Abstract
There is increasing awareness of the effects of Porphyromonas gingivalis on host immune responses. Degradation of cytokines and chemokines by cysteine proteinases has previously been reported. However, the precise mechanisms by which P. gingivalis is able to alter intracellular signaling, and thus proliferation and inflammation, have not been described. We have previously reported suppression of activator protein-1 (AP-1) and degradation of IL-2 by proteinases from P. gingivalis. In the present study, we have analyzed the effects of P. gingivalis on Jurkat T-cell signal transduction and subsequent IL-2 and CXCL8 expression. We found that CXCL8, but not IL-2, gene expression levels were significantly suppressed by viable P. gingivalis. Analysis of intracellular signaling revealed an inhibitory effect of P. gingivalis on c-Jun and c-Fos, but not NFκB (p50 and p65), NFAT or STAT5 expression. This inhibitory effect was not due to suppression of mitogen-activated protein kinase (MAPK) (p38, erk and JNK) gene expression, but was rather due to prevention of protein kinase C (PKC) and p38 phosphorylation, as demonstrated by western blot analysis. Furthermore, SOCS1 and SOCS3 expression levels decreased following treatment of Jurkat T cells with viable P. gingivalis. The results indicate that P. gingivalis is able to suppress inflammatory gene expression by targeting the activity of MAPK pathways in T cells, which was confirmed by using specific inhibitors of NF-κB, PKC, ERK, p38 and JNK.
Keywords: MAPK, Porphyromonas gingivalis, proteinases, T cells
Introduction
The association between the inflammatory conditions of periodontitis and atherosclerosis has been supported by the identification of periodontal pathogens, including Porphyromonas gingivalis, in atherosclerotic plaques.1,2 There is a need to characterize the immune modulatory mechanisms of this pathogen that enable it to evade recognition by immune cells and translocate into atherosclerotic plaques. Recognition of pathogens and subsequent induction of inflammatory mediator release and cellular communication are key steps towards efficient elimination. It is therefore important to examine intracellular events in response to P. gingivalis.
Mitogen-activated protein kinase (MAPK) signaling in T cells plays a central role by regulating cellular proliferation, induction of inflammatory responses and apoptosis. Activation of ERK and p38 are associated with proliferation and inflammation,3,4 while JNK is closely related to induction of apoptosis.5 Signals transduced via these MAPK pathways lead to the assembly and activation of the transcription factor activator protein-1 (AP-1), which is composed of Jun (c-Jun, JunB, JunD) and Fos (c-Fos, FosB, Fra-1, Fra-2).6 The subunits c-Jun and c-Fos contain transcriptional activation domains and are involved in the regulation of broad range of genes, including cytokine and chemokine expression.7 Jun can homodimerize and form heterodimers with Fos and ATF (ATFa, ATF-2, ATF-3), while Fos can only heterodimerize with Jun.8 Early cellular events leading to a phosphorylation cascade of MAPK signaling, includes activation of protein kinase C (PKC) by diacylglycerol and calcium.
The virulence of P. gingivalis is frequently associated with its expression and release of cysteine proteinases. These proteinases are divided into arginine-specific (Rgp) and lysine-specific (Kgp), and have been reported to degrade several cytokines and chemokines. Analysis of gene expression in CD4 and CD8 T cells in response to P. gingivalis revealed predominant downregulatory effects on genes associated with cellular activation and inflammation.9 These effects may be due to P. gingivalis-dependent suppression of AP-1 and NF-κB,10 which are potent inducers of cellular proliferation and inflammatory responses. The transcription factors AP-1 and NF-κB are involved in the induction of a broad range of inflammatory mediators, including IL-2 and CXCL8. Regulation of cytokine and chemokine expression is mediated by members of the suppressor of cytokine signaling (SOCS) family. These proteins are specialized and act to directly suppress the positive feedback actions of cytokines, such as IL-2, IL-4, IL-6 and IFN-γ, and thus regulate cytokine signaling by preventing Jak phosphosrylation and STAT signaling.11 A recent publication by Moffatt and Lamont12 showed that P. gingivalis is able to significantly suppress SOCS3 and SOCS6, which is due to induction of miRNA-203.
Established P. gingivalis growth and infection may alter several signaling pathways and thus impair cellular communication. Analysis of the complexity of these pathways will improve our understanding of the pathogenesis of P. gingivalis and its involvement in the progression of systemic diseases, such as atherosclerosis. The aim of the present study was to characterize the effects of P. gingivalis on the main inflammatory and regulatory pathways, and subsequent inflammatory gene expression, in Jurkat T cells.
Materials and methods
Chemicals
The following chemicals and solutions were used in this study: Krebs–Ringer glucose buffer (120 mM NaCl, 4.9 mM KCl, 1.2 mM MgSO4, 1.7 mM KH2PO4, 8.3 mM Na2HPO4 and 10 mM glucose, pH 7.3); PMA (Phorbol 12-myristate 13-acetate; Sigma, USA); calcium ionophore (Calcium Ionophore A23187 mixed calcium magnesium salt; Sigma); ERK (PD98059; Santa Cruz, Germany); p38 (SB203580; Santa Cruz); NF-κB activation inhibitor (InSolution NF-κB Activation Inhibitor; Calbiochem, USA); JNK inhibitor, (InSolution JNK Inhibitor II; Calbiochem); and PKC Inhibitor (InSolution Bisindolylmaleimide I; Calbiochem).
Cell culture conditions
Jurkat T-cells cells (E6-1, ATCC) were maintained in 90% RPMI 1640 medium (Fisher Scientific, Austria) with 1.5 mM L-glutamine (Invitrogen, USA) and supplemented with 10% fetal bovine serum (Invitrogen). The cells were incubated in a stable environment at 95% air, 5% CO2 and 37 °C.
Bacterial culture conditions and preparation
Porphyromonas gingivalis ATCC 33277 (American Type Culture Collection, Manassas, VA, USA) was grown under anaerobic conditions (80% N2, 10% CO2 and 10% H2) in a chamber at 37 °C (Concept 400 Anaerobic Workstation; Ruskinn Technology Ltd, Leeds, UK). The bacteria were cultured for 3 days in fastidious anaerobe broth (29.7 g/l, pH 7.2) before being washed and resuspended in Krebs–Ringer glucose buffer. The bacterial concentration was adjusted to correlate with approximately 109 CFU/ml, which was determined by viable count where the bacteria were grown on fastidious anaerobe agar (46.0 g/l supplemented with L-tryptophan 0.1 g/liter, pH 7.2; Lab M, Lancashire, UK) for 5 days.
Heat-killed P. gingivalis were prepared following incubation at 70 °C for 1 h. To ensure that the bacteria were killed, 10 µl of the heat-killed suspension was spread on a fastidious anaerobe agar plate and incubated at 37 °C for 5 days. The absence of colony formation was used as an indicator that no viable bacteria were present in the suspension. P. gingivalis was used fresh for every experiment.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed on supernatants from challenged Jurkat T cells to quantify IL-2 (BD OptEIA Set Human IL-2; BD Biosciences, USA) and CXCL8 (Human IL-8 ELISA MAX Deluxe; Nordic Biosite, Sweden) according to the manufacturer's instructions. Briefly, Jurkat T-cells were either pre-treated with P. gingivalis for 1 h followed by stimulation with 50 ng/ml PMA and 1 µg/ml calcium ionophore or stimulated with PMA and calcium ionophore prior to treatment with P. gingivalis for the indicated time periods. The cells were thereafter centrifuged at 95g for 5 min and the supernatants were collected and stored at −80 °C until use.
