Abstract
T-cell regulation by CD52-expressing CD4 T cells appears to operate by two different and possibly synergistic mechanisms. The first is by its release from the cell surface of CD4 T cells that express high levels of CD52 that then binds to the inhibitory sialic acid-binding immunoglobulin-like lectins-10 (Siglec-10) receptor to attenuate effector T-cell activation by impairing phosphorylation of T-cell receptor associated lck and zap-70. The second mechanism appears to be by crosslinkage of the CD52 molecules by an as yet unidentified endogenous ligand that is mimicked by a bivalent anti-CD52 antibody that results in their expansion.
The immune system is designed to protect its host from invading pathogens and yet remain non-reactive to self. Immunological homeostasis is maintained by purging self-reactive lymphocytes by clonal deletion coupled with a regulatory population of lymphocytes that suppress self-reactive lymphocytes that have ‘escaped' clonal deletion. Disruption of the delicate balance of regulatory and effector lymphocytes, is thought to be a major contributor to the development of autoimmune disease. Pioneering studies of Shimon Sakaguchi have led to the firm establishment of CD25+Foxp3 expressing CD4 T lymphocytes as fundamental for immune homeostasis as the loss of this population results in intractable autoimmune disease in man and mouse.1 The transcription factor Foxp3 plays a key role in their development. These cells are a distinct CD4 T-cell lineage that arise in the thymus, but can also be generated in the periphery.
But are there other players in maintaining T-cell homeostasis? The answer appears in the affirmative, and includes players such as IL-10-secreting Tr1 and TGF-β-secreting Th3. cells. Absence of surface markers limited the usefulness of these other regulators. However, the recent report that CD49b and lymphocyte activation gene-3 are highly and stably coexpressed by human and mouse Tr1 has provided an approach for studies of IL-10-secreting Tr1 cells.2
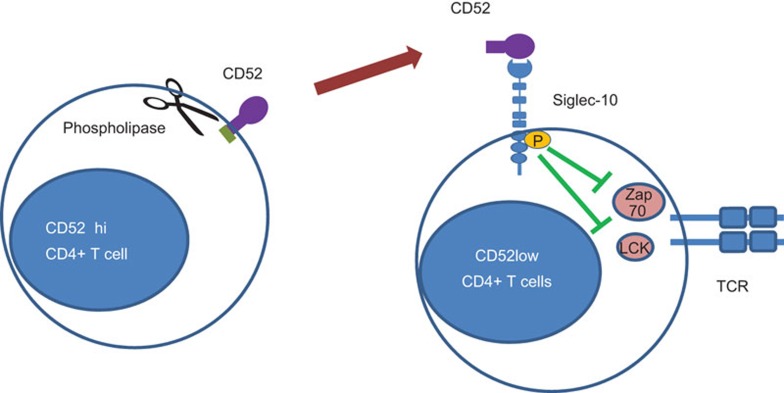
Yet another T-cell regulator has recently gained prominence by a recent report in Nature Immunology of CD4 T cells expressing high levels of CD52.3 CD52 is a short peptide of 12 amino acids in humans linked at its C-terminus to a glycosylphosphatidylinositol (GPI) anchor. Its single N-glycosylation site is occupied by large sialylated polylactossamine on a tetra-antennary fucosylated mannose core.4 Suppression appears to be mediated by soluble CD52, released from its GPI anchor by phospholipase C, which then binds to inhibitory sialic acid-binding immunoglobulin-like lectins-10 (Siglec-10) receptor to inhibit T-cell activation by impairing phosphorylation of T-cell receptor associated kinases Lck and Zap70 (Figure 1). Siglec-10 belongs to a major subfamily of Ig-like lectins that mediate sialic acid recognition by their terminal Ig like domains5 to regulate innate and adaptive immune responses.6 The data suggest that sialic acid structures of CD52 binds to Ig-like domain of Siglec-10 because suppression was lost by cleavage of N-glycans from CD52-Fc by peptide N-glycosidase or by removal of sialic acid residues by neuraminidase. Suppression was also blocked by antibody to the extracellular domain of Siglec-10 and by soluble Siglec-10-Fc. Given that Siglec-10 contain two immunoreceptor tyrosine-based inhibition motifs, it seems likely that recruitment of SHP phosphatases by these motifs impaired phosphorylation of Lck and Zap-70. The observation of a lower frequency and impaired function of these regulators in human type 1 diabetes coupled with the aggravated diabetes following transfer of lymphocytes deleted of this population suggest that these may protect from the development of autoimmune disease. While a loss of function of CD52 was demonstrated in the mouse model of type 1 diabetes, a gain of function with augmented CD52 ameliorating disease was not explored. Also endogenous molecular mechanisms that activate cleavage of the GPI anchor of CD52 to release it in soluble form remains unknown.
