treg, T regulatory cell.
Mutual interaction between Tregs and tolerogenic DCs in the induction of immune tolerance by apoptotic cell administration
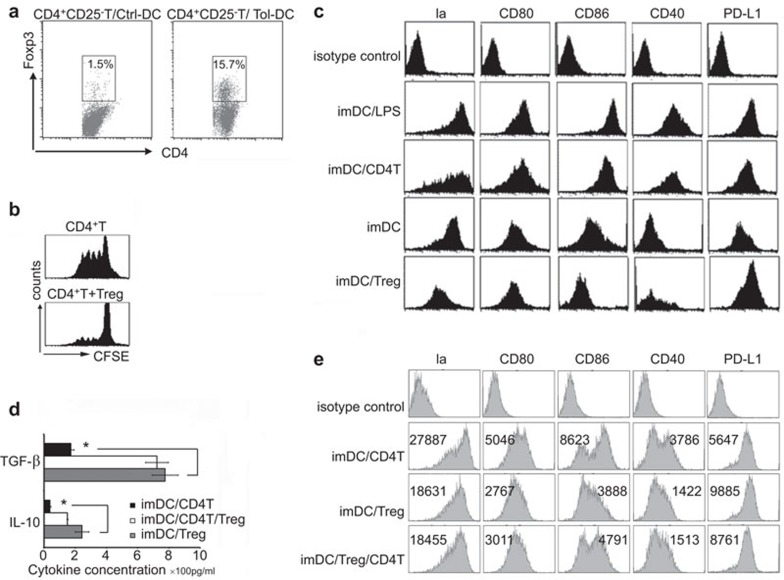
In the Tol-DCs and anti-CD3ε mAb-activated CD4+CD25− T-cell coculture experiment, Tol-DCs could induce the generation of Foxp3+ Tregs (Figure 4a). The conversion induced by Tol-DCs was 15.3%±0.8% while the conversion induced by DCs from age matched control diabetic BALB/c mice receiving no treatment was 1.4%±0.6% (P<0.05) (Figure 4a). Tregs induced by Tol-DCs also had strong suppressive ability in MLR assay that included donor DCs as stimulators and recipient T cells as responders (Figure 4b). Just like the LPS-induced DCs maturation, CD4+ T cells could stimulate maturation of DCs. However, Tregs derived from tolerant mice could help immature BMDC to maintain immature phenotype and induce tolerogenic phenotype on them by downregulating MHC class II molecule, CD80, CD86 and CD40 expression but not PD-L1 expression (Figure 4c), indicating that Tregs can inhibit maturation of DCs and maintain their tolerogenic state. Also, increased TGF-β and IL-10 was observed in the Tregs and DCs coculture supernatants (Figure 4d), indicating that TGF-β and IL-10 may be involved in the induction and maintenance of Tol-DCs. We also found that Tregs could prevent the up-regulation of DCs phenotype induced by CD4+CD25− T cells (Figure 4e).
Figure 4.
Reciprocal interaction between Tol-DCs and Tregs. (a) Tol-DCs derived from tolerant mice could induce Foxp3 expression of CD4+CD25− T cells after incubation for 3 days in presence of 100 ng/ml of anti-CD3ε mAb. DCs from age matched mice had poor ability to induce the conversion. The proportions of Foxp3 positive T cells in CD4+ T cells were indicated. (b) Tregs induced by Tol-DCs could also inhibit T-cell proliferation in MLR. (c) Tregs derived from tolerant mice inhibited phenotypic maturation of DCs. Tregs mediated downregulation of costimulatory molecules but not PD-L1 expression on DCs. The phenotype of DCs was determined by FACS after incubation with Tregs, CD4 T cells or LPS for 3 days. (d) Concentrations of IL-10 and TGF-β in the supernatants of different culture system. Data are based on three independent experiments. *P<0.05. (e) CD4+CD25− T cells could induce the phenotypic maturation of DCs, while Tregs could prevent this maturation. DC, dendritic cell; LPS, lipopolysaccharide; mAb, monoclonal antibody; MLR, mixed-lymphocyte reaction; PD-L1, programmed death 1 ligand; TGF, transforming growth factor; Tol-DC, tolerogenic dendritic cell; Treg, T regulatory cell.
