Abstract
Considerable evidence indicates that type 1 T helper (Th1)- and Th17-mediated immune responses promote the formation of atherosclerotic plaques while that CD4+CD25+Foxp3+ regulatory T cells (Tregs) have a protective effect. However, the functions of diverse CD4+ lymphocyte subsets in plaque rupture remain poorly understood because of a shortage of satisfactory plaque rupture models. Here, we established a murine model of atherosclerotic plaque rupture using a high-fat diet and collar placement on the carotid artery, and triggered plaque rupture by short-term stimulation with a combination of lipopolysaccharide, phenylephrine injection and cold in apolipoprotein E-knockout (ApoE−/−) mice. We investigated the associations between Th1 cells, Th17 cells and Tregs and plaque rupture by PCR, flow cytometry, ELISA and immunohistochemistry. In total, 75% (18/24) of vulnerable plaques, but no stable plaques, showed rupture characteristics. The proportion of Th17 cells was increased among splenocytes after treatment, but the changes in the levels of Th1 cells and Tregs were not related to rupture. Furthermore, the treatment resulted in high levels of interleukin-17 (IL-17) in the serum and in the region of plaque rupture. In vitro, IL-17 increased the level of apoptosis, a major factor associated with plaque rupture, in cultured murine vascular smooth muscle cells. Th17 cells and IL-17 may be involved in the disruption of vulnerable plaques triggered by short-term stimulation with lipopolysaccharide, phenylephrine injection and cold in ApoE−/−mice.
Keywords: atherosclerosis, mice, plaque rupture, Th1, Th17
Introduction
Acute coronary syndrome (ACS) is a principal contributor to high mortality in many countries.1,2,3 Destabilized plaques often lead to sudden plaque rupture with thrombosis under acute stress conditions and are acknowledged as the major causes of ACS.4,5 It is known that inflammatory processes play key roles in the development of atherosclerotic lesions and ACS, but the potential mechanisms connecting inflammation with plaque rupture have not yet been elucidated.6,7
Accumulating evidence demonstrates that CD4+ T cells are associated with atherogenesis. Type 1 T helper (Th1) cells promote plaque formation, whereas regulatory T cells (Tregs) have a protective function. The role of Th2 cells in atherogenesis is still debatable.8,9,10,11 It has been found that a new CD4+ T subset, Th17 cells, which produce interleukin-17A (IL-17A), is implicated in the pathogenesis of atherosclerosis.12,13,14,15,16,17,18 IL-17, a major effector molecule for Th17 cells, has been detected in human atherosclerotic plaques.19,20,21 An elevated plasma level of IL-17 and an imbalanced ratio of Th17 to Tregs have been detected in ACS patients, suggesting an underlying effect of Th17 on plaque destabilization and possibly the occurrence of ACS.22 Although roles of different CD4+ T-cell subsets in plaque formation have been widely investigated, their effects on plaque rupture remain poorly understood.
Investigating the functions of CD4+ T-cell subsets in plaque rupture requires a suitable animal model of plaque rupture. Although apolipoprotein E-knockout (ApoE−/−) mice are commonly used as a plaque rupture model,23,24,25,26,27,28,29,30,31,32,33 this model is unsatisfactory due to the low rate of plaque rupture and the lack of evidence of atherosclerotic thrombosis. von der Thusen and colleagues found that phenylephrine administration significantly increased the plaque rupture rate in p53-transformed ApoE−/− mice and found one confirmed case of plaque rupture with thrombosis.30 More recently, a combination of stress induced by lipopolysaccharide (LPS) for 4–8 weeks after a high-fat diet and the placement of a perivascular collar greatly increased the rate of plaque rupture and thrombosis in carotid arteries.32 However, this model is still not ideal due to ethical problems (electric foot shock) and the lack of an exact time point for plaque rupture because of the long-term stimulation, which makes it difficult to analyze plaque rupture.
In the present study, we established a murine model of atherosclerotic plaque rupture using a high-fat diet and collar placement on the carotid artery in ApoE−/− mice. We triggered plaque rupture by short-term stimulation with the combination of treatment with LPS, the injection of phenylephrine and the exposure of mice to cold. We then investigated the roles of T-cell subsets in plaque rupture at 6 and 14 weeks after collar placement.
Materials and methods
Reagents and antibodies
Phenylephrine, ionomycin, phorbol myristate acetate and LPS were from Sigma-Aldrich (St Louis, MO, USA). The PE-Cy5-conjugated anti-mouse CD4 antibody, FITC-conjugated anti-mouse CD25 and interferon-γ (IFN-γ), were from BD Biosciences (San Jose, CA, USA). Brefeldin A (BFA). The Foxp3 and IL-17A monoclonal antibodies were from eBioscience (San Diego, CA, USA).