Analysis of cytokine and chemokine regulation was examined by using inhibitors targeting specific gene regulatory proteins, including PKC, NF-κB, ERK, p38 and JNK. Briefly, Jurkat T cells were incubated with two final concentrations of each inhibitor, 20 nM and 20 µM for 2 h, prior to stimulation with 50 ng/ml PMA and 1 µg/ml calcium ionophore for 24 h. Supernatants were collected as mentioned above and ELISA was performed to determine IL-2 and CXCL8 levels.
Western blot analysis
Jurkat T cells were harvested in RIPA buffer supplemented with Halt protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL, USA). The cells were homogenized with a syringe and needle. DC protein assay (Bio-Rad Laboratories, Hercules, CA, USA) was used to measure samples protein concentration. Equal amounts of protein were mixed with Laemmli buffer (Sigma-Aldrich, USA) and boiled for 5 min at 100 °C. The samples (5–10 µg) were separated by SDS–PAGE (AnyKD TGX gel) and transferred to Immun-Blot polyvinylidene fluoride membrane (Bio-Rad Laboratories). The polyvinylidene fluoride membrane was blocked with 2% ECL advance blocking agent (GE Healthcare, Amersham Place, UK). Phospho-PKC (ζ Thr410) was detected using a rabbit monoclonal antibody (Cell Signaling Technology Danvers, MA, USA), diluted 1∶3000. Phospho-p38 was detected using a rabbit polyclonal antibody (Santa Cruz Biotechnology Inc.), diluted 1∶1000. SOCS3 protein was detected using a rabbit polyclonal antibody (Abcam, Cambridge, UK), diluted 1∶500. GAPDH was detected with a rabbit polyclonal antibody (Santa Cruz Biotechnology Inc.), diluted 1∶15000. As a secondary antibody, a goat polyclonal to rabbit IgG (HRP) was used (Abcam). The blots were developed using the western blotting detection reagent ECL advance (GE Healthcare).
Reverse transcription quantitative PCR (RT-qPCR)
RT-qPCR was used to determine gene expression levels of selected surface receptors, intracellular signaling proteins and target genes (Table 1), in response to viable and heat-killed P. gingivalis. Briefly, Jurkat T cells were pre-treated with P. gingivalis for 1 h, followed by stimulation with PMA/Ionophore for 24 h. RNA was extracted using GeneJET RNA Purification Kit (Fermentas, Sweden) according to the manufacturer's recommendations. Reverse transcription was performed using iScript cDNA Synthesis Kit (Biorad, Sweden). Thermal cycling conditions for SYBR Green (Maxima SYBR Green/ROX qPCR Master Mix; Fermentas) consisted of a denaturation step at 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Gene expression was analyzed using a 7900 HT real-time PCR instrument (Applied Biosystems, Sweden). The obtained Ct values were normalized against GAPDH. Relative quantification of gene-expression was determined by using the ΔΔCt method. Fold change was generated by using the equation 2ΔΔCt.
Table 1. Primer sequences used for gene expression analysis in T cells.
| Gene | Oligo | Primer sequence | Tm (°C) |
|---|---|---|---|
| GAPDH | Forward | GTCTCCTCTGACTTCAACAGCG | 62.1 |
| Reverse | ACCACCCTGTTGCTGTAGCCAA | 62.1 | |
| IL-2 | Forward | AGAACTCAAACCTCTGGAGGAAG | 60.6 |
| Reverse | GCTGTCTCATCAGCATATTCACAC | 61 | |
| SOCS1 | Forward | TTCGCCCTTAGCGTGAAGATGG | 62.1 |
| Reverse | TAGTGCTCCAGCAGCTCGAAGA | 62.1 | |
| SOCS3 | Forward | CATCTCTGTCGGAAGACCGTCA | 62.1 |
| Reverse | GCATCGTACTGGTCCAGGAACT | 62.1 | |
| NFAT1 | Forward | GATAGTGGGCAACACCAAAGTCC | 62.4 |
| Reverse | TCTCGCCTTTCCGCAGCTCAAT | 62.1 | |
| c-Jun | Forward | CCTTGAAAGCTCAGAACTCGGAG | 62.1 |
| Reverse | TGCTGCGTTAGCATGAGTTGGC | 62.1 | |
| c-Fos | Forward | GCCTCTCTTACTACCACTCACC | 62.1 |
| Reverse | AGATGGCAGTGACCGTGGGAAT | 62.1 | |
| p50 | Forward | GCAGCACTACTTCTTGACCACC | 62.1 |
| Reverse | TCTGCTCCTGAGCATTGACGTC | 62.1 | |
| p65 | Forward | TGAACCGAAACTCTGGCAGCTG | 62.1 |
| Reverse | CATCAGCTTGCGAAAAGGAGCC | 62.1 | |
| STAT5a | Forward | GTTCAGTGTTGGCAGCAATGAGC | 62.1 |
| Reverse | AGCACAGTAGCCGTGGCATTGT | 62.1 | |
| CXCL8 | Forward | GAGAGTGATTGAGAGTGGACCAC | 62.4 |
| Reverse | CACAACCCTCTGCACCCAGTTT | 62.1 | |
| CD28 | Forward | GAGAAGAGCAATGGAACCATTATC | 59.3 |
| Reverse | TAGCAAGCCAGGACTCCACCAA | 62.1 | |
| CD3 | Forward | GCATTTTCGTCCTTGCTGTTGGG | 62.4 |
| Reverse | GGTCATCTTCTCGATCCTTGAGG | 62.4 | |
| PAR2 | Forward | CTCCTCTCTGTCATCTGGTTCC | 62.1 |
| Reverse | TGCACACTGAGGCAGGTCATGA | 62.1 | |
| p38α | Forward | GAGCGTTACCAGAACCTGTCTC | 62.1 |
| Reverse | AGTAACCGCAGTTCTCTGTAGGT | 60.6 | |
| p38β | Forward | CAGAAGGACCTGAGCAGCATCT | 62.1 |
| Reverse | GTACTGGCTGAAGTAGGCGTGG | 64.0 | |
| p38γ | Forward | CAGTTCCTCGTGTACCAGATGC | 62.1 |
| Reverse | CACAGTCTTCGTTCACAGCCAG | 62.1 | |
| p38δ | Forward | GTCATTGGGCTCCTGGATGTCT | 62.1 |
| Reverse | CACCAGGTACTGGATCTTCTCC | 62.1 | |
| ERK1 | Forward | TGGCAAGCACTACCTGGATCAG | 62.1 |
| Reverse | GCAGAGACTGTAGGTAGTTTCGG | 62.4 | |
| ERK2 | Forward | ACACCAACCTCTCGTACATCGG | 62.1 |
| Reverse | TGGCAGTAGGTCTGGTGCTCAA | 62.1 | |
| JNK1 | Forward | GACGCCTTATGTAGTGACTCGC | 62.1 |
| Reverse | TCCTGGAAAGAGGATTTTGTGGC | 60.6 | |
| JNK2 | Forward | TACGTGGTGACACGGTACTACC | 62.1 |
| Reverse | CACAACCTTTCACCAGCTCTCC | 62.1 |
Statistical analysis
Statistical significant differences were determined using two-tailed Student's t-test and one-way ANOVA followed by Bonferroni's multiple comparison test on at least five independent experiments (*P<0.05; **P<0.01; ***P<0.001).