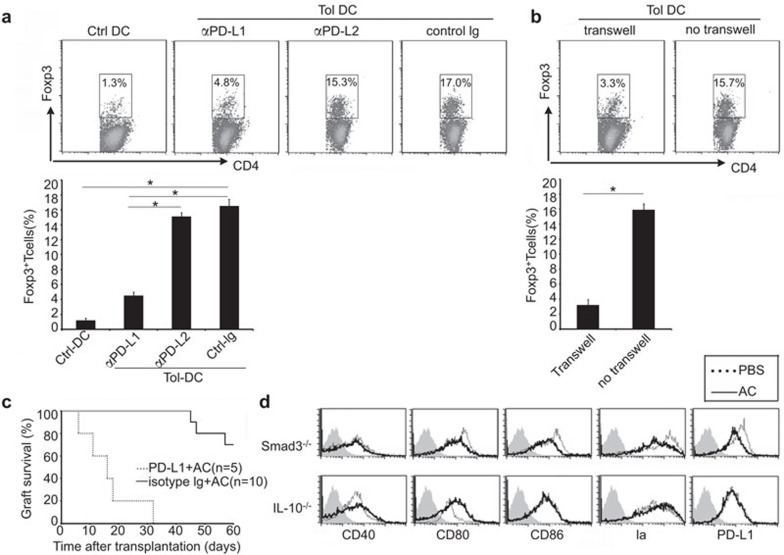
PD-L1 expression on DCs is required for the induction of regulatory T cells and TGF-β and IL-10 are required for induction and maintenance of tolerogenic DCs
As observed in previous experiments, the expression of PD-L1 was upregulated on the surface of Tol-DCs (Figure 2b). In coculture with CD4+CD25− T cells, Tol-DCs promoted Foxp3 expression, while splenic DCs from age-matched control mice did not have this ability (16.5%±0.9% vs. 1.2%±0.3%, P<0.05) (Figure 5a). Tol-DCs induced Tregs expansion was inhibited by antibody against PD-L1 (4.5%±0.5%, P<0.05) but not antibody against PD-L2 (15.1%±0.5%) (Figure 5a). Physical separation of Tol-DCs from T cells in a transwell experiment also prevented Tregs expansion (3.2%±0.7% vs. 15.9%±0.8%, P<0.05) (Figure 5b). These results suggest that surface expression of PD-L1 is crucial in the Tregs induction. A neutralizing antibody against PD-L1 in diabetic mice receiving islet transplantation abrogated the tolerance induced by apoptotic cell infusion (Figure 5c). As shown in Figure 5D, infusion with apoptotic cells did not induce the generation of Tol-DCs in mice deficient in signal of TGF-β (Smad3−/− transgenic mice) or IL-10 (IL-10−/− transgenic mice), indicating that TGF-β and IL-10 are required for the induction and maintenance of tolerogenic DCs by apoptotic cell administration.
Figure 5.
PD-L1 expression on DCs is required for the induction of Tregs, while IL-10 and TGF-β are required for the maintenance of tolerogenic DCs phenotype. (a, b) CD4+CD25− T cells were cultured with control DCs or Tol-DCs in presence of antibodies against PD-L1, PD-L2 or isotype control, and transwell was used to separate CD4+CD25− T cells and Tol-DCs. Data are based on three independent experiments.(c) Intraperitoneal injection of anti-PD-L1 mAb (100 µg) 24 h before apoptotic cell administration could abrogate induction of transplantation tolerance by apoptotic cells (56.9±1.7 days versus 16.6±2.9 days, n=5, P<0.01). (d) Administration of apoptotic cells could not induce the generation of Tol-DCs in Smad3−/− mice and IL-10−/− mice. There is no phenotype changes observed after apoptotic cell administration. Syngenic mice without apoptotic cell administration were used as control (dashed line). Data are based on three independent experiments. DC, dendritic cell; mAb, monoclonal antibody; PD-L1, programmed death 1 ligand; TGF, transforming growth factor; Tol-DC, tolerogenic dendritic cell; Treg, T regulatory cell.
Discussion
Donor apoptotic cells could induce transplantation tolerance via expansion of Tregs. Recent studies showed that apoptotic DCs could induce immune tolerance via reuptake by viable DCs.7,46 It has been found that the uptake of apoptotic DCs suppresses subsequent LPS-induced maturation, induces differentiation of naive T cells into Tregs via TGF-β, but does not affect surface phenotypes of DCs. Using an islet transplantation mouse model, we demonstrated that apoptotic cells could convert DCs into Tol-DCs, and the Tol-DCs induced expansion of Tregs through PD-L1, and Tregs facilitate the maintenance of Tol-DCs perhaps through TGF-β and IL-10. Consequently, the mutual interaction of Tol-DCs and Tregs contributes to the induction of immune tolerance by apoptotic cell administration.
It has been shown that upon ingestion of apoptotic T cells, immature DCs could secret TGF-β and induce Tregs in vivo.47 Apoptotic splenocytes consist of apoptotic T cells, B cells, apoptotic DCs and many other cell types. Different cells secret different cytokines and act on different molecular targets.3,48 We speculate that other apoptotic cells in the spleen might also contribute to Tol-DCs induction. Specific role of each component of apoptotic splenocytes needs to be evaluated. Also, whether mixed apoptotic splenocytes could produce synergistic action is an issue of importance.