Animals and carotid collar placement
Male ApoE−/− mice (8 weeks old; 21–25 g) were purchased from Peking University (Beijing, China) and were housed in an environment with a 12-h dark cycle and a constant temperature (24 °C). Mice were divided into four groups: the control group, which was fed a regular diet (n=5); the high-fat diet group, which was fed a high-fat diet (0.25% cholesterol and 15% cocoa butter) (n=5) starting at 8 weeks of age; the collar placement group, in which a perivascular silica collar (3-mm long, 0.3-mm diameter) was placed on the left common carotid artery (average adventitial diameter of 0.5 mm)29 at 10 weeks (n=5); and the high-fat diet plus collar placement group (n=72). The animal studies were performed according to the Animal Management Rules of the Chinese Ministry of Health (document no. 55, 2001) and were authorized by the Animal Care and Utilization Committee of Shandong University.
Triggering plaque rupture
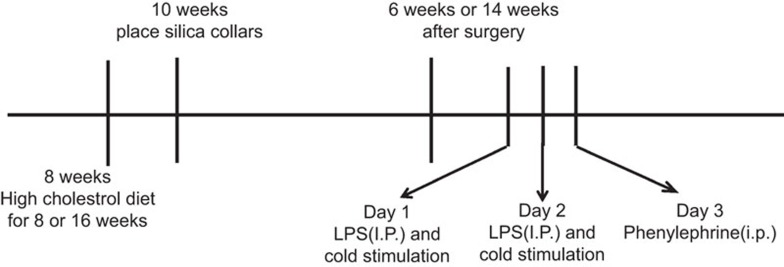
At 6 and 14 weeks after collar placement, ApoE−/− mice (n=72) in both the collar placement and high-fat diet groups were randomly divided into two groups: the control group, in which mice were intraperitoneally injected with 0.9% sodium chloride solution for 3 days without cold stimulation, and the treatment group, in which mice were stimulated with ice water (0 °C, not submerged) for 5–10 min/time/day and intraperitoneally injected with 1 mg/kg/day LPS for 2 days, followed by injection with 8 µg/kg phenylephrine in a volume of 0.2 ml phosphate-buffered saline on the third day (Figure 1).
Figure 1.
Schematic diagram of the trigger stimulation of atherosclerotic plaque rupture in ApoE−/− mice. Mice were fed a high-cholesterol diet from 8 weeks old, and atherosclerotic lesions in the left common carotid artery were induced at 10 weeks of age by the perivascular placement of a constrictive collar. At 6 and 14 weeks after collar placement, the mice in the interventional group were stimulated with ice water for 5–10 min daily and injected intraperitoneally with 1 mg/kg/day lipopolysaccharide for 2 days, followed by injection with 8 µg/kg phenylephrine on the third day to trigger plaque disruption. At 12 h after the last challenge, all mice were anesthetized, and plaque rupture was detected. ApoE−/−, apolipoprotein E-knockout; i.p., intraperitoneal injection; LPS, lipopolysaccharide.
Serum biological measurements
At the end of experiments, the mice were killed after being anesthetized by an intraperitoneal pentobarbital injection. Blood specimens were obtained by retroorbital bleeding. Serum was separated, and the levels of total cholesterol, triglycerides and high-density lipoprotein cholesterol in the serum were measured using enzymatic methods after the precipitation of ApoB. The level of low-density lipoprotein cholesterol was calculated using the Friedewald formula.
Histology and immunohistochemistry
Mice were perfused with phosphate-buffered saline and 4% polyformaldehyde under physiological pressure, and then the left common carotid arteries were removed. After the removal of the silica collar, each vessel was fixed in 4% polyformaldehyde overnight and embedded in OCT compound. Serial cryosections, 5 µm, were cut along the carotid artery proximal to the collar and were routinely stained with hematoxylin and eosin, Oil-red O for lipids, and Masson's trichrome for collagen. Corresponding sections on separate slides were stained with a rabbit polyclonal anti-alpha-smooth muscle actin antibody (anti-αSMC; Abcam, Cambridge, MA, USA) and a rabbit polyclonal anti-mouse anti-IL-17 antibody (H-132; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Histology staining was quantified using a Nikon microscope. The plaque size, cap area and core area were analyzed using Image-Pro Plus 6.0, and the cap-to-core area ratio was calculated. The initial point was the surface of the endothelial cells, and the endpoint was the side of the intima near the vascular wall.
ELISA
Heparinized blood specimens were separated by centrifugation, and the plasma was stored at −80 °C. The IL-17A concentration in the plasma was measured using an ELISA kit (eBioscience). The lower limit of detection was 4.0 pg/ml. Plasma samples were diluted with phosphate-buffered saline for calibration curve ranges. The assays involved 2 concentrations of cytokines in plasma for all steps. The average intra- and interassay coefficients of variation were always <6%.
