Abstract
Approaches for controlling inflammatory responses and reducing the mortality rate of septic patients remain clinically ineffective; new drugs need to be identified that can induce anti-inflammatory responses. Ephedrine hydrochloride (EH) is a compound that is widely used in cardiovascular diseases, especially to treat hypotension caused by either anesthesia or overdose of antihypertensive drugs. In this study, we reported that EH also plays an important role in the control of the inflammatory response. EH increased IL-10 and decreased proinflammatory cytokine (IL-6, tumor-necrosis factor (TNF)-α, IL-12 and IL-1β) expression in primary peritoneal macrophages and Raw264.7 cells treated with peptidoglycan (PGN), a Gram-positive cell wall component. The anti-inflammatory role of EH was also demonstrated in an experimental mouse model of peritonitis induced by intraperitoneal PGN injection. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway was found to be responsible for the EH-mediated increase in IL-10 production and decrease in IL-6 expression. Therefore, our results illustrated that EH can help maintain immune equilibrium and diminish host damage by balancing the production of pro- and anti-inflammatory cytokines after PGN challenge. EH may be a new potential anti-inflammatory drug that can be useful for treating severe invasive Gram-positive bacterial infection.
Keywords: anti-inflammation, EH, ephedrine hydrochloride, IL-10, peptidoglycan, PGN, phosphatidylinositol 3-kinase, PI3K
Introduction
Sepsis is caused by pathological infection accompanied by systemic inflammatory response syndrome. It has been reported that sepsis is the second leading cause of death in intensive care units.1 Gram-negative (G−) bacteria were the predominant organisms causing sepsis from 1979 to 1987; however, Gram-positive (G+) bacteria are increasingly common causes of sepsis in these two decades.1,2 In 2000, G+ bacteria reportedly accounted for 52.1% of new incidences of sepsis, with G− bacteria accounting for 37.6% polymicrobial infections, anaerobes and fungi accounted for the remaining cases.1 Although the mortality rates of septic patients caused by G− bacteria have declined in recent years, they remain steady for cases caused by G+ bacteria. Therefore, effective drugs are clearly needed for the treatment of severe G+ bacterial infection.
The G+ bacterium Staphylococcus aureus is by far the most common cause of severe invasive infection and sepsis. The excessive and harmful production of cytokines is involved in the pathogenesis of sepsis. Inflammatory responses to S. aureus mainly depend on the recognition of peptidoglycan (PGN), the main component of the G+ bacterial cell wall, and bacterial lipoproteins by Toll-like receptor (TLR) 2/6 heterodimers and other receptors.3 PGN ligation initiates innate immune responses by activating the mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3-kinase (PI3K), and the transcription factor nuclear factor κB (NF-κB) and its downstream signaling pathways, inducing the production of proinflammatory cytokines (IL-6, tumor-necrosis factor (TNF)-α, IL-1β, etc.), nitric oxide and the anti-inflammatory cytokine IL-10. The equilibrium between pro- and anti-inflammatory cytokines determines the extent of host immune responses.4 To control the overwhelming and harmful inflammatory responses in sepsis, effective drugs are urgently needed that can increase IL-10 while inhibiting proinflammatory cytokine expression in response to S. aureus.
Ephedrine hydrochloride (EH) is a compound from ephedrine, derived from Ephedra sinica (known in Chinese as Ma Huang). Ephedrine plays a classical role as an α- and β-adrenergic agonist, stimulating the central nervous system, dilating bronchial tubes, elevating blood pressure and increasing the heart rate.5 We previously reported that EH protects mice from endotoxic shock by promoting production of IL-10 and inhibiting proinflammatory cytokine secretion in response to lipopolysaccharide (LPS), the main component of G− bacterial cell wall.6 Therefore, we investigated how EH regulates the responses to the G+ bacterial cell wall component PGN.
In this study, we demonstrated that EH can promote PGN-triggered production of IL-10 as well as inhibit the production of the proinflammatory cytokines IL-6, TNF-α, and IL-1β in primary peritoneal macrophages and Raw264.7 cells. EH also promoted PGN-induced phosphorylation of Akt and glycogen synthase kinase (GSK)-3β at Thr308 and Ser9, respectively, accounting for EH-mediated regulation of IL-10 and IL-6 production. In parallel, the anti-inflammatory role of EH was observed in a PGN-induced mouse model of peritonitis. Thus, EH may be an effective drug for treating severe G+ bacterial infection.
Materials and methods
Mice and reagents
Female C57BL/6J mice (6–8 weeks old) were obtained from the Joint Ventures Sipper BK Experimental Animal Co. (Shanghai, China). All mice were raised in a pathogen-free facility. All animal experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Scientific Investigation Board of Second Military Medical University (Shanghai, China). Anti-β-actin and horseradish peroxidase-coupled secondary antibodies were acquired from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Phospho-antibodies against extracellular signal-regulated kinase p44/p42 (ERK1/2, Thr202/Tyr204), c-Jun N-terminal kinase (JNK)/stress-associated protein kinase (Thr183/Tyr185), p38 (Thr180/Tyr182), Akt (Ser473), Akt (Thr308), GSK-3α/β (Ser21/9), IKKα/β (Ser176/180) and the p65 subunit (Ser536) of NF-κB, and corresponding antibodies against total proteins were obtained from Cell Signaling Technology (Beverly, MA, USA). PD98059, SB203580, LY294002 and PDTC were purchased from Calbiochem (San Diego, CA, USA). Antimouse IL-10 neutralizing antibody (AB-417-NA) was purchased from R&D Systems (Minneapolis, MN, USA). PGN from S. aureus was purchased from Fluka (St Louis, MO, USA). EH (molecular formula: C10H15NO·HCl; molecular weight: 202) (30 mg/ml) was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). DMSO, thioglycolate and dexamethasone (DXM) (molecular formula: C22H29FO5; molecular weight: 392) (Figure 1A) (1 mg/ml) were obtained from Sigma (St Louis, MO, USA).