Figure 1.
T-cell regulation by soluble CD52 binding to Siglec-10. CD52 release from its GPI anchor by phospholipase binds via its terminal sialic acid domain to the terminal Ig domain of Siglec-10. Phosphatase activation by the ITIM motifs of Siglec-10 impairs phosphorylation of T-cell receptor associated lck and zap70. GPI, glycosylphosphatidylinositol; ITIM, immunoreceptor tyrosine-based inhibition motif; Siglec-10, sialic acid-binding immunoglobulin-like lectins-10.
Siglec-10 was cloned from a human dendritic cell library in 20017 and was also identified by database mining of an asthmatic eosinophil EST library.8 It is expressed in peripheral blood mononuclear cells including monocytes, dendritic cells and B lymphocytes. Given its broad immune distribution, suppression by CD52 released from CD52-expressing regulatory CD4 T cells may not be restricted to T cells only, but may also extend to a broader population of peripheral blood mononuclear cells that include dendritic cells, monocytes and B lymphocytes. As human Siglec-10 also binds to vascular adhesion protein-1 to act as its substrate and to mediate lymphocyte binding to the endothelium,9 binding of CD52 to siglec-10 could also potentially modify the inflammatory microenvironment.
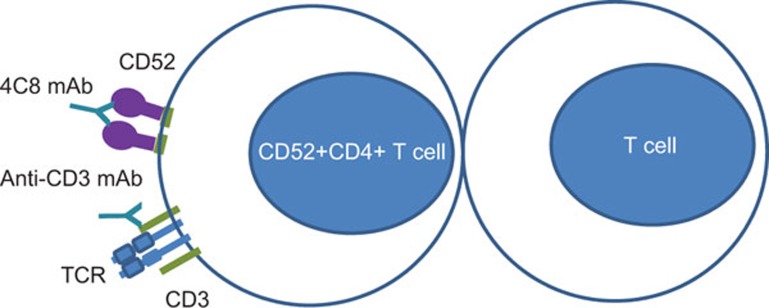
The CD52 story may be even more complex than revealed by the above studies. Earlier reports suggest that CD52 recognized by monoclonal antibody 4C8 can also act as a novel costimulatory molecule for the induction of CD4 regulatory T cells .The 4C8 IgG3 monoclonal antibody was first reported for its capacity to inhibit the post-adhesive transendothelial migration of T cells through human endothelial cell monolayers.10 In a subsequent study, CD4 T cells expanded by stimulation with anti-CD3 and costimulation with anti-4C8 expressed high levels of IL-2 that suppressed in a contact-dependent manner.11 The suppressor cells were shown to develop from CD4+CD25−CD45RO+ precursor cells. These anti-CD52 induced regulatory T cells suppressed proliferation of CD4 and CD8 T cells provided with polyclonal or allogeneic stimulation.12 Co-injection of regulatory T cells expanded in culture by IL-2 and anti-CD52 suppressed lethal graft vs. host disease in severe combined immunodeficiency disorder recipients caused by human peripheral blood mononuclear cells. As 4C8 is an IgG3 monoclonal antibody, it probably acts as a costimulator by crosslinking CD52 molecules on the cell surface, to expand CD4 T cells that suppresses in a contact-dependent manner (Figure 2). The epitope for this antibody as with the CAMPATH antibody13 likely lies on an amino-acid sequence proximal to the terminal sialic acids that bind to the terminal Ig domain of Siglec-10. A natural ligand that crosslinks CD52 remains unknown. Nonetheless the situation here is analogous to the costimulatory expansion of CD4+Foxp3+ regulatory T cells by monoclonal antibody to the tumor-necrosis factor (TNF) receptor superfamily member 25 that is constitutively and highly expressed by these regulatory T cells.14 The expansion, dependent on T-cell receptor engagement with MHC class II antigen, prevents allergic lung inflammation. The TNF receptor superfamily member 25 ligand in this instance has been identified as sTLIA, the constitutive expression of which in transgenic mice expands these regulatory T cells.15 Given that TNF receptor superfamily member 25 is also expressed on conventional CD4 T cells and there are multiple isoforms of this molecule, it has been suggested that the antibody that costimulates regulatory T-cell expansion may bind to an epitope preferentially expressed on regulatory T cells.
Figure 2.