Flow cytometry
Splenocytes were prepared from ApoE−/− mice after treatment and incubated with 25 ng/ml phorbol myristate acetate, 1 µg/ml ionomycin and 10 µg/ml BFA for 4 h at 37 °C under 5% CO2. The cells were washed with washing buffer and stained with a PE-Cy5-conjugated anti-CD4 antibody, fixed using a fixation and permeabilization kit (eBioscience), and then exposed to PE-conjugated anti-IL-17 and FITC-conjugated anti-IFN-γ antibodies. To analyze Tregs, cells were labeled with PE-Cy5-conjugated anti-CD4 and FITC-conjugated anti-CD25 antibodies, fixed and permeabilized with Foxp3 staining buffer (eBioscience), and then stained with an anti-Foxp3 antibody. Apoptosis was detected using an annexin V-FITC/propidium iodide apoptosis detection kit (Promega, Madison, WI, USA) and flow cytometry (Becton and Dickinson, Franklin Lakes NJ, USA). The acquisition and analysis of the flow cytometry data were performed with a FACSCalibur Flow Cytometer and CellQuest software (BD Biosciences, Mountain View, CA, USA) and a Cytomics FC500 system (Beckman Coulter, Brea, CA, USA).
Reverse transcription-polymerase chain reaction (RT-PCR) assay
Total RNA in the carotid arterial walls was extracted using a Trizol kit (Invitrogen, Carlsbad, CA, USA). The relative mRNA levels of RORγt and T-bet were analyzed by RT-PCR. The primer sequences for RORγt were sense, 5′- GCGGAGCAGACACACTTACA-3′, and antisense, 5′-TTGGCAAACTCCACCACATA-3′ (582 bp), and those for T-bet were sense, 5′-TTCCCATTC CTGTCCTTCAC- 3′ and antisense, 5′-CCTCTGGCTCTCCATCATTC-3′ (346 bp). The cycle conditions were 45 s at 94 °C, 30 s at 58 °C, and 30 s at 72 °C for 28 cycles. The RT-PCR products were analyzed by electrophoresis in 2% agarose gels, and the amount of transcript was quantified using a Scion Imager (Bio-Rad, Philadelphia, PA, USA).
Cell culture and treatment
The Movas cell line, a murine smooth muscle cell (SMC) line, was obtained from the Qi Lu Hospital of Shandong University (Jinan, China). These cells were maintained in culture as an adherent monolayer in DMEM (Gibco, Invitrogen) supplemented with 10% fetal bovine serum, 50 U/ml penicillin and 50 mg/ml streptomycin and incubated at 37 °C under 5% CO2. After the cells had attached to the culture plate, they were treated with 25, 50 or 100 µg/ml IL-17 for 24, 48 or 72 h.
Hoechst staining assay
Cell morphology was analyzed by fluorescence microscopy. Cells adhered to coverslips were fixed in cold methanol for 5 min, air dried, stained with Hoechst-33 258 (2.5 mg/ml in Milli-Q water, 2 min), and then examined under an Olympus BX61 microscope. Images were captured using an Olympus DP50 digital camera.
Statistical analysis
All analyses were performed with SPSS 11.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean±s.e.m. Nonparametric ANOVA and unpaired t tests were used for the comparison of continuous data. A P<0.05 was considered statistically significant.
Results
Model of atherosclerotic plaque rupture in ApoE−/− mice
To investigate the role of T cells in plaque rupture, we first established a murine ruptured plaque model by improving previous methods.10 We induced carotid atherosclerotic plaques in ApoE−/− mice by feeding the mice a high-fat diet starting at 8 weeks old and placing a collar on the carotid artery at 10 weeks old. Plaque vulnerability was estimated after 6 and 14 weeks by calculating the sizes of the plaque, necrotic core and fibrous cap, and ratio of the fibrous cap to the necrotic core. Body weight was lower in the treatment group than in the control groups at both time points (P<0.05). However, the control and treatment groups did not differ with respect to the serum lipid levels at 6 or 14 weeks (Table 1). The status of the mice after strong stimulation was similar to the clinical status of patients with ACS.32 In addition, mice in the high-fat diet group, the collar placement group and the control group had no plaques in the carotid arteries (Supplementary Figure 1).