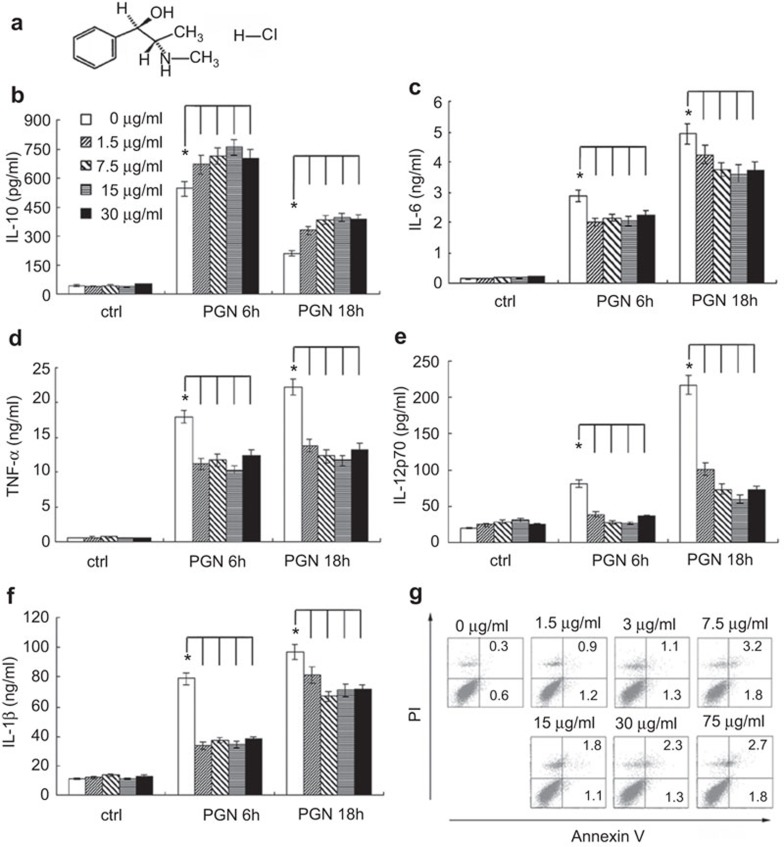
Figure 1.
EH promotes PGN-induced IL-10 and inhibits proinflammatory cytokine production by mouse peritoneal macrophages, but does not induce cellular apoptosis. (a) Chemical structure of EH. Mouse peritoneal macrophages (3.5×105 cells/350 µl) were stimulated by various concentrations of EH in the presence or absence of PGN (25 µg/ml) for the indicated time period. The concentrations of IL-10 (b), IL-6 (c), TNF-α (d), IL-12p70 (e) and IL-1β (f) in the supernatants were measured by ELISA. Data are shown as the mean±s.d. of three independent experiments. * indicates P<0.05. (g) Mouse primary peritoneal macrophages were treated with the indicated concentrations of EH for 24 h. Cells were then harvested and labeled with PI and Annexin V, and analyzed by FACS. Similar results were obtained in three independent experiments. EH, ephedrine hydrochloride; PGN, peptidoglycan; PI, propidium iodide; s.d., standard deviation; TNF, tumor-necrosis factor.
Cell culture
Thioglycolate-elicited mouse primary peritoneal macrophages were prepared from female C57BL/6J mice (6–8 weeks old) and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum as previously described.7 The mouse macrophage-like cell line RAW264.7 was obtained from ATCC (Manassas, VA, USA), and was cultured as previously described.8
Detection of cytokine production
ELISA kits for murine IL-6, TNF-α, IL-12p70, IL-10 and IL-1β were purchased from R&D Systems (Minneapolis, MN, USA). IL-6, TNF-α, IL-12p70, IL-10 and IL-1β levels were measured in the supernatants. IL-6, TNF-α, IL-10 and IL-1β levels in the sera were also measured by ELISA.
Nitric oxide measurement
Nitric oxide (NO) production was assayed by measuring nitrite concentrations with the Griess assay (Sigma) as previously described.9
Western blot analysis
Cells were lysed with M-PER Protein Extraction Reagent (Pierce, Rockford, IL, USA) supplemented with protease inhibitor cocktail (Calbiochem). Protein concentrations were then measured by bicinchoninic acid assay (Pierce). Equal amounts of extracts were separated by 10% SDS-PAGE, transferred onto nitrocellulose membranes, and then blotted as described previously.7
Flow cytometry assay
Peritoneal macrophages were treated with the indicated concentrations of EH for 24 h. Cells were then harvested and labeled with Annexin-V-FITC and propidium iodide provided by Calbiochem, following the manufacturer's instructions. Samples were examined by a flow cytometer (FACSCalibur; BD Bioscience, Franklin Lakes, NJ, USA) as previously described.7 The data were analyzed using CellQuest software (Becton Dickinson, San Jose, CA, USA).