Results
Suppression of cytokine/chemokine accumulation
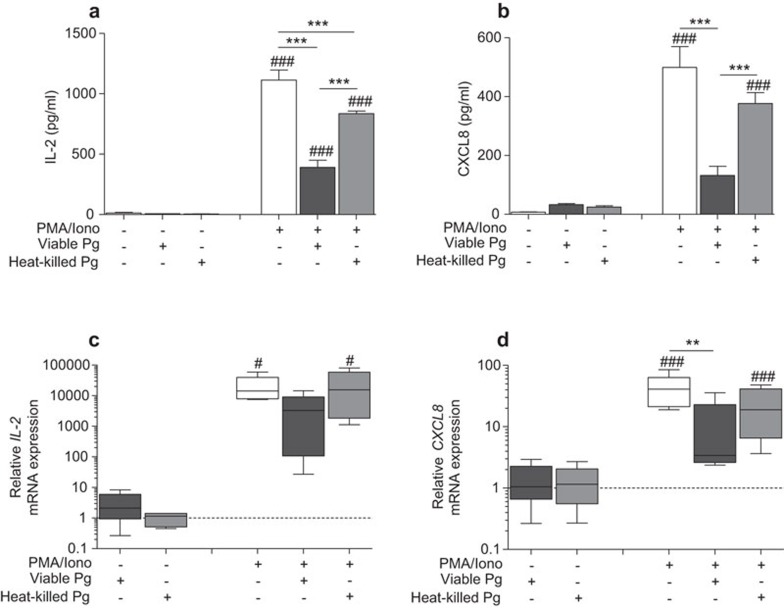
Stable cytokine and chemokine responses are required for optimal activation of T cells and for the establishment of a functional cellular communication system. The ability of Jurkat T cells to express and release IL-2 and CXCL8 was determined in response to P. gingivalis treatment (Figure 1). Treatment with viable or heat-killed P. gingivalis caused a significant decrease in IL-2 protein levels following stimulation with PMA/Ionophore (∼2.8- and ∼1.3-fold, respectively), while basal levels were not affected by either type of treatment (Figure 1a). Furthermore, CXCL8 basal levels were not altered by either viable or heat-killed bacteria (Figure 1b), while PMA/Ionophore-induced CXCL8 release and accumulation was significantly suppressed by viable (∼3.7-fold) but not heat-killed P. gingivalis. This prompted us to determine IL-2 and CXCL8 transcript levels, which were analyzed by RT-qPCR. The results showed a ∼five and ∼fourfold suppression of the PMA/Ionophore-induced IL-2 (Figure 1c) and CXCL8 (Figure 1d) mRNA levels, respectively, by viable P. gingivalis. However, CXCL8 mRNA levels but not IL-2 mRNA levels were significantly suppressed. Basal transcript levels were not affected by the bacteria.
Figure 1.
Viable P. gingivalis suppresses il-2 and cxcl8 gene expression and prevents protein accumulation. Jurkat T cells, at a cell density of 106 cells/ml, were treated with 108 CFU/ml of viable or heat-killed P. gingivalis (MOI: 100) for 1 h prior to stimulation with 50 ng/ml PMA and 1 µg/ml calcium ionophore for 24 h. IL-2 (a) and CXCL8 (b) accumulated levels were determined in cell-culture supernatants by ELISA. Relative gene expression levels of IL-2 (c) and CXCL8 (d) were analyzed by RT-qPCR and presented in box plots showing the median, 25th and 75th percentiles in boxes and the 10th and 90th percentiles as whiskers. Viable P. gingivalis treatment caused a significant inhibition of inflammatory gene expression and protein accumulation. Dotted lines (c, d) indicate basal control levels that were arbitrarily set to 1. Results are presented from at least five independent experiments. Statistically significant differences were determined by one-way ANOVA followed by Bonferroni's multiple comparison test (#/*P<0.05; ##/**P<0.01; ###/***P<0.001, #: significance from the negative control; *: significance from the positive control PMA/Iono). ELISA, enzyme-linked immunosorbent assay; MOI, multiplicity of infection; RT-qPCR, reverse transcription quantitative PCR.
Degradation of CXCL8 by gingipains
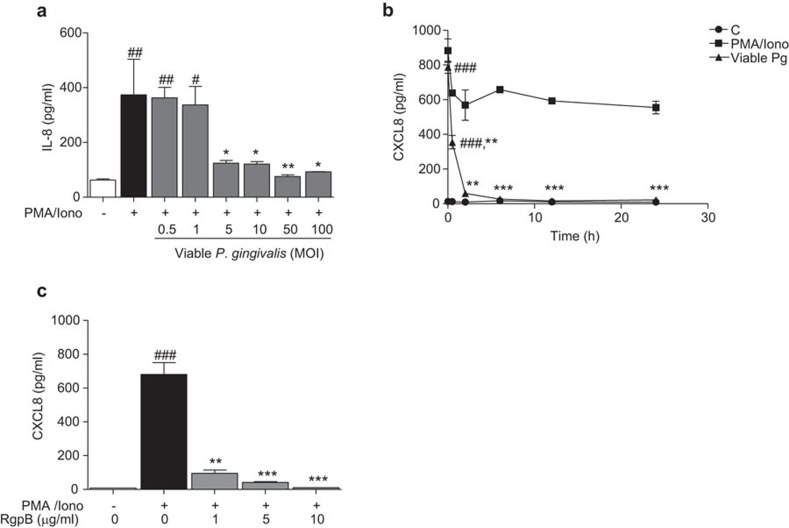
Inhibition of CXCL8 accumulation by viable P. gingivalis was further assessed to determine whether this action was dose and time-dependent. Pre-exposure of Jurkat T-cells to an increasing concentration of viable P. gingivalis, prior to stimulation with PMA/Ionophore, was observed to decrease CXCL8 secretion in a dose-dependent manner (Figure 2a). A final concentration of 5×106 CFU/ml (multiplicity of infection (MOI): 5) was sufficient to reduce CXCL8 levels by threefold. We then aimed to determine whether this inhibition was due to the enzymatic activity of P. gingivalis-derived gingipains. Jurkat T cells were pre-stimulated with PMA/Ionophore for 24 h to induce CXCL8 accumulation, followed by exposure to viable P. gingivalis for the indicated time periods (Figure 2b). Basal levels were constant at all time points, with an average of ∼12 pg/ml. CXCL8 levels in the PMA/Ionophore-stimulated cell suspension increased to ∼880 pg/ml and thereafter decreased to stable levels with an average of ∼650 pg/ml. However, the addition of viable P. gingivalis to PMA/Ionophore-stimulated groups resulted in a drastic drop in CXCL8 levels. These results were further confirmed by using purified RgpB, which suppressed the PMA/Ionophore-induced CXCL8 protein levels in a dose-dependent manner (Figure 2c).