Table 1. Basic characteristics and biochemical parameters of mice.
| Variables | 6 weeks after surgery | 14 weeks after surgery | ||
|---|---|---|---|---|
| Control (n=12) | Treatment (n=12) | Control (n=24) | Treatment (n=24) | |
| Body weight (g) | 25.52±0.53 | 23.84±0.46* | 32.12±0.71 | 29.98±0.55* |
| Total cholesterol (mmol/l) | 7.67±1.38 | 7.53±1.41 | 18.16±1.22 | 18.17±1.28 |
| Triglycerides (mmol/l) | 1.56±0.06 | 1.49±0.13 | 1.99±0.10 | 2.05±0.18 |
| LDL cholesterol (mmol/l) | 5.21±0.19 | 5.20±0.25 | 5.78±0.06 | 5.76±0.08 |
| HDL cholesterol (mmol/l) | 1.78±0.15 | 1.81±0.19 | 2.48±0.24 | 2.87±0.12 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Data are mean±s.d.
*P<0.05 vs control
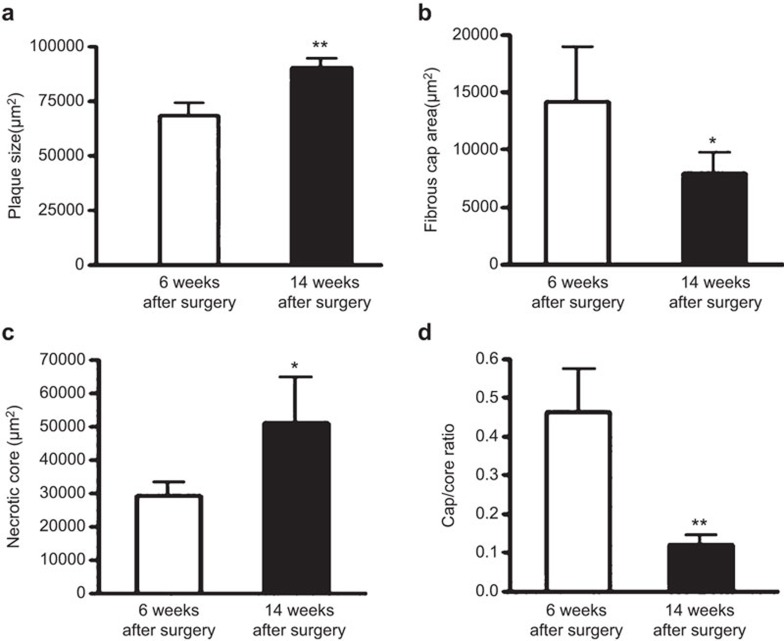
The sizes of the plaques and necrotic cores were significantly larger at 14 weeks than 6 weeks after collar placement (plaque size: 90,625±4,125 µm2 vs 68,750±6,250 µm2, P<0.01; necrotic core: 52,340±14,300 µm2 vs 29,883±3,890 µm2, P<0.05) (Figure 2). However, the fibrous cap area and the ratio of the fibrous cap to the necrotic core were smaller at 14 weeks than 6 weeks (fibrous cap area: 8,690±1,635 µm2 vs 13,850±4,900 µm2, P<0.05; ratio of the cap to the core: 0.12±0.034 vs 0.47±0.17, P<0.01). Therefore, the plaques present at 14 weeks had vulnerable features, suggesting that the plaques were prone to rupture.
Figure 2.
Plaque status in ApoE−/− mice at 6 and 14 weeks after collar placement. Plaque size (a), fibrous cap area (b), necrotic core area (c) and ratio of the cap to the core (d) in ApoE−/− at 6 and 14 weeks after collar placement (6 weeks, n=12; 14 weeks, n=24). Data are the mean±s.e.m. *P<0.05, **P<0.01. ApoE−/−, apolipoprotein E-knockout.
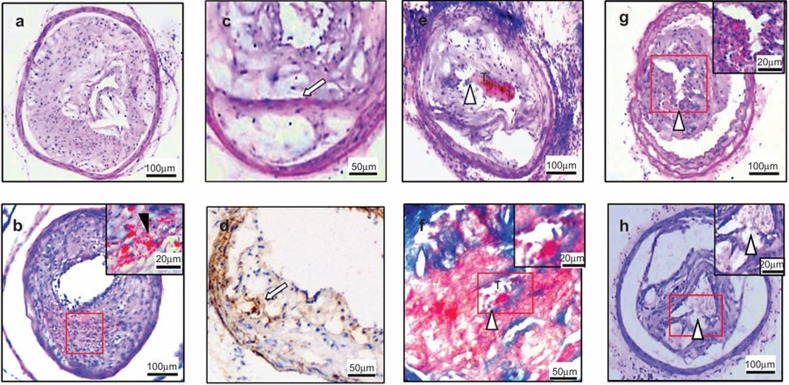
Next, we analyzed the effect of short-term stimulation with LPS, phenylephrine and cold on plaque rupture. At the end of 6 weeks after collar placement, plaques in the treatment group did not rupture, although 83.3% showed intraplaque hemorrhages (Figure 3, Table 2). However, at the end of 14 weeks, 45.8% of the treated mice (11/24) had cap breakage, 20.8% (5/24) had cap breakage with thrombosis, 8.3% (2/24) had cap breakage with core extrusion and 4.2% (1/24) had a buried fibrous cap. Only one mouse in the control group had a buried fibrous cap. Excluding the buried fibrous cap, 75% of the treated mice had ruptured plaques. Therefore, short-term strong stimulation effectively induced the disruption of vulnerable but not stable plaques, and thus this treatment procedure provides a model of atherosclerotic plaque rupture for the study of immunological mechanisms.