PGN-induced mouse model of peritonitis
An experimental mouse model of peritonitis was induced by intraperitoneal injection of PGN. PGN was prepared in sterile phosphate-buffered saline, and sonicated for 1 h before use. Female C57BL/6J mice (6–8 weeks old) were injected with PGN (5 mg/kg body weight, i.p.) and different concentrations of EH. Blood samples were drawn by cardiac puncture after 4 h and clotted overnight at 4 °C. Serum samples were collected after centrifugation for cytokine analysis. Phosphate-buffered saline and DXM (7 mg/kg body weight, equivalent to the clinical dose) were chosen as negative and positive controls, respectively.10 The synergetic role of combined DXM and low-dose EH was verified in vivo.
Statistical analysis
The results are presented as the mean±standard deviation (s.d.) or standard error (s.e.) as indicated. Comparisons between two groups were performed by Student's t-test analyses. Statistical significance was determined at P<0.05.
Results
EH promotes PGN-triggered IL-10 production and inhibits proinflammatory cytokine production in macrophages, but does not induce cellular apoptosis alone at examined concentrations
Macrophages are the main cell population involved in the pathogenesis of sepsis. Therefore, we investigated whether EH may regulate the inflammatory responses of macrophages to PGN. EH (1.5–30 µg/ml, equivalent to 7.5–150 µM) and PGN (25 µg/ml) were simultaneously added to the cell culture supernatant of peritoneal macrophages. Cytokines were measured in the supernatants after 6 or 18 h of treatment by ELISA. The results showed that EH promoted PGN-induced production of IL-10 in macrophages (Figure 1b) but inhibited the production of IL-6, TNF-α, IL-12p70 and IL-1β (Figure 1c–f). The PGN-induced production of nitric oxide remained unchanged (data not shown).
To exclude the possibility that EH may be causing cellular apoptosis, we examined the apoptotic sensitivity of primary peritoneal macrophages to EH. EH was added at various concentrations (1.5–75 µg/ml, equivalent to 7.5–375 µM) into the cell culture supernatant, and incubated cells for 24 h. The results of fluorescence-activated cell sorting (FACS) showed that all tested concentrations of EH (up to 75 µg/ml) could not induce significant apoptosis of peritoneal macrophages (Figure 1g).
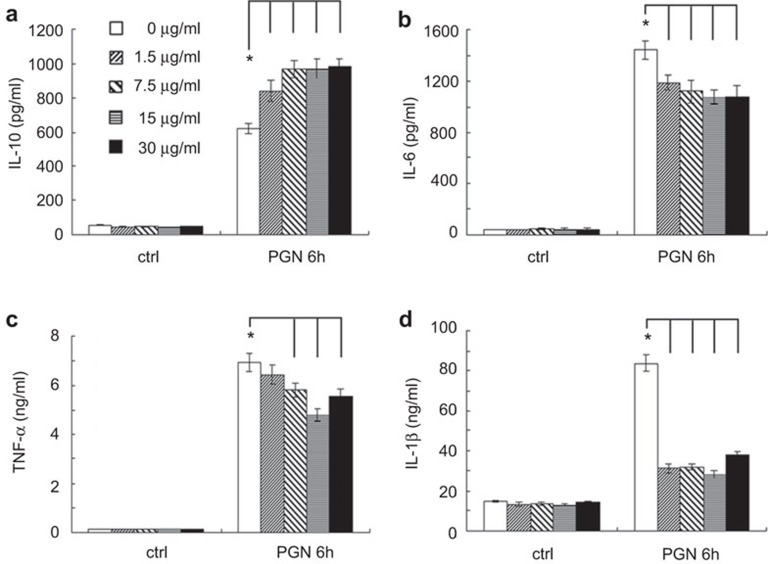
The anti-inflammatory role of EH was also observed in the mouse macrophage-like cell line Raw264.7 (Figure 2). Cumulatively, these data demonstrated that PGN-induced immune responses in macrophages are inhibited by EH-mediated modulation of cytokine production.
Figure 2.
EH promotes PGN-induced production of IL-10 and inhibits proinflammatory cytokine production in Raw264.7 cells. Plates (24-well) were seeded at 2×105 cells/well. After 24 h, cells were stimulated by various concentrations of EH in the presence or absence of PGN (25 µg/ml) for 6 h. IL-10 (a), IL-6 (b), TNF-α (c) and IL-1β (d) levels were measured by ELISA in the supernatants. The data are presented as the mean±s.d. of three independent experiments. * indicates P<0.05. EH, ephedrine hydrochloride; PGN, peptidoglycan; s.d., standard deviation; TNF, tumor-necrosis factor.