Results from the current study demonstrated that the PD-L1 pathway is necessary for Tregs expansion induced by DCs.49 Tol-DCs failed to induce Foxp3 expression when separated physically from CD4+ T cells, indicating that Tregs induction require cell-cell contact with Tol-DCs. PD-1 is a member of the CD28 and T cell-associated antigen-4 immunoglobulin superfamily and interacts with PD-L1 (CD274) and PD-L2 (CD273). PD-L1 is widely distributed on leukocytes and non-hematopoietic cells, whereas PD-L2 is expressed exclusively on DCs and monocytes.50 Our results indicated that Tregs expansion induced by Tol-DCs depend on surface expression of PD-L1. Blockade of the PD-L2, in contrast, did not affect Tregs expansion. A recent study suggested that PD-1-PD-L1 interaction could induce T cell anergy by preventing the conjugation of DCs to T cells; and blockade of PD-L1 promotes prolonged T cell–DC interactions and T-cell activation.51 Accordingly, T cells could not aggregate around Tol-DCs that express high level of PD-L1 permanently, but separate Tol-DCs and T cells will abrogate Tol-DCs induced Foxp3 expression. So within this context, we speculate that Tregs induction requires low levels of MHC molecules and costimulatory factors. We also speculate that repeated brief conjugation with Tol-DCs might be more conductive for Tregs induction. Alternatively, PD-1/PD-L1 pathway could induce Foxp3 expression; but further experiments are required to address this question in the future.
A previously study showed that Tregs were more mobile than naïve T cells and could aggregate around DCs and inhibit their maturation.34 Consistent with previous studies of BMDCs,24,25,52,53 we demonstrated that Tregs producing IL-10 and TGF-β are needed for the maintenance of Tol-DCs. TGF-β and IL-10 could also be produced by apoptotic cells as well as Tol-DCs themselves.5,20 Our findings suggest that Tregs secreting TGF-β and IL-10 might be more important in the maintenance rather than the induction of Tol-DCs. Depletion of the Tregs or DCs was temporary in the current study, but was sufficient to prevent tolerance induction. Apoptotic cells were administered once before transplantation but could induce long time tolerance, suggesting that once initiated, the feedback nature of the Treg–Tol-DCs interaction becomes self-sustaining and sufficient for the development of immune tolerance.
In conclusion, in the current study we demonstrate that transfusion with apoptotic splenocytes prior to pancreatic islet transplantation could significantly prolong the survival of islet engraftment by inducing generation of Tol-DCs and Tregs. Elimination of either DCs or Tregs prevented the development of immune tolerance. Another important finding is the reciprocal interaction between Tol-DCs and Tregs in the induction of immune tolerance by apoptotic cells: Tol-DCs from tolerant mice can promote the generation of Tregs via the PD-1/PD-L1 pathway; Tregs can maintain the Tol-DCs phenotype perhaps by providing more IL-10 and TGF-β. Our results provide new mechanistic explanation for the induction of immune tolerance by infusion with apoptotic cells.
Acknowledgments
This work was supported by the National High Biotechnology Research and Development Program of China (2012AA020901 and 2006AA02A239), National Natural Science Foundation of China (81230074 and 30801000) and National Key Basic Research Program of China (2013CB530503).
The authors declare no competing financial interests.