Figure 3.
Effect of strong combined stimulation on plaque rupture. (a) Intact fibrous cap in the control group. (b) Intraplaque hemorrhage without plaque rupture in plaques from treated mice. (c and d) Buried fibrous caps (white arrow) (c, HE staining; d, α-smooth actin immunostaining). (e and f) Fibrous cap disruption associated with luminal thrombus (white arrowheads show cracks in the fibrous caps, T shows the thrombus) (e, HE staining, f, Masson's trichrome staining). (g and h) Cap break with intraplaque hemorrhage. HE, hematoxylin and eosin.
Table 2. Histological evidence of plaque rupture.
| 6 weeks after surgery | 14 weeks after surgery | |||
|---|---|---|---|---|
| Control (n=12) | Treatment (n=12) | Control (n=24) | Treatment (n=24) | |
| IPH | 0 (0%) | 10 (83.3%) | 0 (0%) | 4 (16.7%) |
| Cap breakage±IPH | 0 (0%) | 0 (0%) | 0 (0%) | 11 (45.8%) |
| Cap breakage+thrombosis | 0 (0%) | 0 (0%) | 0 (0%) | 5 (20.8%) |
| Cap breakage+core extrusion | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8.3%) |
| Buried fibrous cap | 0 (0%) | 0 (0%) | 1 (4.2%) | 1 (4.2%) |
| Total plaque rupture | 0 (0%) | 0 (0%) | 0 (0%) | 18 (75%) |
Data are the number (%).
IPH: presence of intraplaque hemorrhage but no cracks in the neointima and no thrombus.
Cap breakage, presence of fibrous cap disruption.
Buried fibrous cap, presence of healing of a plaque rupture.
Total plaque rupture: presence of cap breakage with or without luminal thrombosis, intraplaque hemorrhage, and core extrusion.
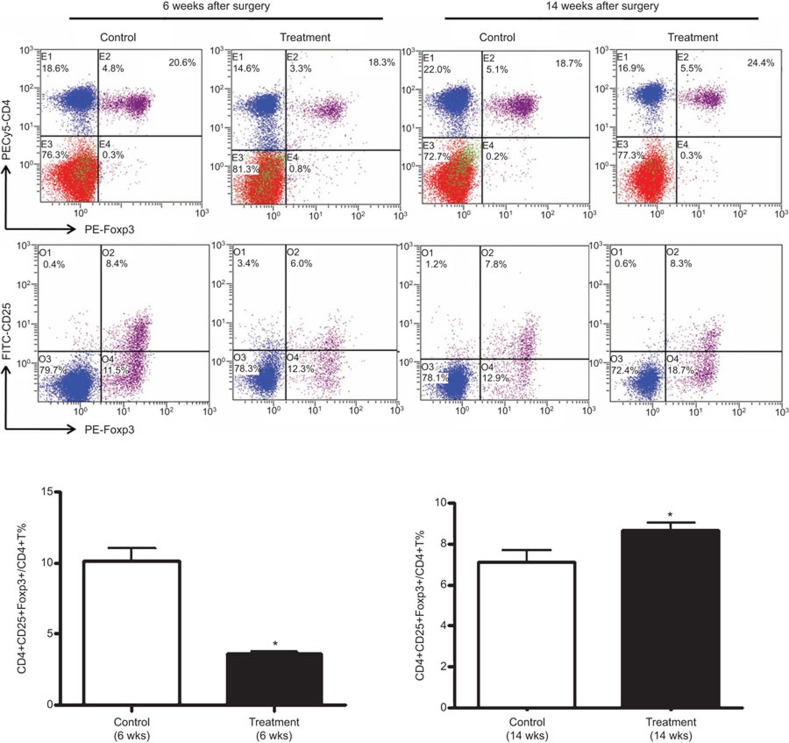
Combined treatment increased CD4+CD25+Foxp3+ Tregs with plaque rupture
To investigate whether CD4+ T-cell subsets are involved in plaque rupture in our model, we first analyzed the effect of combined stimulation on different CD4+ T-cell subsets. Combined stimulation reduced the percentage of CD4+CD25+Foxp3+ Tregs at 6 weeks after collar placement (no plaque rupture) but increased this percentage at 14 weeks (a high rate of rupture) compared with the percentages in the control mice (6 weeks: 4.27%±0.33% vs 10.38%±1.26%, P<0.05; 14 weeks: 8.97%±0.74% vs 7.42%±1.35%, P<0.05) (Figure 4). The change in the proportion of CD4+CD25+Foxp3+ Tregs did not induce plaque rupture but may have been induced by the plaque rupture.