Figure 2.
Inhibition of CXCL8 accumulation is caused by P. gingivalis-derived proteolytic enzymatic activity. Jurkat T cells (106 cells/ml) were pre-treated with increasing concentrations of viable P. gingivalis (MOI: 0.5, 1, 5, 10, 50 and 100) for 1 h followed by stimulation with 50 ng/ml PMA and 1 µg/ml calcium ionophore for 24 h. CXCL8 expression decreased with increasing bacterial concentrations (a). Jurkat T cells were either left untreated or stimulated with 50 ng/ml PMA and 1 µg/ml calcium ionophore for 24 h to induce CXCL8 accumulation, followed by exposure to viable P. gingivalis (viable Pg, 108 CFU/ml, MOI: 100) for the indicated times. Pre-accumulated CXCL8 levels decreased in a time-dependent manner. Statistical analyses show the differences between the PMA/Iono+viable Pg groups to their respective negative (C) and positive control (PMA/Iono) at each specific time point. The positive controls were statistically significant from the negative controls at all time points (b). Purified RgpB cleaved CXCL8 protein in a dose-dependent manner (c). Results are from four independent experiments. (#/*P<0.05; ##/**P<0.01; ###/***P<0.001, #: significance from the negative control; *: significance from the positive control PMA/Iono, one-way ANOVA with Bonferroni's multiple comparison test). MOI, multiplicity of infection.
P. gingivalis targets MAPK signaling in T cells
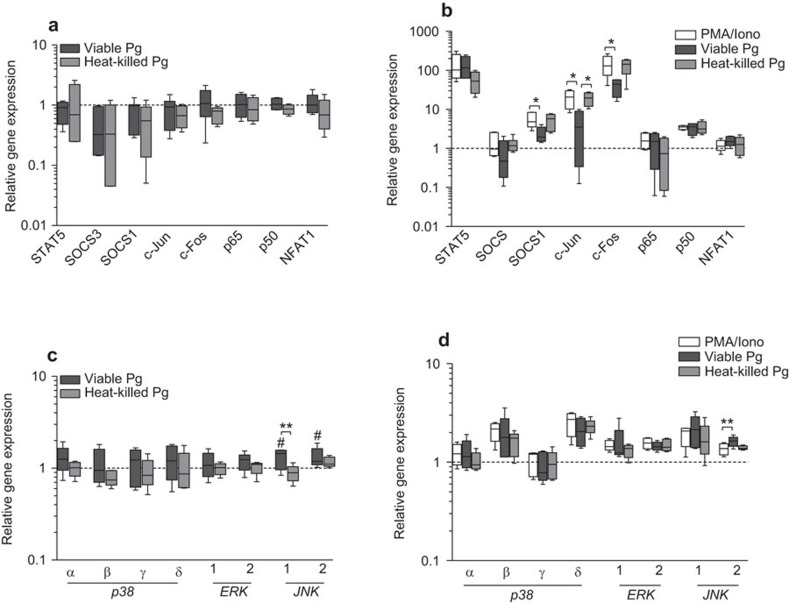
The observed effects of P. gingivalis on IL-2 and CXCL8 gene and protein expression encouraged us to analyze the intracellular mechanisms by which P. gingivalis can alter cellular communication and induction of inflammation. The levels of an array of transcriptional regulators in Jurkat T cells were investigated in response to viable and heat-killed P. gingivalis. Basal gene expression levels were not altered by either viable or heat-killed bacteria (Figure 3a). However, since significant alteration of T cell-derived inflammatory responses by P. gingivalis is mainly associated with the activated state of T cells, we aimed to determine the levels of these genes after stimulation with PMA/Ionophore, prior to treatment with P. gingivalis. All genes, except socs3, p65 and nfat1, were significantly induced by PMA/Ionophore. Treatment with viable bacteria significantly suppressed SOCS1, c-Jun and c-Fos, while heat-killed bacteria did not affect the induced levels of any of the analyzed genes (Figure 3b). Suppression of c-Jun and c-Fos was further investigated by analyzing the upstream MAPKs p38 (α, β, γ, δ), ERK (1, 2) and JNK (1, 2). Viable P. gingivalis treatment of Jurkat T cells elevated JNK1 and JNK2 basal levels (Figure 3c). Furthermore, PMA/Ionophore stimulation caused a significant induction of p38β, p38δ, ERK2, JNK1 and JNK2 (Figure 3d). However, the addition of P. gingivalis did not affect the PMA/Ionophore-induced transcript levels of these MAPKs, except for JNK2, which was elevated in response to viable bacteria (Figure 3d).
Figure 3.
P. gingivalis inhibits MAPK and AP-1 signaling. Jurkat T cells were either treated with viable or heat-killed P. gingivalis (MOI: 100) for 24 h (a, c) or pre-treated with viable or heat-killed P. gingivalis (MOI: 100) for 1 h prior to stimulation with 50 ng/ml PMA and 1 µg/ml calcium ionophore for 24 h (b, d). Selected intracellular signaling molecules were analyzed by RT-qPCR and the results are presented in box plots showing the median, 25th and 75th percentiles in boxes and the 10th and 90th percentiles as whiskers. Basal level gene expression was not altered by the bacteria (a), while SOCS1, as well as the two AP-1 subunits c-Jun and c-Fos, were suppressed in Jurkat T cells in response to viable P. gingivalis and PMA/Ionophore stimulation (b). Suppression of c-Jun and c-Fos was further assessed by analyzing the expression levels of p38 (α, β, γ, δ), ERK (1, 2) and JNK (1, 2) (c, d). Viable P. gingivalis elevated JNK1 and JNK2 basal levels (c). PMA/Ionophore stimulation significantly induced p38δ, ERK2 and JNK2 gene expression and addition of either viable or heat-killed P. gingivalis did not affect any of the analyzed MAPKs (d). Results are presented from at least five independent experiments. Statistically significant differences were determined by one-way ANOVA followed by Bonferroni's multiple comparison test (#/*P<0.05; ##/**P<0.01). Dotted lines indicate control levels that were arbitrarily set to 1. AP-1, activator protein-1; MAPK, mitogen-activated protein kinase; MOI, multiplicity of infection; RT-qPCR, reverse transcription quantitative PCR.