Expansion of CD52 expressing T cells by antibody crosslinkage and TCR activation by anti-CD3 antibody. TCR, T-cell receptor.
CD52-expressing CD4 T cells differ from CD25+Foxp3+ regulatory CD4 T cells in a number of important aspects. It differs in its distribution in that CD52 is also found in sperm as its major maturation-associated membrane protein.16 It is also more widely distributed in the immune system as it is expressed by all lymphocytes, monocytes and dendritic cells.17,18 It is also expressed by eosinophils but not by neutrophils.18 Despite its expression in lymphocytes and sperm and the report that the N-glycans of a CD52 isoform is associated with human infertility,19 CD52 gene knockout generates no discernable phenotype in the immune and reproductive systems.20 This stands in contrast to the fatal autoimmune disease phenotype arising from the phenotype arising from knockout of CD2521 or of the Foxp3 transcriptional regulator.1,22 The developmental pathway of these T cells in the thymus and/or in the periphery is also not known.
CD52 is also the molecular target of CAMPATH-1, a rat monoclonal antibody raised against human lymphocyte proteins by Hermann Waldmann in the Cambridge Pathology Department.23 CAMPATh1 was first developed to prevent graft vs. host disease. Its first incarnation was as an IgM, complement fixing rat monoclonal antibody to human lymphocytes that depletes more than 99% of lymphocytes in bone marrow before transplantation.23 The latest incarnation of this reagent is CAMPATH-1H, a humanized antibody of IgG1 isotype that is also known as alemtuzumab.24 It is used to treat the T-cell lymphoproliferative diseases and to deplete lymphocytes in bone marrow transplants to prevent graft vs. host disease.
In the context of the autoimmune diseases, alemtuzumab has also been successfully used to reduce clinical and paraclinical measures of disease activity in relapsing–remitting multiple sclerosis, a putative autoimmune disease of the nervous system and shown to be more efficacious than interferon-β.25,26 However, the treatment can be complicated by the development of autoimmune disease and in particular thyroid autoimmune disease27 in about a third of patients.28 This complication may reflect the deletion of CD52-expressing regulatory T cells by alemtuzumab. In the light of recent findings on CD52, a rational approach to treat multiple sclerosis and perhaps other putative autoimmune diseases is to explore the therapeutic potential of applying CD52-Fc to target CD52–Siglec-10 interactions to suppress pathogenic autoreactive T-cell clones. Other fusion proteins such as TNFR-Fc (Etanercept)29 have been successfully exploited to treat autoimmune diseases such as rheumatoid arthritis.30 An alternative therapeutic approach, given the findings with antibody to CD52 in the expansion of CD52-expressing regulatory T cells, a non-depleting anti-CD52 antibody, could potentially be of benefit in the in vivo expansion of these regulators in the control of the autoimmune diseases. In vivo expansion of Foxp3 regulatory CD4 T cells have been shown to ameliorate atherosclerosis development and progression.31
References
- Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- Bandala-Sanchez E, Zhang Y, Reinwald S, Dromey JA, Lee BH, Qian J, et al. T cell regulation mediated by interaction of soluble CD52 with the inhibitory receptor Siglec-10. Nat Immunol. 2013;14:741–748. doi: 10.1038/ni.2610. [DOI] [PubMed] [Google Scholar]
- Hale G. Synthetic peptide mimotope of the CAMPATH-1 (CD52) antigen, a small glycosylphosphatidylinositol-anchored glycoprotein. Immunotechnology. 1995;1:175–187. doi: 10.1016/1380-2933(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Varki A, Angata T. Siglecs—the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang W, Wan T, Zhang J, Chen T, Yu Y, et al. Cloning and characterization of Siglec-10, a novel sialic acid binding member of the Ig superfamily, from human dendritic cells. J Biol Chem. 2001;276:28106–28112. doi: 10.1074/jbc.M100467200. [DOI] [PubMed] [Google Scholar]