Figure 4.
Tregs in ApoE−/− mice with or without stimulation. (a) Flow cytometry analysis of the proportion of Tregs in the splenocytes of ApoE−/− mice. (b) Quantification of Tregs in the spleens of ApoE−/− mice at 6 and 14 weeks after collar placement (n=5 mice in each group). Data are mean±s.e.m. *P<0.05, **P<0.01. ApoE−/−, apolipoprotein E-knockout; n.s., not significant; Treg, regulatory T cell.
Combined treatment increased IL-17 release from Th17 cells with high a plaque rupture rate
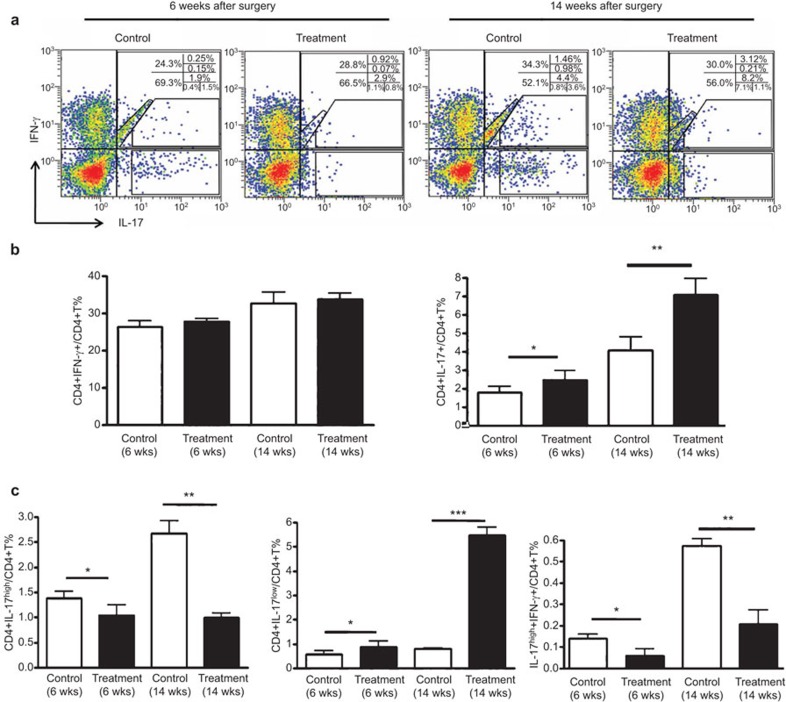
The associations of Th1 and Th17 cells with atherosclerotic plaque ruptures were also assessed. As shown in Figure 5a and b, the proportion of Th1 cells (IL-17−IFN-γ+ CD4+) did not differ between the control and treatment groups both at 6 weeks and 14 weeks after collar placement (6 weeks: 26.34%±0.41% vs 29.23%±0.24%, P>0.05; 14 weeks: 32.45%±1.33% vs 33.60%±0.86%, P>0.05). There were two populations of Th17 cells (IL-17+IFN-γ−CD4+), Th17high cells with a high fluorescence intensity and Th17low cells with a low fluorescence intensity (Figure 5a). Although the proportion of Th17low cells was significantly increased in the treatment group compared with the control groups (6 weeks: 0.84%±0.26% vs 0.53%±0.17%, P<0.05 and 14 weeks: 5.37%±0.72% vs 0.91%±0.04%, P<0.001), the percentage of Th17high cells was lower in the treatment group than in the control groups (6 weeks 1.04%±0.74% vs 1.46%±0.57%, P<0.05 and 14 weeks: 1.13%±0.38% vs 2.62%±1.25%, P<0.01) (Figure 5c). In addition, the proportion of IL-17high+IFN-γ+ double-positive CD4+ T cells was also significantly lower in the treatment group than in the control groups at both 6 and 14 weeks (6 weeks: 0.07%±0.04% vs 0.13%±0.02%, P<0.05 and 14 weeks: 0.21%±0.06% vs 0.57%±0.04%, P<0.01). These results suggest that pre-existing IL-17 in Th17 cells may be released and new Th17 produced soon after stimulation.
Figure 5.