MAPK signaling pathways are the main regulators of cytokine expression in T cells
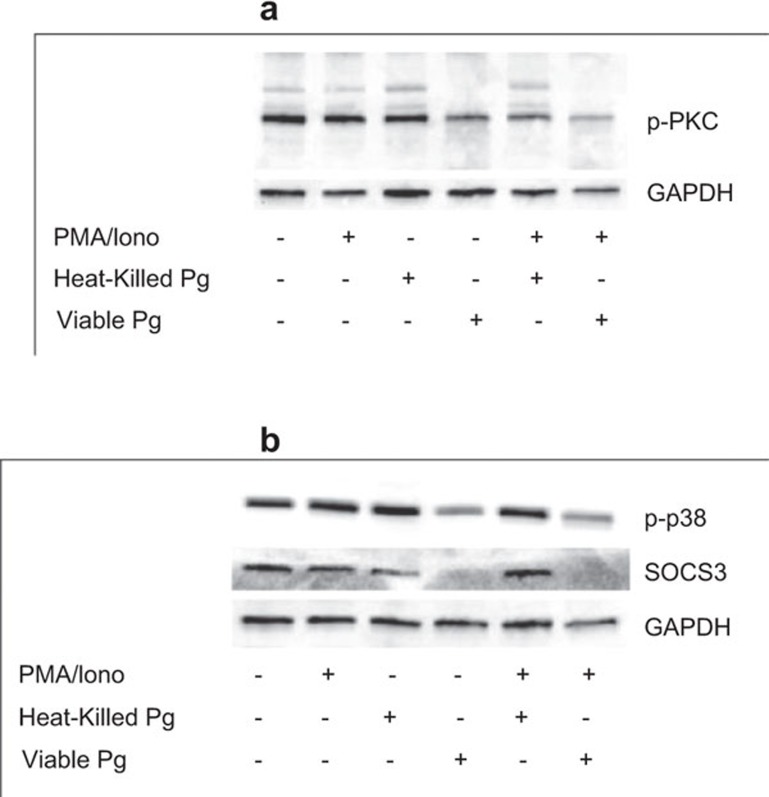
Activation of MAPK signaling pathways has previously been shown to be mediated by the upstream kinase PKC. The observed suppression of c-jun and c-fos gene expression may therefore be due to P. gingivalis-dependent inhibition of PKC activation. This hypothesis was tested by determining the levels of phosphorylated PKC following treatment of Jurkat T cells with P. gingivalis. The levels of phosphorylated PKC decreased in response to viable, but not heat-killed P. gingivalis, with and without PMA/Ionophore stimulation (Figure 4a). As a consequence, phosphorylation of p38, which is one of the downstream targets of PKC, was also suppressed by viable bacteria (Figure 4b). Furthermore, the levels of SOCS3 were markedly reduced by viable P. gingivalis, both with and without stimulation (Figure 4b).
Figure 4.
Inhibition of MAPK signaling is due to suppression of PKC and p38 phosphorylation. Jurkat T cells were treated with viable or heat-killed P. gingivalis (MOI: 100) for 24 h or pre-treated with viable or heat-killed P. gingivalis (MOI: 100) for 1 h prior to stimulation with 50 ng/ml PMA and 1 µg/ml calcium ionophore for 24 h. Phospho-PKC (a) and phospho-p38 and SOCS3 (b) were suppressed by viable, but not heat-killed, P. gingivalis. Representative results from three independent experiments. GAPDH was used as a loading control. MAPK, mitogen-activated protein kinase; MOI, multiplicity of infection; PKC, protein kinase C; SOCS, suppressor of cytokine signaling.
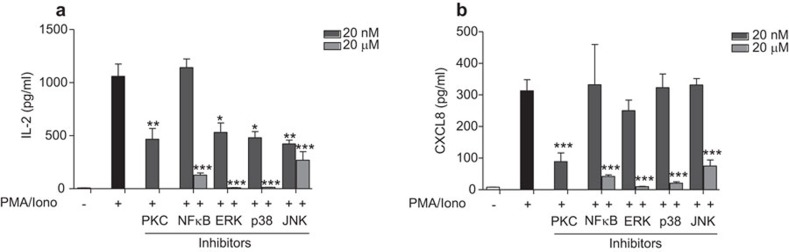
The results indicate that P. gingivalis is able to suppress IL-2 and CXCL8 gene expression and protein accumulation by targeting MAPK signaling, through PKC. In order to verify these findings, we used specific inhibitors of PKC, NF-κB and the three main MAPK pathways, namely ERK, p38 and JNK, to further determine the mechanism of cytokine regulation. The inhibitor of PKC, with a final concentration of 20 nM, resulted in a ∼55% reduction in IL-2 secretion (Figure 5a) and ∼70% reduction in CXCL8 secretion (Figure 5b). However, since PKC is involved in the regulation of both NF-κB and MAPK signaling, we aimed to determine the contribution of each pathway to cytokine and chemokine expression. The inhibitor of the transcription factor NF-κB, with a final concentration of 20 nM, did not alter IL-2 or CXCL8 levels, while a final concentration of 20 µM significantly reduced both (Figure 5). Furthermore, inhibition of the three MAP kinases, ERK, p38 and JNK, all reduced IL-2 levels in a dose-dependent manner (Figure 5a). Suppression of ERK and p38 with 20 µM of the inhibitors decreased IL-2 expression to basal levels, whereas JNK was less effective. Furthermore, CXCL8 expression was also observed to be more potently suppressed following inhibition of ERK and p38 than JNK (Figure 5b). These results indicate that MAPK signaling, in particular ERK and p38, are the main regulators of IL-2 and CXCL8 expression.
Figure 5.
IL-2 and CXCL8 expression is mainly dependent on MAPK signaling. Regulation of cytokine and chemokine expression was assessed by using inhibitors for NF-κB, PKC, ERK, p38 and JNK. Jurkat T cells were incubated with the indicated concentrations of inhibitors for 2 h prior to stimulation with 50 ng/ml PMA and 1 µg/ml calcium ionophore for 24 h. MAPK signaling, in particular ERK and p38, were found to be important regulators of IL-2 (a) and CXCL8 (b) expression. Results are presented from three independent experiments. Statistically significant differences from the positive control PMA/Iono were determined by Student's t-test (*P<0.05; **P<0.01; ***P<0.001). MAPK, mitogen-activated protein kinase; PKC, protein kinase C.
Surface receptor expression in response to P. gingivalis
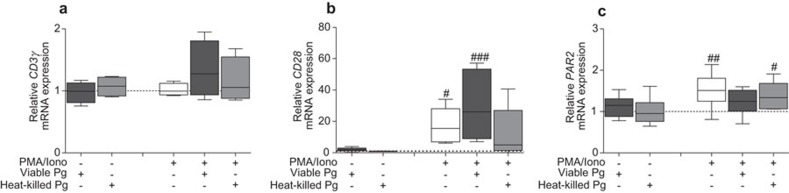
In addition to altering intracellular signaling, P. gingivalis has, previously been shown to affect the expression and availability of surface receptors on T cells. The inhibition of intracellular signaling may therefore be due to reduced antigen recognition. We aimed to determine the effects of P. gingivalis on CD3γ, CD28 and PAR2 gene expression. Gene expression levels of these surface receptors were not affected by P. gingivalis, neither at the basal levels nor after stimulation with PMA/Ionophore (Figure 6). However, CD28 and PAR2, but not CD3γ, transcript levels were significantly elevated following stimulation with PMA/Ionophore.
Figure 6.
