The balance between follicular helper T cells (TFH cells) and follicular regulatory T (Treg) cells (TFR cells) is one of the key factors for the development of humoral immune responses. How the balance is regulated, however, is largely unknown. In a recent issue of Nature Immunology, Sage et al. revealed a critical role of the programmed death-1 (PD-1)/PD-L1 pathway in TFR cell generation and function,1 which provides a novel insight into the mechanisms how TFH and TFR cells regulate antibody production.
Germinal centers (GCs) are sites within lymph nodules, where B lymphocytes rapidly proliferate and differentiate into plasma cells and memory B cells during humoral immune responses. These events are precisely regulated by interactions between cognate B cells, TFH cells, TFR cells and follicular dendritic cells.2,3,4,5 TFH cells are a specialized CD4+ helper subset, which provides help to B cells. TFR cells are a newly identified CD4+ Treg subset in GCs, which downregulates the GC response.6,7 TFH and TFR cells are defined by expression of the CXC-chemokine receptor 5 (CXCR5) which directs them to B-cell follicles in GCs via gradients of the CXC-chemokine ligand 13 (CXCL13).1,2,8 TFH and TFR cells are present not only in lymph nodes but also in peripheral blood. PD-1 is a well-known inhibitory receptor for humoral immune responses9 and can be expressed by T cells, B cells, macrophages and dendritic cells.10 TFR cells express more PD-1 than TFH cells.1 B cells and dendritic cells express the PD-1 ligands, PD-L1 and PD-L2.10
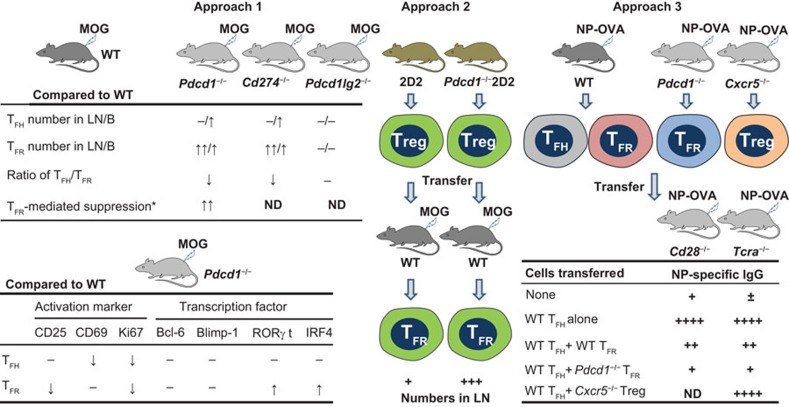
To uncover how the balance between TFH and TFR cells controls humoral immunity, Sage et al. attempted to clarify the individual role of PD-1 expressed on TFH and TFR cells. Table 1 summarizes the major cells, molecules, antigens and knockout/transgenic mice and Figure 1 outlines the three main approaches and the findings of this work.
Table 1. Cell types, molecules, antigens and mice.
| Name | Feature | |
|---|---|---|
| Cell | TFH cell | Follicular helper T cell, CD4+CXCR5+Foxp3−ICOS+Bcl-6+CD19− and PD-1+GITR−. TFH cells are essential for GC formation and for B cell activation/differentiation into plasma cells |
| TFR cell | Follicular regulatory T cell, CD4+CXCR5+Foxp3+ICOS+Bcl-6+CD19− and PD-1+GITR+. TFR cells can inhibit TFH cell function and naive T- and B-cell activation | |
| CD4+ Treg cell | Treg cell, CD4+CXCR5−Foxp3+ and GITR+ | |
| Molecule | PD-1 | PD-1 (CD279), also called PDCD-1. Well-known inhibitory receptor and expressed by T cells, B cells, macrophages and dendritic cells |
| PD-L1 | Ligand 1 of PD-1 (CD274), also called B7 homolog-1 (B7-H1) | |
| PD-L2 | Ligand 2 of PD-1 (CD273), also called B7-DCPD-L1 and PD-L2 are type I transmembrane proteins related to the CD28 ligands, but do not bind CD28 or CTLA-4 (CD152). B cells and dendritic cells in GCs express both PD-L1 and PD-L2 | |
| CXCR5 | CXC-chemokine receptor 5 (CD185), a marker of TFH and TFR cells in a follicular program | |
| CXCL13 | CXC-chemokine ligand 13, also called BLC. CXCL13 can direct the migration and relocation of CXCR5+ B cells, TFH cells and TFR cells into follicles | |
| GITR | Glucocorticoid-induced tumor-necrosis factor receptor (CD357, TNFRSF-18), a type 1 transmembrane protein of the TNF receptor superfamily. GITR is expressed on TFR cells but not on TFH cells, and is used as a marker to isolate TFR cells for functional study | |
| CD28 | Well-known T-cell costimulatory molecule. Its natural ligands are CD80 (B7-1) and CD86 (B7-2). CD28 provides essential costimulatory signals for TFH and TFR cells | |
| ICOS | Inducible T-cell costimulator (CD278). Its ligand is CD275 (B7H). ICOS provides essential costimulatory signals for TFH and TFR cells | |
| Antigen | MOG | Myelin oligodendrocyte glycoprotein peptide (amino acids 35–55) emulsified in complete Freund's adjuvant. |
| NP-OVA | The hapten NP (4-hydroxy-3-nitrophenylacetyl) conjugated to ovalbumin. | |
| Mouse | WT | WT C57BL/6 |
| Pdcd1−/− | Deficient in PD-1 | |
| Cd274−/− | Deficient in PD-L1 | |
| Pdcd1lg2−/− | Deficient in PD-L2 | |
| Cxcr5−/− | Deficient in CXCR5, lacking a follicular program | |
| Cd28−/− | Deficient in CD28, lacking TFH and TFR cells | |
| Icos−/− | Deficient in ICOS, lacking TFH and TFR cells | |
| Tcra−/− | Deficient in TCR α-chain, lacking TFH and TFR cells | |
| 2D2 | Transgenic mice, expressing a MOG-specific TCR and Foxp3-GFP | |
| Pdcd1−/−2D2 | With the properties of both Pdcd1−/− and 2D2 mice | |
Abbreviations: BLC, B lymphocyte chemoattractant; GC, germinal center; GFP, green fluorescent protein; PD, programmed death; PDCD-1, programmed cell death-1; TCR, T-cell receptor; Treg, regulatory T; WT, wild-type.