Th1 and Th17 cell proportions in ApoE−/− splenocytes with or without stimulation. (a) Flow cytometry analysis of the proportions of Th1 and Th17 cells in ApoE−/− splenocytes. Splenocytes were incubated with phorbol myristate acetate (25 ng/ml), ionomycin (1 µg/ml) and the blocking reagent Brefeldin A (10 µg/ml) at 37 °C and 5% CO2 for 4 h and then assayed by flow cytometry to determine the number of CD4+IFN-γ+ (Th1) and CD4+IL-17+ (Th17) cells. (b) Quantification of Th1 and Th17 cells in splenocytes at 6 and 14 weeks after collar placement. (c) Quantification of Th17low, Th17high and IL-17high+IFN-γ+T cells in splenocytes at 6 and 14 weeks after collar placement. Data are mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001. ApoE−/−, apolipoprotein E-knockout; IFNγ, interferon-γ IL-17, interleukin-17.
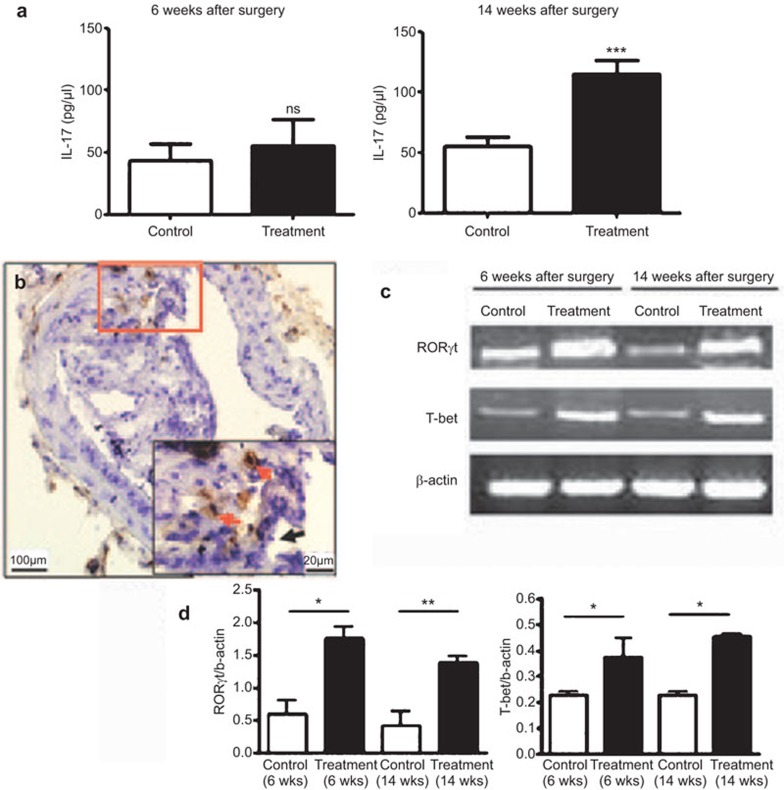
To confirm our hypothesis, we measured the serum level of IL-17 by ELISA. At 14 weeks, the level of IL-17 was higher in the treatment group than in the control groups after collar placement, with no difference at 6 weeks (Figure 6a). These results suggest that IL-17 is an important factor related to plaque rupture. To further determine the relationship between plaque rupture and Th17 and Th1 cells, we analyzed the levels of RORγt, a transcription factor controlling Th17 differentiation, and T-bet, the Th1 master transcription factor, by RT-PCR, and we determined the level of IL-17 in the carotid arterial walls by immunohistochemistry. The mRNA levels of RORγt and T-bet were high in the arterial walls of treated mice, and IL-17 expression was observed in the region around the ruptured plaques (Figure 6b–d). These results suggest that pro-inflammatory Th17 cells and IL-17 are involved in plaque rupture.
Figure 6.
Expression of IL-17 in mouse serum and the RORγt, T-bet and IL-17 levels in arterial walls. (a) Serum level of IL-17 (n=5 mice in each group). ***P<0.001. (b) Expression of IL-17 in the carotid vascular walls detected by immunohistochemical staining (red arrows show IL-17, black arrow shows cap break). (c) RT-PCR analysis of the mRNA levels of RORγt and T-bet in arterial walls. (d) Quantification (n=5 mice). Data are the mean±s.e.m. *P<0.05, **P<0.01. IL-17, interleukin-17; n.s., not significant; RT-PCR, reverse transcription polymerase chain reaction; Th17, type 17 T helper.