Analysis of receptor expression levels in response to P. gingivalis. Jurkat T cells (106 cells/ml) were treated with 108 CFU/ml of viable or heat-killed P. gingivalis (MOI: 100) for 1 h prior to stimulation with 50 ng/ml PMA and 1 µg/ml calcium ionophore for 24 h. Gene expression for CD3γ (a), CD28 (b) and PAR2 (c) was analyzed by RT-qPCR and the results are presented in box plots showing the median, 25th and 75th percentiles in boxes and the 10th and 90th percentiles as whiskers. The receptors CD28 and PAR2 were shown to be inducible by PMA/Iono. Results are presented from at least five independent experiments. Dotted lines indicate basal control levels that were arbitrarily set to 1. Statistically significant differences were determined one-way ANOVA followed by Bonferroni's multiple comparison test (#P<0.05; ##P<0.01; ###P<0.001, #: significance from the negative control). MOI, multiplicity of infection; RT-qPCR, reverse transcription quantitative PCR.
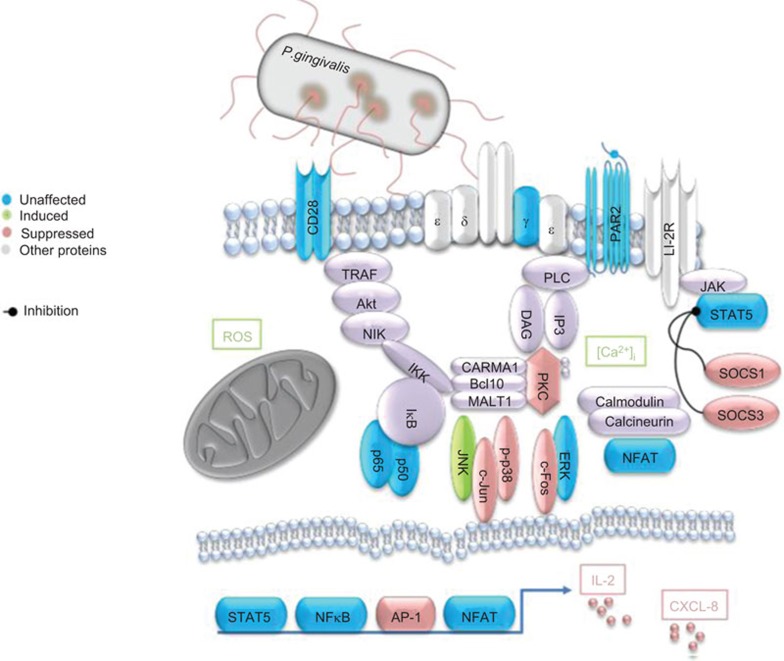
The analyzed genes and proteins, and their interplay with other signaling molecules, are summarized in Figure 7. Although several signaling complexes remained elevated and not affected by P. gingivalis, it is evident that the effects on these pathways are complex and that the primary target of P. gingivalis is MAPK signaling.
Figure 7.
Review of affected signaling pathways in T cells in response to P. gingivalis. The figure summarizes the effects of P. gingivalis on PMA/Ionophore-induced signaling in T cells. MAPK signaling is targeted by P. gingivalis, and as a consequence, gene expression of inflammatory mediators is also affected, including IL-2 and CXCL8. P. gingivalis-induced ROS and [Ca2+]i has previously been reported by our group.15 MAPK, mitogen-activated protein kinase; ROS, reactive oxygen species.
Discussion
Periodontal pathogenesis involves diverse immunoregulatory effects, and analysis of the pathogen–host cell interactions would improve our understanding of these effects and enable characterization of the involved signaling pathways and development of alternative treatment strategies. T cells are recruited to the site of infection in the periodontal pocket at an early stage. However, their contribution and subsequent immunoregulatory mechanisms after encountering periodontal pathogens, such as P. gingivalis, have not fully elucidated.
Membrane bound and secreted gingipains from P. gingivalis have diverse effects on host cell-derived inflammatory mediators, including degradation of TNF,13 IL-4 and IL-5.14 We have previously reported that the T-cell-derived cytokine IL-2 is targeted for degradation by gingipains in Jurkat T cells and isolated lymphocytes.15 Here we report that P. gingivalis is able to suppress CXCL8 gene expression and protein accumulation, due to degradation by gingipains. Cytokine and chemokine expression in T cells has previously been shown to be strictly regulated by MAPK signaling and the transcription factor AP-1. Analysis of IL-2 promoter regulation revealed the importance of AP-1, but not NF-κB, in the induction of IL-2 transcription. Mutation of the AP-1 site markedly reduced IL-2 expression, while mutation of the NF-κB site did not affect IL-2 release.16
Furthermore, pre-accumulated CXCL8 levels rapidly decreased in response to viable P. gingivalis. The ability of this pathogen to alter CXCL8 protein levels was shown to be dependent on the proteolytic activity of gingipains. The diverse enzymatic activity of gingipains has previously been reported to target IL-6,17 TNF13 and the T cell-derived cytokines IL-4 and IL-5.14 However, P. gingivalis affects different host inflammatory markers. Oda and colleagues18 showed that stimulation of peripheral blood mononuclear cells with outer membrane proteins from P. gingivalis resulted in a significant elevation of IL-17 mRNA and protein expression. The effects of P. gingivalis on T cells could, as a consequence, impair T cell-mediated chemotaxis and activation of the antibody-based adaptive immunity.
Characterization of intracellular signaling in T cells in response to different periodontal pathogens is important for obtaining sufficient knowledge about their role in systemic diseases, such as periodontitis and atherosclerosis. P. gingivalis was shown to decrease SOCS1 and SOCS3 expression, while STAT5a was not affected. Analysis of the regulatory role of SOCS1 in T cells has been reported to strictly control the activated state of STAT5A/B in response to IL-2.19 T-cells deficient in SOCS1 failed to control the elevated levels of phosphorylated STAT5A/B and the cells displayed increased proliferation, which was antigen-independent. The apparent necessity of regulatory mechanisms for controlling inflammation is important in order to prevent development of chronic inflammatory conditions. Furthermore, SOCS3 has been shown, in a similar manner as SOCS1, to act as a negative regulator of cytokine signaling in T cells. This negative feedback mechanism of SOCS3 is mediated by suppressing STAT5 phosphorylation, through association and prevention of Jak1 phosphorylation.20 STAT5a expression levels remained elevated and were not affected by P. gingivalis in the present study, which may be due to suppression of these negative regulatory mechanisms of SOCS. The action of SOCS proteins has been mainly associated with STAT signaling; however, other mechanisms have been proposed for a cross-talk between SOCS and NF-κB pathway. Ryo and colleagues showed that SOCS1 binds to and facilitates p65 degradation.21 This indicates that P. gingivalis is able to eliminate the negative feedback action of SOCS proteins and that these proteins are important in controlling cellular activity and proliferation through STAT5A/B and NF-κB signaling. The ability of P. gingivalis to suppress SOCS3 has recently been reported to be due to upregulation of miRNA-203, which inhibited SOCS3 and promoted STAT signaling.12 Targeting miRNAs may therefore be a beneficial treatment strategy for diseases such as periodontitis and atherosclerosis, following an established P. gingivalis infection. However, DNA from P. gingivalis has previously been shown to induce socs1 gene expression.22 The immunosuppressive effects of P. gingivalis are widely accepted, however, further investigations are needed to clarify the effects of different P. gingivalis-derived compounds on the host immune response and to unravel the complexity of these regulatory intracellular mechanisms.