Figure 1.
The major experimental approaches and findings which reveal the critical role of the PD-1/PD-L1 pathway in TFR generation and function. *, TFR-mediated suppression includes the inhibition of responder T- and B-cell proliferation, and the inhibition of IgG production. See Table 1 for the mice and antigens used. B, blood; LN, lymph nodes; MOG & NP-OVA, antigens; ND, not detected; PD-1, programmed death-1; −, no significant difference.
In the first approach, the authors determined the frequency and number of TFH cells (CD4+CXCR5+Foxp3−ICOS+CD19−) and TFR cells (CD4+CXCR5+Foxp3+ICOS+CD19−) in 4 strains of C57BL/6 mice: wild-type (WT), PD-1-deficient (Pdcd1−/−), PD-L1-deficient (Cd274−/−) and PD-L2-deficient (Pdcd1lg2−/−), these mice being immunized with MOG. Deficiency of PD-1 or PD-L1, but not PD-L2, resulted in more TFR cells than TFH cells in lymph nodes and blood. Compared to WT TFR cells, PD-1-deficient TFR cells displayed a potent capacity to inhibit both the proliferation of responder T and B cells and the TFH cell-mediated IgG production in vitro. The expressions of activation markers (CD25, CD69 and Ki67) and transcription factors (Bcl-6, Blimp-1, RORγt and IRF4) by TFH and TFR cells were also compared between Pdcd1−/− and WT mice, as shown in Figure 1. The data indicate that the PD-1/PD-L1 pathway controls not only the generation and function of TFR cells but also the ratio of TFH to TFR cells. In addition, Cd28−/− and Icos−/− mice showed considerable deficiency of TFH and TFR cells, suggesting that CD28 and ICOS provide essential costimulatory signals for TFH and TFR cell development. Like WT TFR cells, PD-1-deficient TFR cells can home to GCs.
In the second approach, the authors investigated the role of PD-1/PD-L1 pathway in the differentiation of TFR cells from CD4+Foxp3+CXCR5− Treg cells. They used 2D2 and Pdcd1−/−2D2 mice that obtained transgenic expressions of a MOG-specific TCR and Foxp3-green fluorescent protein (GFP). The MOG-specific GFP+ Treg cells purified from these mice were transferred into WT mice and the recipients were further immunized with MOG. In the mice receiving PD-1− Treg cells, the GFP+ TFR cells (CD4+Foxp3+CXCR5+) in draining lymph nodes were found at higher frequency and higher absolute number than in the mice received PD-1+ Treg cells. These data confirm that the PD-1/PD-L1 pathway controls the differentiation of CD4+Foxp3+CXCR5− Treg cells into TFR cells.
In the third approach, the authors evaluated whether TFR cells in blood could suppress antibody production in vivo. WT, Pdcd1−/− and Cxcr5−/− mice were immunized with NP-OVA. WT TFH cells (CD4+ICOS+CXCR5+GITR−CD19−), WT and PD-1-deficient TFR cells (CD4+ICOS+CXCR5+GITR+CD19−) and CXCR5-deficient Treg cells (CD25+CD62L+) were sorted from blood 8 days later. The isolated cells, in various combinations, were transferred into Cd28−/− mice and into TCR α-chain knockout (Tcra−/−) mice. These mice cannot produce their own TFH and TFR cells, and so any follicular T cells in their bodies must have come from the transferred TFH and TFR cells. The recipients were then immunized with NP-OVA and the titers of NP-specific IgG were measured. In both recipient mice, the transferred WT TFH cells promoted antibody production, whereas the transferred WT TFR cells strongly inhibited the antibody production induced by the TFH cells. Particularly, the transferred PD-1-deficient TFR cells displayed much stronger suppressive ability than the WT TFR cells. The TFR-mediated inhibition depends on the ‘follicular program' because CXCR5-deficient Treg cells failed to inhibit the antibody production induced by the WT TFH cells. The TFR cell-mediated inhibition was further confirmed by quantification of CD138+ plasma cells in Cd28−/− and Tcra−/− mice after the transfer of WT TFH and TFR cells. These in vivo results demonstrate that TFR cells in blood can home to lymph nodes and potently inhibit antibody production.
Taken together, the PD-1/PD-L1 pathway plays a critical role in TFR generation and function. Signaling via PD-1/PD-L1 inhibits TFR cell differentiation from CD4+Foxp3+ Treg cells and controls their suppressive ability, thus changing the balance between TFH and TFR cells. These findings provide new insights into the regulation of humoral immunity, and suggest that TFR cells and associated molecules could be used as targets for the treatment of autoimmune diseases.
References
- Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Tarlinton DM. Germinal center B and follicular helper T cells: siblings, cousins or just good friends. Nat Immunol. 2011;12:472–477. doi: 10.1038/ni.2019. [DOI] [PubMed] [Google Scholar]
- Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunol Rev. 2013;252:146–155. doi: 10.1111/imr.12031. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010;237:72–89. doi: 10.1111/j.1600-065X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]