Elevated production of IL-10 is not responsible for the EH-mediated inhibition of proinflammatory cytokine production
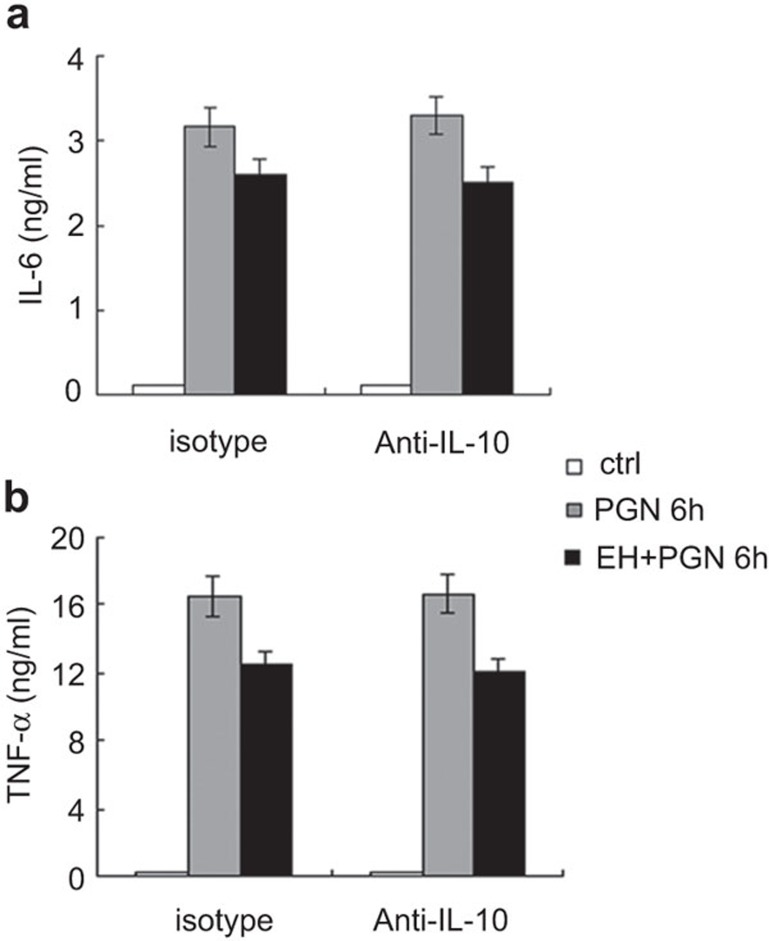
It has been reported that IL-10 can inhibit TNF-α by targeting transcriptional elongation mediated by cyclin-dependent kinase 9.11 Therefore, we investigated whether the increased production of the regulatory cytokine IL-10 could be the mechanism of action for EH-mediated inhibition of IL-6 and TNF-α production. After pre-treatment of peritoneal macrophages with isotype or neutralizing antibody for IL-10 (10 µg/ml), cells were stimulated by PGN in the presence or absence of EH for 6 h. As shown in Figure 3, pre-treatment with anti-IL-10 antibody did not rescue IL-6 (Figure 3a) or TNF-α (Figure 3b) secretion. These data indicated that EH upregulates IL-10 expression and downregulates proinflammatory cytokine expression independently.
Figure 3.
EH-mediated inhibition of PGN-induced proinflammatory cytokines does not depend on IL-10 production. Mouse peritoneal macrophages (3.5×105 cells/350 µl) were plated the day before stimulation. After pre-treatment with anti-IL-10 neutralizing antibody (10 µg/ml) for 30 min, cells were stimulated with PGN in the presence or absence of EH for 6 h. The secretion of IL-6 (a) and TNF-α (b) in the supernatants was examined by ELISA. The data are shown as the mean±s.d. of three independent experiments. EH, ephedrine hydrochloride; PGN, peptidoglycan; s.d., standard deviation; TNF, tumor-necrosis factor.
EH facilitates activation of the PI3K/Akt/GSK-3β pathway, accounting for the elevated IL-10 production and the decreased IL-6 production
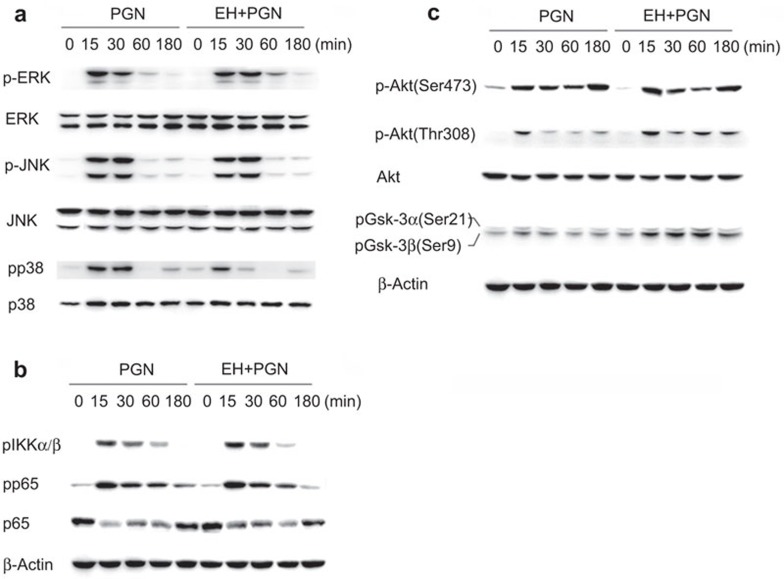
Stimulation by PGN activates TLR2, and myeloid differentiation protein 88 (MyD88) is adapted and thus the adapter complex is formed, leading to the JNK, ERK1/2, p38 MAPK and NF-κB activation, and production of cytokines. The PI3K/Akt/GSK-3β signaling pathway also participates in PGN-induced production of IL-10 and proinflammatory cytokines.12,13 Thus, we examined the phosphorylation of these two signaling pathways via Western blot. The results showed that EH treatment inhibited PGN-induced p38 phosphorylation (Figure 4a) and facilitated phosphorylation of Akt (Thr308) and GSK-3β (Ser9) (Figure 4c). EH did not affect PGN-induced phosphorylation of ERK, JNK (Figure 4a), IKKα/β and NF-κB p65 subunit (Figure 4b).
Figure 4.