We have shown that P. gingivalis suppresses PKC and p38 activity, and c-Jun and c-Fos expression. These effects were primarily observed with viable, but not heat-killed, P. gingivalis. This may be due to loss of bacterial virulence, including fimbriae and enzymatic activity of proteinases, caused by denaturation following incubation of the bacteria for 1 h at 70 °C. Viable, but not heat-killed, P. gingivalis has previously been shown to induce apoptosis in gingival epithelial cells,23 indicating the importance of these virulence factors in P. gingivalis-mediated pathogenesis. Furthermore, the subunits c-Jun and c-Fos are members of the AP-1 family of transcription factors, which play a central role in cellular proliferation and induction of inflammatory responses.6 The activity of these transcriptional regulators is controlled by the MAPKs JNK, p38 and ERK. Analysis of different the MAPKs in response to P. gingivalis treatment showed increased JNK gene expression, without affecting other MAPKs. Phosphorylation of p38 in HUVECs has previously been shown to correspond to early activation (30 min), followed by a drastic suppression (>1 h) in response to P. gingivalis ATCC 33277.24 However, stimulation of macrophages with proteinases, for 90 min, activated p38α, JNK2 and ERK1/2, which in turn induced TNF and CXCL8 secretion.25 Dommisch and colleagues26 showed that induction of MIP-3α/CCL20 by P. gingivalis is mediated by PLC, p38 and NF-κB. MAPK signaling pathways appear to be differentially regulated by P. gingivalis, and its derived virulence factors, in different types of cells.
Gene regulation by AP-1 is mediated by a wide range of stimuli, including cytokines, growth factors, cellular stress and bacterial infections.8 Reiera-Sans and Behrens27 investigated the role of c-Jun in thymic lymphopoiesis in mice and showed that deletion of c-Jun caused an arrest in αβ T-cell differentiation, while γδ T-cell generation was promoted. Furthermore, Choi and colleagues10 showed that treatment of human umbilical vein endothelial cells with P. gingivalis resulted in an upregulation of MCP-1 levels, which was mediated by AP-1 and NF-κB. However, MCP-1 levels decreased with increasing bacterial concentrations. Both AP-1 and NF-κB corresponded to early activation (<1 h) in response to P. gingivalis, and their activity was thereafter drastically reduced. Activation of AP-1 and NF-κB, and subsequent cytokine expression, requires PKC, which is rapidly recruited upon recognition of an antigen by T-cell receptor.28 PKC-dependent activation of NF-κB has been shown to be strictly controlled by a protein complex consisting of MALT1, Bcl10 and CARMA1. Deletion in any of these proteins impairs antigen receptor dependent activation of NF-κB.29,30 PKC depletion has been demonstrated to almost revoke IL-2 expression.16 We found that inhibition of PKC, ERK and p38 were more potent at suppressing IL-2 and CXCL8 secretion in P. gingivalis-treated T cells, compared to inhibition of NF-κB or JNK. These observations indicate that PKC and the transcriptional regulators c-Jun and c-Fos (AP-1) play a pivotal role in the induction of inflammatory responses in T cells.
Analysis of surface receptor expression in response to P. gingivalis revealed an increase in CD28 basal levels, while CD3γ and PAR2 were not affected. Determination of the levels of surface receptors following challenge of T cells with gingipains has been shown to increase CD28, but not CD3 expression.31 However, others have shown that CD3ζ chain was significantly downregulated by P. gingivalis.32,33 Recognition of an antigen, and subsequent recruitment and assembly of signaling molecules, is an important step towards attaining optimal cellular responses. Synergistic signals from the costimulatory receptor CD28 are required for optimal T-cell activation, and their absence results in cellular anergy.34 Furthermore, Kitamura et al.35 showed that proteases released from P. gingivalis cleaved CD4 and CD8 on T cells, and thus impaired downstream signal transduction and T-cell function. Furthermore, PAR2 is expressed on several types of cells, including fibroblasts, epithelial cells and T cells.36,37 P. gingivalis-derived gingipains have been reported to cleave, and thus activate PAR2,38 suggesting a role for PAR2 in the induction of chronic inflammation in periodontitis.
It is evident that P. gingivalis specifically targets PKC and MAPK signaling, and as a consequence cytokine and chemokine expression is altered. The signaling protein PKC and the transcription factor AP-1 have previously been described as important regulators of cytokine expression in T cells, including IL-2. Suppression of c-Jun and c-Fos, and as a consequence suppression of cytokine and chemokine expression, appears to be mediated by P. gingivalis-dependent prevention of MAPK activity and thus phosphorylation, rather than suppression of MAPK gene expression. Unraveling these pathways will provide a better understanding of the mechanisms used by P. gingivalis to colonize, proliferate and cause disease. This will enable identification of new strategies targeting and restricting the ability of this pathogen to proliferate, invade and survive within host cells. However, more research is needed to determine the precise mechanism(s) by which P. gingivalis is able to selectively inhibit MAPK signaling, without altering other pathways such as NF-κB.
Acknowledgments
This work was supported by grants from the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Fund for Research without Animal Experiments, the Swedish Heart and Lung Association, the Foundation of Olle Engkvist and the Foundation of Längmanska Kulturfonden.
The authors declare no conflict of interest.