- Whitney G, Wang S, Chang H, Cheng KY, Lu P, Zhou XD, et al. A new Siglec family member, Siglec-10, is expressed in cells of the immune system and has signaling properties similar to CD33. Eur J Biochem. 2001;268:6083–6096. doi: 10.1046/j.0014-2956.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- Kivi E, Elima K, Aalto K, Nymalm Y, Auvinen K, Koivunen E, et al. Human Siglec-10 can bind to vascular adhesion protein-1 and serves as its substrate. Blood. 2009;114:5385–5392. doi: 10.1182/blood-2009-04-219253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama J, Yoshio T, Suzuki K, Kitagawa S, Iwamoto M, Kamimura T, et al. Characterization of the 4C8 antigen involved in transendothelial migration of CD26hi T cells after tight adhesion to human umbilical vein endothelial cell monolayers. J Exp Med. 1999;189:979–990. doi: 10.1084/jem.189.6.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama J, Kaga S, Kano S, Minota S. A novel costimulation pathway via the 4C8 antigen for the induction of CD4+ regulatory T cells. J Immunol. 2002;169:3710–3716. doi: 10.4049/jimmunol.169.7.3710. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Masuyama J, Sohma Y, Inazawa H, Horie K, Kojima K, et al. CD52 is a novel costimulatory molecule for induction of CD4+ regulatory T cells. Clin Immunol. 2006;120:247–259. doi: 10.1016/j.clim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Hale G. CD52 (CAMPATH1) J Biol Regul Homeost Agents. 2001;15:386–391. [PubMed] [Google Scholar]
- Schreiber TH, Wolf D, Tsai MS, Chirinos J, Deyev VV, Gonzalez L, et al. Therapeutic treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010;120:3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraban VY, Ferdinand JR, Al-Shamkhani A.Expression of TNFRSF25 on conventional T cells and tregs J Clin Invest 2011121463–464.author reply 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff C. CD52 is the ‘major maturation-associated' sperm membrane antigen. Mol Hum Reprod. 1996;2:9–17. doi: 10.1093/molehr/2.1.9. [DOI] [PubMed] [Google Scholar]
- Ratzinger G, Reagan JL, Heller G, Busam KJ, Young JW. Differential CD52 expression by distinct myeloid dendritic cell subsets: implications for alemtuzumab activity at the level of antigen presentation in allogeneic graft–host interactions in transplantation. Blood. 2003;101:1422–1429. doi: 10.1182/blood-2002-04-1093. [DOI] [PubMed] [Google Scholar]
- Elsner J, Hochstetter R, Spiekermann K, Kapp A. Surface and mRNA expression of the CD52 antigen by human eosinophils but not by neutrophils. Blood. 1996;88:4684–4693. [PubMed] [Google Scholar]
- Diekman AB, Norton EJ, Klotz KL, Westbrook VA, Shibahara H, Naaby-Hansen S, et al. N-linked glycan of a sperm CD52 glycoform associated with human infertility. FASEB J. 1999;13:1303–1313. doi: 10.1096/fasebj.13.11.1303. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Yamagata K, Hasuwa H, Inano E, Ikawa M, Okabe M. CD52, known as a major maturation-associated sperm membrane antigen secreted from the epididymis, is not required for fertilization in the mouse. Genes Cells. 2008;13:851–861. doi: 10.1111/j.1365-2443.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Hale G, Bright S, Chumbley G, Hoang T, Metcalf D, Munro AJ, et al. Removal of T cells from bone marrow for transplantation: a monoclonal antilymphocyte antibody that fixes human complement. Blood. 1983;62:873–882. [PubMed] [Google Scholar]
- Gribben JG, Hallek M. Rediscovering alemtuzumab: current and emerging therapeutic roles. Br J Haematol. 2009;144:818–831. doi: 10.1111/j.1365-2141.2008.07557.x. [DOI] [PubMed] [Google Scholar]
- Robertson NP. Alemtuzumab for multiple sclerosis: a new age of immunotherapy. J Neurol. 2013;260:343–345. doi: 10.1007/s00415-012-6786-x. [DOI] [PubMed] [Google Scholar]
- Wiendl H, Kieseier B. Multiple sclerosis: reprogramming the immune repertoire with alemtuzumab in MS. Nat Rev Neurol. 2013;9:125–126. doi: 10.1038/nrneurol.2013.2. [DOI] [PubMed] [Google Scholar]
- Cossburn M, Pace AA, Jones J, Ali R, Ingram G, Baker K, et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology. 2011;77:573–579. doi: 10.1212/WNL.0b013e318228bec5. [DOI] [PubMed] [Google Scholar]
- Costelloe L, Jones J, Coles A. Secondary autoimmune diseases following alemtuzumab therapy for multiple sclerosis. Expert Rev Neurother. 2012;12:335–341. doi: 10.1586/ern.12.5. [DOI] [PubMed] [Google Scholar]
- Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991;174:1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M, Maini RN. Lasker clinical medical research award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9:1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, et al. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation. 2012;126:1256–1266. doi: 10.1161/CIRCULATIONAHA.112.099044. [DOI] [PubMed] [Google Scholar]