EH inhibits PGN-induced p38 phosphorylation and facilitates activation of the PI3K/Akt/GSK-3β pathway. (a) Mouse peritoneal macrophages (1×106 cells) were plated overnight and then stimulated with PGN (25 µg/ml) in the presence or absence of EH (7.5 µg/ml) for the indicated time period. Phospho-ERK, JNK and p38, along with the corresponding total ERK, JNK and p38, were detected by Western blot. Similar results were obtained in three independent experiments. (b) Mouse peritoneal macrophages were treated as in Figure 5A, and phospho-IKKα/β, phospho-p65, total p65 and β-actin were detected by Western blot. Similar results were obtained in three independent experiments. (c) Peritoneal macrophages were treated as previously described. Phospho-Akt at Ser473 and Thr308, total Akt, phospho-GSK-3β and β-actin were detected by Western blot. Similar results were obtained in three independent experiments. EH, ephedrine hydrochloride; ERK, extracellular signal-regulated kinase; GSK-3β, glycogen synthase kinase 3β JNK, c-Jun N-terminal kinase; PGN, peptidoglycan; PI3K, phosphatidylinositol 3-kinase.
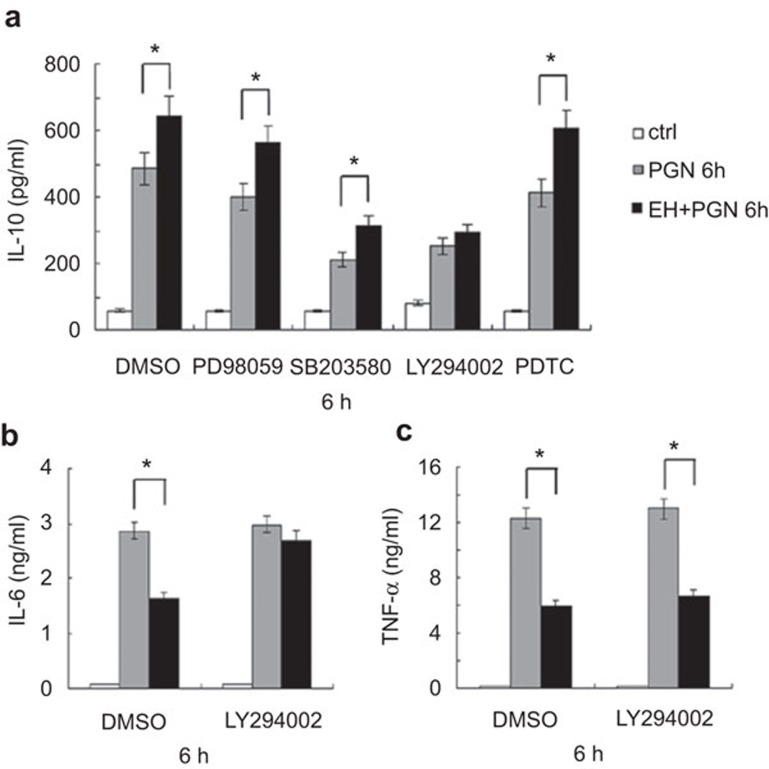
IL-10 expression is regulated by several signaling pathways after TLR2 activation in macrophages, including ERK, p38, PI3K/Akt/GSK-3β and NF-κB.12 We sought to identify the pivotal signaling pathway responsible for EH-mediated elevation of IL-10 production. To this end, peritoneal macrophages were pre-treated for 30 min with either DMSO, the ERK inhibitor PD98059, the p38 inhibitor SB203580, the PI3K inhibitor LY294002 or the NF-κB inhibitor PDTC, followed by stimulation for 6 h as the figure described. As shown in Figure 5a, the EH-mediated increase in IL-10 expression was only abolished in the presence of LY294002. GSK-3β is a repressor of IL-10 production at the transcriptional level, and its activity can be inhibited by Akt-mediated phosphorylation at Ser9.12 Pre-treatment with LY294002 also abolished the EH-mediated decrease in IL-6 secretion (Figure 5b), but the EH-mediated decrease in TNF-α secretion was not significantly affected (Figure 5c). Taken together, EH promotes PGN-induced IL-10 expression and inhibits IL-6 expression mainly through the PI3K signaling pathway; however, there may be other underlying mechanisms involved in the regulation of TNF-α expression.
Figure 5.
Blockade of PI3K abrogates EH-induced IL-10 expression and decreased IL-6 expression in macrophages. (a) Mouse peritoneal macrophages (3×105 cells/300 µl) were seeded the day before stimulation. Cells were pretreated for 30 min with DMSO, ERK inhibitor PD98059 (10 µM), p38 inhibitor SB203580 (10 µM), PI3K inhibitor LY294002 (20 µM) or NF-κB inhibitor PDTC (20 µM), and then stimulated with PGN (25 µg/ml) and EH (7.5 µg/ml) as indicated for 6 h. IL-10 was measured in the supernatants by ELISA. The data are shown as the mean±s.d. of three independent experiments. * indicates P<0.05. (b) Cells were pre-treated for 30 min with DMSO, PI3K inhibitor LY294002 (20 µM), and then stimulated as indicated for 6 h. IL-6 (B) and TNF-α (c) levels in the supernatants were measured by ELISA. The data are shown as the mean±s.d. of three independent experiments. * indicates P<0.05. EH, ephedrine hydrochloride; ERK, extracellular signal-regulated kinase; PGN, peptidoglycan; PI3K, phosphatidylinositol 3-kinase; NF-κB, nuclear factor-κb; s.d., standard deviation; TNF, tumor-necrosis factor.