References
- Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. 1999;138 5 Pt 2:S534–S536. doi: 10.1016/s0002-8703(99)70294-2. [DOI] [PubMed] [Google Scholar]
- Brodala N, Merricks EP, Bellinger DA, Damrongsri D, Offenbacher S, Beck J, et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2005;25:1446–1451. doi: 10.1161/01.ATV.0000167525.69400.9c. [DOI] [PubMed] [Google Scholar]
- Holmstrom TH, Schmitz I, Soderstrom TS, Poukkula M, Johnson VL, Chow SC, et al. MAPK/ERK signaling in activated T cells inhibits CD95/Fas-mediated apoptosis downstream of DISC assembly. EMBO J. 2000;19:5418–5428. doi: 10.1093/emboj/19.20.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Salojin KV, Gao JX, Cameron MJ, Bergerot I, Delovitch TL. p38 mitogen-activated protein kinase mediates signal integration of TCR/CD28 costimulation in primary murine T cells. J Immunol. 1999;162:3819–3829. [PubMed] [Google Scholar]
- Wilson DJ, Fortner KA, Lynch DH, Mattingly RR, Macara IG, Posada JA, et al. JNK, but not MAPK, activation is associated with Fas-mediated apoptosis in human T cells. Eur J Immunol. 1996;26:989–894. doi: 10.1002/eji.1830260505. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Khalaf H, Jass J, Olsson PE. Differential cytokine regulation by NF-kappaB and AP-1 in Jurkat T-cells. BMC Immunol. 2010;11:26. doi: 10.1186/1471-2172-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117 Pt 25:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- Gemmell E, Drysdale KE, Seymour GJ. Gene expression in splenic CD4 and CD8 cells from BALB/c mice immunized with Porphyromonas gingivalis. . J Periodontol. 2006;77 4:622–633. doi: 10.1902/jop.2006.050211. [DOI] [PubMed] [Google Scholar]
- Choi EK, Park SA, Oh WM, Kang HC, Kuramitsu HK, Kim BG, et al. Mechanisms of Porphyromonas gingivalis-induced monocyte chemoattractant protein-1 expression in endothelial cells. FEMS Immunol Med Microbiol. 2005;44:51–58. doi: 10.1016/j.femsim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Sporri B, Kovanen PE, Sasaki A, Yoshimura A, Leonard WJ. JAB/SOCS1/SSI-1 is an interleukin-2-induced inhibitor of IL-2 signaling. Blood. 2001;97:221–226. doi: 10.1182/blood.v97.1.221. [DOI] [PubMed] [Google Scholar]
- Moffatt CE, Lamont RJ. Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect Immun. 2011;79:2632–2637. doi: 10.1128/IAI.00082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins CC, Platt K, Potempa J, Travis J. Inactivation of tumor necrosis factor-alpha by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis. Implications of immune evasion. J Biol Chem. 1998;273:6611–6614. doi: 10.1074/jbc.273.12.6611. [DOI] [PubMed] [Google Scholar]
- Tam V, O'Brien-Simpson NM, Chen YY, Sanderson CJ, Kinnear B, Reynolds EC. The RgpA–Kgp proteinase–adhesin complexes of Porphyromonas gingivalis Inactivate the Th2 cytokines interleukin-4 and interleukin-5. Infect Immun. 2009;77:1451–1458. doi: 10.1128/IAI.01377-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf H, Bengtsson T. Altered T-cell responses by the periodontal pathogen Porphyromonas gingivalis. PLoS ONE. 2012;7:e45192. doi: 10.1371/journal.pone.0045192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J, Valge-Archer VE, Sinskey AJ, Rao A. The AP-1 site at −150 bp, but not the NF-kappa B site, is likely to represent the major target of protein kinase C in the interleukin 2 promoter. J Exp Med. 1992;175:853–862. doi: 10.1084/jem.175.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banbula A, Bugno M, Kuster A, Heinrich PC, Travis J, Potempa J. Rapid and efficient inactivation of IL-6 gingipains, lysine- and arginine-specific proteinases from Porphyromonas gingivalis. . Biochem Biophys Res Commun. 1999;261:598–602. doi: 10.1006/bbrc.1999.1075. [DOI] [PubMed] [Google Scholar]
- Oda T, Yoshie H, Yamazaki K. Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL-17 but not receptor activator of NF-kappaB ligand in vitro. Oral Microbiol Immunol. 2003;18:30–36. doi: 10.1034/j.1399-302x.2003.180105.x. [DOI] [PubMed] [Google Scholar]
- Cornish AL, Chong MM, Davey GM, Darwiche R, Nicola NA, Hilton DJ, et al. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J Biol Chem. 2003;278:22755–22761. doi: 10.1074/jbc.M303021200. [DOI] [PubMed] [Google Scholar]
- Cohney SJ, Sanden D, Cacalano NA, Yoshimura A, Mui A, Migone TS, et al. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol Cell Biol. 1999;19:4980–4988. doi: 10.1128/mcb.19.7.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G, et al. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell. 2003;12:1413–1426. doi: 10.1016/s1097-2765(03)00490-8. [DOI] [PubMed] [Google Scholar]
- Taubman MA, Han X, Larosa KB, Socransky SS, Smith DJ. Periodontal bacterial DNA suppresses the immune response to mutans streptococcal glucosyltransferase. Infect Immun. 2007;75:4088–4096. doi: 10.1128/IAI.00623-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulou PG, Galicia JC, Benakanakere MR, Garcia CA, Potempa J, Kinane DF. Porphyromonas gingivalis induce apoptosis in human gingival epithelial cells through a gingipain-dependent mechanism. BMC Microbiol. 2009;9:107. doi: 10.1186/1471-2180-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zheng H, Zhao J, Lin L, Li C, Liu J, et al. Porphorymonas gingivalis induces intracellular adhesion molecule-1 expression in endothelial cells through the nuclear factor-kappaB pathway, but not through the p38 MAPK pathway. J Periodontal Res. 2011;46:31–38. doi: 10.1111/j.1600-0765.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- Grenier D, Tanabe S. Porphyromonas gingivalis gingipains trigger a proinflammatory response in human monocyte-derived macrophages through the p38alpha mitogen-activated protein kinase signal transduction pathway. Toxins (Basel) 2010;2:341–352. doi: 10.3390/toxins2030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommisch H, Chung WO, Jepsen S, Hacker BM, Dale BA. Phospholipase C, p38/MAPK, and NF-kappaB-mediated induction of MIP-3alpha/CCL20 by Porphyromonas gingivalis. Innate Immun. 2010;16:226–234. doi: 10.1177/1753425909339237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera-Sans L, Behrens A. Regulation of alphabeta/gammadelta T cell development by the activator protein 1 transcription factor c-Jun. J Immunol. 2007;178:5690–5700. doi: 10.4049/jimmunol.178.9.5690. [DOI] [PubMed] [Google Scholar]
- Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- Narayan P, Holt B, Tosti R, Kane LP. CARMA1 is required for Akt-mediated NF-kappaB activation in T cells. Mol Cell Biol. 2006;26:2327–2336. doi: 10.1128/MCB.26.6.2327-2336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt E, Wegener E, Heissmeyer V, Rao A, Krappmann D. Degradation of Bcl10 induced by T-cell activation negatively regulates NF-kappa B signaling. Mol Cell Biol. 2004;24:3860–3873. doi: 10.1128/MCB.24.9.3860-3873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun PL, Decarlo AA, Chapple CC, Collyer CA, Hunter N. Binding of Porphyromonas gingivalis gingipains to human CD4+ T cells preferentially down-regulates surface CD2 and CD4 with little affect on co-stimulatory molecule expression. Microb Pathog. 2005;38:85–96. doi: 10.1016/j.micpath.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bronstein-Sitton N, Cohen-Daniel L, Vaknin I, Ezernitchi AV, Leshem B, Halabi A, et al. Sustained exposure to bacterial antigen induces interferon-gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nat Immunol. 2003;4:957–64. doi: 10.1038/ni975. [DOI] [PubMed] [Google Scholar]
- Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Matono S, Aida Y, Hirofuji T, Maeda K. Gingipains in the culture supernatant of Porphyromonas gingivalis cleave CD4 and CD8 on human T cells. J Periodontal Res. 2002;37:464–468. doi: 10.1034/j.1600-0765.2002.01364.x. [DOI] [PubMed] [Google Scholar]
- Hansen KK, Saifeddine M, Hollenberg MD. Tethered ligand-derived peptides of proteinase-activated receptor 3 (PAR3) activate PAR1 and PAR2 in Jurkat T cells. Immunology. 2004;112:183–190. doi: 10.1111/j.1365-2567.2004.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzhausen M, Spolidorio LC, Vergnolle N. Role of protease-activated receptor-2 in inflammation, and its possible implications as a putative mediator of periodontitis. Mem Inst Oswaldo Cruz. 2005;100 Suppl 1:177–180. doi: 10.1590/s0074-02762005000900030. [DOI] [PubMed] [Google Scholar]
- Lourbakos A, Yuan YP, Jenkins AL, Travis J, Andrade-Gordon P, Santulli R, et al. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenicity. Blood. 2001;97:3790–3797. doi: 10.1182/blood.v97.12.3790. [DOI] [PubMed] [Google Scholar]