References
- Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834–1842. doi: 10.1002/lt.21953. [DOI] [PubMed] [Google Scholar]
- MacDonald A. Improving tolerability of immunosuppressive regimens. Transplantation. 2001;72:S105–S112. [PubMed] [Google Scholar]
- Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15:243–250. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- Sun E, Gao Y, Chen J, Roberts AI, Wang X, Chen Z, et al. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ. 2004;11:1258–1264. doi: 10.1038/sj.cdd.4401500. [DOI] [PubMed] [Google Scholar]
- Kushwah R, Oliver JR, Zhang J, Siminovitch KA, Hu J. Apoptotic dendritic cells induce tolerance in mice through suppression of dendritic cell maturation and induction of antigen-specific regulatory T cells. J Immunol. 2009;183:7104–7118. doi: 10.4049/jimmunol.0900824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinclauss F, Perruche S, Masson E, de Carvalho BM, Biichle S, Remy-Martin JP, et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13:41–52. doi: 10.1038/sj.cdd.4401699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki M, Esquivel CO, Martinez OM, Strober S, Uemoto S, Krams SM. Induced tolerance to rat liver allografts involves the apoptosis of intragraft T cells and the generation of CD4+CD25+FoxP3+ T regulatory cells. Liver Transpl. 2010;16:147–154. doi: 10.1002/lt.21963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Bonham CA, Peng L, Liang X, Chen Z, Wang L, Ma L, et al. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-kappa B oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J Immunol. 2002;169:3382–3391. doi: 10.4049/jimmunol.169.6.3382. [DOI] [PubMed] [Google Scholar]
- Sun W, Wang Q, Zhang L, Pan J, Zhang M, Lu G, et al. TGF-beta1 gene modified immature dendritic cells exhibit enhanced tolerogenicity but induce allograft fibrosis in vivo. . J Mol Med. 2002;80:514–523. doi: 10.1007/s00109-002-0346-2. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang Q, Liu Y, Sun Y, Ding G, Fu Z, et al. Effective induction of immune tolerance by portal venous infusion with IL-10 gene-modified immature dendritic cells leading to prolongation of allograft survival. J Mol Med. 2004;82:240–249. doi: 10.1007/s00109-003-0521-0. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu Y, Wang J, Ding G, Zhang W, Chen G, et al. Induction of allospecific tolerance by immature dendritic cells genetically modified to express soluble TNF receptor. J Immunol. 2006;177:2175–2185. doi: 10.4049/jimmunol.177.4.2175. [DOI] [PubMed] [Google Scholar]
- Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–146. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- Chauveau C, Remy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- O'Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–F9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Konrad A, Iqbal N, Hatton RD, Weaver CT, Elson CO. Generation of antigen-specific, Foxp3-expressing CD4+ regulatory T cells by inhibition of APC proteosome function. J Immunol. 2005;174:2787–2795. doi: 10.4049/jimmunol.174.5.2787. [DOI] [PubMed] [Google Scholar]
- Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. . Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, et al. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J Immunol. 2010;184:1765–1775. doi: 10.4049/jimmunol.0902133. [DOI] [PubMed] [Google Scholar]
- Taner T, Hackstein H, Wang Z, Morelli AE, Thomson AW. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am J Transplant. 2005;5:228–236. doi: 10.1046/j.1600-6143.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. . J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Guo Z, Zhang M, Wang J, Chen G. Cao, X Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood. 2006;108:1189–1197. doi: 10.1182/blood-2006-01-007187. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Yu Y, Yang X, Cao X. SOCS3 promotes TLR4 response in macrophages by feedback inhibiting TGF-beta1/Smad3 signaling. Mol Immunol. 2008;45:1405–1413. doi: 10.1016/j.molimm.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xu S, Han Y, Cao X. Apoptotic cells attenuate fulminant hepatitis by priming Kupffer cells to produce interleukin-10 through membrane-bound TGF-β. Hepatology. 2011;53:306–316. doi: 10.1002/hep.24029. [DOI] [PubMed] [Google Scholar]
- Bittencourt MC, Perruche S, Contassot E, Fresnay S, Baron MH, Angonin R, et al. Intravenous injection of apoptotic leukocytes enhances bone marrow engraftment across major histocompatibility barriers. Blood. 2001;98:224–230. doi: 10.1182/blood.v98.1.224. [DOI] [PubMed] [Google Scholar]
- Chen W, Han C, Xie B, Hu X, Yu Q, Shi L, et al. Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell. 2013;152:467–478. doi: 10.1016/j.cell.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437–438. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- Tokita D, Shishida M, Ohdan H, Onoe T, Hara H, Tanaka Y, et al. Liver sinusoidal endothelial cells that endocytose allogeneic cells suppress T cells with indirect allospecificity. J Immunol. 2006;177:3615–3624. doi: 10.4049/jimmunol.177.6.3615. [DOI] [PubMed] [Google Scholar]
- Scumpia PO, McAuliffe PF, O'Malley KA, Ungaro R, Uchida T, Matsumoto T, et al. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol. 2005;175:3282–3286. doi: 10.4049/jimmunol.175.5.3282. [DOI] [PubMed] [Google Scholar]
- Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, Riley EM. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J Immunol. 2007;178:4136–4146. doi: 10.4049/jimmunol.178.7.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwah R, Wu J, Oliver JR, Jiang G, Zhang J, Siminovitch KA, et al. Uptake of apoptotic DC converts immature DC into tolerogenic DC, which induce differentiation of Foxp3+ regulatory T cells. Eur J Immunol. 2010;40:1022–1035. doi: 10.1002/eji.200939782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Jonuleit H, Wiendl H. Fulfilling the dream: tolerogenic dendritic cells to treat multiple sclerosis. Eur J Immunol. 2012;42:569–572. doi: 10.1002/eji.201242402. [DOI] [PubMed] [Google Scholar]
- Burrell B, Nakayama Y, Xu J, Brinkman C, Bromberg J. Regulatory T cell induction, migration, and function in transplantation. J Immunol. 2012;189:4705–4711. doi: 10.4049/jimmunol.1202027. [DOI] [PMC free article] [PubMed] [Google Scholar]