EH promotes IL-10 production and inhibits proinflammatory cytokine production in vivo
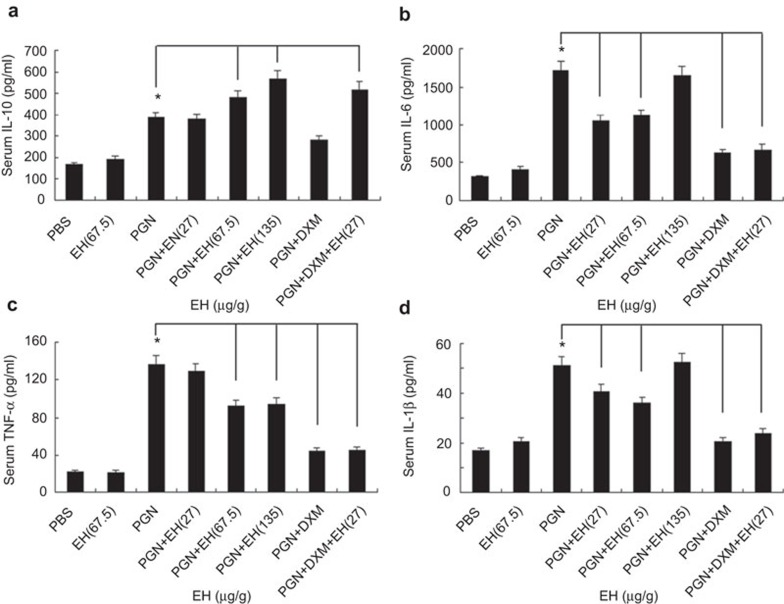
PGN-induced peritonitis is often used as an experimental model of peritonitis caused by G+ bacterial infection.14 To evaluate the anti-inflammatory potential of EH in vivo, we carried out PGN-induced peritonitis mouse models with i.p. injection of EH. Glucocorticoids (e.g., DXM) are regularly used to treat sepsis or septic shock. DXM can lower plasma cytokines (IL-1β, TNF-α, etc.) and has demonstrated anti-inflammatory effects in shock.15 Thus, we utilized DXM as an effective drug control in PGN-induced peritonitis. We chose the following doses of EH: 27, 67.5 and 135 µg/g, based on evaluation of serum cytokines. These doses were equivalent to 3, 7.5 and 15 µg/ml, respectively, in in vitro cellular experiments. Paralleling results obtained with macrophages, EH-treated mice produced significantly higher levels of IL-10 in sera when compared to control mice. Low-dose EH (27 µg/g) did not increase IL-10 expression alone, but could act synergistically with DXM to increase IL-10 levels (Figure 6a). EH treatment decreased IL-6 and IL-1β production only at lower doses (Figure 6b and d), and decreased TNF-α production at higher doses (Figure 6c). These data showed that EH increases serum IL-10 and decreases IL-6, IL-1β and TNF-α secretion induced by PGN in vivo. Furthermore, EH and DXM synergistically induced IL-10 expression.
Figure 6.
EH promotes IL-10 production and inhibits proinflammatory cytokine secretion in PGN-induced mouse model of peritonitis. C57BL/6J mice were injected intraperitoneally with PGN (5 mg/kg; Sigma) in the presence of different concentrations of EH, DXM (7 mg/kg; Sigma), or combinational application of DXM and EH. Mice were euthanized 4 h after injection and blood samples were clotted overnight at 4 °C and the concentration of various cytokines in the sera were examined by ELISA. The data are shown as the mean±s.e. of eight mice per group. * indicates P<0.05. DXM, dexamethasone; EH, ephedrine hydrochloride; PGN, peptidoglycan; s.e., standard error.
Discussion
TLR2 or other receptors (e.g., C5α) are activated upon recognition of G+ bacteria (e.g., S. aureus) or G+ bacterial components (e.g., PGN), resulting in the activation of signaling pathways (e.g., ERK1/2, JNK, p38 MAPKs, PI3K/Akt and NF-κB) that account for a large amount of proinflammatory cytokine production and a relatively small amount of IL-10 production.4,9 The overwhelming expression of proinflammatory cytokines can lead to various severe diseases such as sepsis, multiple organ failure or even death.16,17 In contrast, the anti-inflammatory cytokine IL-10 plays an indispensable role in maintaining homeostasis to avoid host damage.12 When faced with an acute, life-threatening infection, it is essential to maintain a moderate equilibrium between pro- and anti-inflammatory cytokine production.
In our study, we investigated EH as a potential regulator of the balance of pro- and anti-inflammatory cytokine expression in the context of G+ bacterium-induced infection. We demonstrated that EH plays an anti-inflammatory role by promoting PGN-induced IL-10 expression and decreasing the production of proinflammatory cytokines (IL-6, TNF-α, etc.) in macrophages. IL-6 and TNF-α have been considered the primary mediators of sepsis,18 and a positive correlation has been found between multiple organ failure and serum levels of IL-6 and TNF-α.19,20 Previously, blocking IL-6 was reported to improve the survival of mice in sepsis. Therefore, we examined the secretion of these cytokines in a PGN-induced mouse model of peritonitis to confirm the protective role of EH in vivo. We chose DXM as a positive control, which is regularly used to treat sepsis or septic shock. As shown in Figure 6, DXM dramatically inhibited IL-6, TNF-α and IL-1β expression in sera, but had only a weak effect on IL-10 levels in vivo. However, EH played an impressive role in promoting the PGN-induced secretion of IL-10. PI3K physically interacts with the glucocorticoid receptor (GR) through two consensus YxxM binding motifs within the GR.21 In human fibroblasts, DXM protects cells from apoptosis via activation of PKB/Akt.22 DXM may enhance Akt activation by low doses of EH, thus playing a synergistic role in the expression of IL-10 while remaining a strong inhibitor of proinflammatory cytokine production. Therefore, differential modulation of pro- and anti-inflammatory cytokines by EH may be an effective therapeutic strategy for preventing undesirable PGN-induced inflammatory damage. The combination of EH and DXM may be helpful in overcoming the obstacle of decreasing the mortality rate of sepsis caused by G+ bacteria.
In monocytes, macrophages and mDCs, accumulating evidence showed that negative regulation of IL-12 and positive regulation of IL-10 by PI3K/Akt activation serves as a potential ‘safety mechanism' for controlling the extent of cellular responses to pathogens.23,24 GSK-3 is a main target of Akt and normally blocks IL-10 expression by acting on the transcription factors activator protein 1 and cAMP response element-binding protein.12 The activity of GSK-3 can be inhibited by Akt-mediated phosphorylation of GSK-3α and GSK-3β at Ser21 and Ser9, respectively. Here, we provide evidence that EH activates the PI3K/Akt (Thr308)/GSK-3β (Ser9) pathway to increase PGN-induced IL-10. Keck et al.25 reported that the activation of PI3K/Akt attenuates IL-6 secretion in response to TLR signaling in bone marrow-derived macrophages by controlling p38 MAPK phosphorylation. We also demonstrated in our study that inhibition of PI3K activity by LY294002 prevented the EH-mediated decrease in IL-6 secretion. Thus, EH facilitates PGN-induced activation of the PI3K/Akt signaling pathway, accounting for increased IL-10 expression and decreased IL-6 expression. Decreased p38 MAPK phosphorylation may also account for the EH-mediated decrease in TNF-α expression, but TNF-α production could not be rescued by LY294002. These results suggest that there may be an underlying PI3K-independent mechanism in the regulation of TNF-α expression by EH.
Ephedrine is a vasopressor drug and its primary use is to treat anesthesia-induced hypotension, sympathectomy or hypotension resulting from overdose of antihypertensive drugs.26 As an activator of the α- and β-adrenoceptors, ephedrine acts as a strong vasoconstrictor, increasing blood pressure and heart rate.27 The data in this study demonstrate that EH regulates the expression of IL-10 and IL-6 via PI3K activation, but regulates TNF-α expression through other mechanisms. α- and β-adrenoceptors may be coexpressed in macrophages, and EH may cause different effects through them.
Previously, we reported that EH plays an anti-inflammatory role in endotoxic shock. EH protects mice against LPS challenge by decreasing LPS-induced inflammatory cytokines (IL-6, TNF-α, IL-12, etc.) and increasing IL-10 expression.6 Upon LPS (TLR4 agonist) stimulation, EH increases IL-10 secretion in macrophages by elevating p38 phosphorylation. Here, we demonstrated that EH promotes IL-10 and impairs IL-6 production following PGN (TLR2 agonist) challenge via the PI3K/Akt pathway. The different regulatory mechanisms affected by EH to control IL-10 expression may be due to differences in crosstalk between EH to the signaling pathways downstream of different TLRs.28 Regardless of whether mice were challenged with LPS or PGN, DXM had a small impact on anti-inflammatory IL-10 expression. In contrast, EH enhances IL-10 secretion significantly in both LPS- and PGN-challenged mouse models. Taken together, these results suggest that EH is a promising adjunct to DXM for the treatment of autoimmunological or severe infectious diseases, including refractory mixed infections of G+ and G− bacteria.
Further work should focus on finding the exact molecular target of EH. The structure of EH could be modified to enhance its anti-inflammatory function and abolish the unwanted vasoconstrictive effects.
Acknowledgments
This work was supported by grants from the National Key Basic Research Program of China (2010CB529901 and 2010CB530600), the National Natural Science Foundation of China (31100619 and 30972705), the China Postdoctoral Science Foundation (20110490186), the Chen-guang Plan Project of Shanghai Educational Municipal Education Commission (11CG48 and szy10004), the Specialized Research Fund for the Doctoral Program of Higher Education (20113107120014) and the Leading Academic Discipline Project of Shanghai Municipal Education Commission (J50301) (Yuejuan Zheng, Shanghai University of T.C.M).
The authors declare no competing financial interests.
References
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Prabhakara R, Harro JM, Leid JG, Keegan AD, Prior ML, Shirtliff ME. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect Immun. 2011;79:5010–5018. doi: 10.1128/IAI.05571-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S, Bunk S, Meergans T, Doninger B, Stich K, Stulnig T, et al. Apolipoprotein B100 is a suppressor of Staphylococcus aureus-induced innate immune responses in humans and mice. Eur J Immunol. 2012;42:2983–2989. doi: 10.1002/eji.201242564. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Andraws R, Chawla P, Brown DL. Cardiovascular effects of ephedra alkaloids: a comprehensive review. Prog Cardiovasc Dis. 2005;47:217–225. doi: 10.1016/j.pcad.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Guo Z, He W, Yang Y, Li Y, Zheng A, et al. Ephedrine hydrochloride protects mice from LPS challenge by promoting IL-10 secretion and inhibiting proinflammatory cytokines. Int Immunopharmacol. 2012;13:46–53. doi: 10.1016/j.intimp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Zheng Y, An H, Yao M, Hou J, Yu Y, Feng G, et al. Scaffolding adaptor protein Gab1 is required for TLR3/4- and RIG-I-mediated production of proinflammatory cytokines and type I IFN in macrophages. J Immunol. 2010;184:6447–6456. doi: 10.4049/jimmunol.0901750. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P, et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat Immunol. 2011;12:416–424. doi: 10.1038/ni.2015. [DOI] [PubMed] [Google Scholar]
- Xu S, Liu X, Bao Y, Zhu X, Han C, Zhang P, et al. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps-SHP-2 pathway. Nat Immunol. 2012;13:551–559. doi: 10.1038/ni.2283. [DOI] [PubMed] [Google Scholar]
- Li L, Whiteman M, Moore PK. Dexamethasone inhibits lipopolysaccharide-induced hydrogen sulphide biosynthesis in intact cells and in an animal model of endotoxic shock. J Cell Mol Med. 2009;13:2684–2692. doi: 10.1111/j.1582-4934.2008.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallie T, Ricchetti G, Horwood NJ, Feldmann M, Clark AR, Williams LM. IL-10 inhibits transcription elongation of the human TNF gene in primary macrophages. J Exp Med. 2010;207:2081–2088. doi: 10.1084/jem.20100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Mullaly SC, Kubes P. The role of TLR2 in vivo following challenge with Staphylococcus aureus and prototypic ligands. J Immunol. 2006;177:8154–8163. doi: 10.4049/jimmunol.177.11.8154. [DOI] [PubMed] [Google Scholar]
- Tuluc F, Meshki J, Kunapuli SP. Membrane lipid microdomains differentially regulate intracellular signaling events in human neutrophils. Int Immunopharmacol. 2003;3:1775–1790. doi: 10.1016/j.intimp.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Fry DE. Sepsis, systemic inflammatory response, and multiple organ dysfunction: the mystery continues. Am Surg. 2012;78:1–8. [PubMed] [Google Scholar]
- Wang W, Lim J, Li J. Synergistic and feedback signaling mechanisms in the regulation of inflammation in respiratory infections. Cell Mol Immunol. 2012;9:131–135. doi: 10.1038/cmi.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol. 1998;275:R269–R277. doi: 10.1152/ajpregu.1998.275.1.R269. [DOI] [PubMed] [Google Scholar]
- Geppert A, Steiner A, Zorn G, Delle-Karth G, Koreny M, Haumer M, et al. Multiple organ failure in patients with cardiogenic shock is associated with high plasma levels of interleukin-6. Crit Care Med. 2002;30:1987–1994. doi: 10.1097/00003246-200209000-00007. [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis EJ, Bolanos N, Laoutaris G, Papadakis V, Koussoulas V, Perrea D, et al. Immunomodulatory intervention in sepsis by multidrug-resistant Pseudomonas aeruginosa with thalidomide: an experimental study. . BMC Infect Dis. 2005;5:51. doi: 10.1186/1471-2334-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia S, Benítez D, Núñez LE, Jewell CM, Langjahr P, Candia E, et al. Phosphatidylinositol 3-kinase interacts with the glucocorticoid receptor upon TLR2 activation. J Cell Mol Med. 2011;15:339–349. doi: 10.1111/j.1582-4934.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis B, Lüth A, Kleuser B. Dexamethasone protects human fibroblasts from apoptosis via an S1P3-receptor subtype dependent activation of PKB/Akt and Bcl XL. Pharmacol Res. 2010;61:449–459. doi: 10.1016/j.phrs.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Weichhart T, Säemann MD. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann Rheum Dis. 2008;67:iii70–74. doi: 10.1136/ard.2008.098459. [DOI] [PubMed] [Google Scholar]
- Polumuri SK, Toshchakov VY, Vogel SN. Role of phosphatidylinositol-3 kinase in transcriptional regulation of TLR-induced IL-12 and IL-10 by Fcγ receptor ligation in murine macrophages. J Immunol. 2007;179:236–246. doi: 10.4049/jimmunol.179.1.236. [DOI] [PubMed] [Google Scholar]
- Keck S, Freudenberg M, Huber M. Activation of murine macrophages via TLR2 and TLR4 is negatively regulated by a Lyn/PI3K module and promoted by SHIP1. J Immunol. 2010;184:5809–5818. doi: 10.4049/jimmunol.0901423. [DOI] [PubMed] [Google Scholar]
- Kee VR. Hemodynamic pharmacology of intravenous vasopressors. Crit Care Nurse. 2003;23:79–82. [PubMed] [Google Scholar]
- Gonzalez ER, Kannewurf BS, Hess ML.Inotropic therapy and the critically ill patient.In: Grenvik A, Ayres SM, Holbrook PR, Shoemaker WC, editors. 4th edn. Textbook of Critical Care. Philadelphia, PA; WB Saunders; 2000; 1123–1130. [Google Scholar]
- Zhang C, Bai N, Zhang Z, Liang N, Dong L, Xiang R, et al. TLR2 signaling subpathways regulate TLR9 signaling for the effective induction of IL-12 upon stimulation by heat-killed Brucella abortus. Cell Mol Immunol. 2012;9:324–333. doi: 10.1038/cmi.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]