IL-17 increased the apoptosis of the cultured murine vascular SMCs
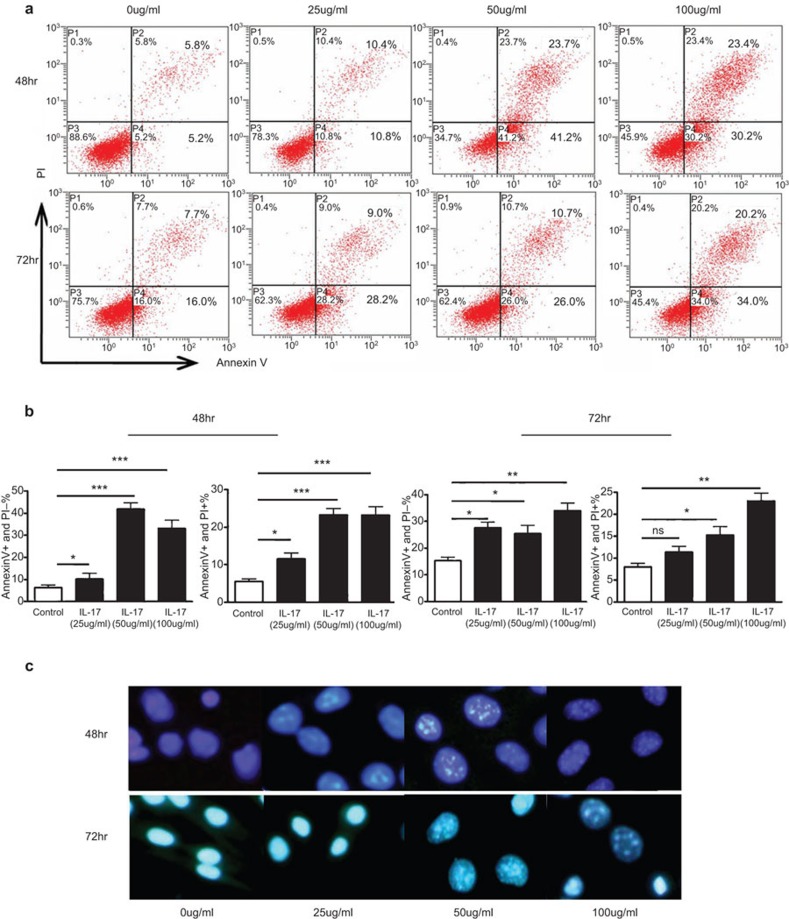
To explore the mechanism by which IL-17 increases the rupture of atherosclerotic plaques, we treated cultured murine vascular SMCs (Movas cells) with different concentrations of IL-17 for different times and analyzed cell apoptosis by flow cytometry. Cells treated with 50 or 100 µg/ml IL-17A for 48 or 72 h became shrunken and rounded, with condensed and broken nuclei (Figure 7c). Apoptotic bodies were also observed by fluorescence microscopy and with Hoechst 33258 staining. The number of apoptotic cells (annexin V+ P−) was markedly increased after treatment with 50 or 100 µg/ml IL-17A for 48 or 72 h (Figure 7a and b). In addition, IL-17 also promoted cell death (annexin V+ propidium iodide+) (Figure 7a and b). Therefore, IL-17 is able to promote the apoptosis and death of SMCs in vitro, suggesting that IL-17-induced apoptosis is involved in atherosclerotic plaque rupture.
Figure 7.
Apoptosis of Movas smooth muscle cells in the presence of different concentrations of IL-17 for different times. Movas cells were treated with IL-17 at 25, 50 or 100 µg/ml for 24, or and 72 h. (a) Flow cytometry analysis of the apoptosis of Movas cells treated with different concentrations of IL-17 for different times using annexin V/propidium iodide staining. (b) Quantification. (c) Apoptotic bodies visualized by Hoechst staining (control: n=5; IL-17: n=5). Data are mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001. IL-17, interleukin-17; n.s., not significant.
Discussion
The roles of different CD4+ T-cell subsets in the formation of atherosclerotic plaques are well characterized. However, their effects on plaque rupture are poorly understood because of the lack of a satisfactory murine plaque rupture model. To investigate the possible roles of CD4+ T-cell subsets in plaque rupture, we improved the previous methods.22,23,24,25,26,27,28,29,30,31,32,33 In our model, we addressed the intrinsic vulnerability and extrinsic forces that contribute to plaque rupture at the same time. Vulnerable plaques were induced with a high-fat diet and collar placement in ApoE−/− mice for 14 weeks. Short-term combination stimulation effectively disrupted vulnerable plaques (14 weeks after collar placement) but not stable plaques (6 weeks after collar placement) in mice, suggesting that the effective induction of plaque rupture depends on strong inducing and triggering factors and on the status of plaque.
ACS is an inflammatory disease that involves T-cell subsets.34 CD4+ T cells are involved in the development of atherosclerosis and also have important effects on plaque instability. These cells can convert a chronic disease into a serious acute disorder with ensuing thromboembolism.35 CD4+ T cells are the prominent T lymphocyte subset in atherosclerotic plaques and are primarily localized at the sites of plaque rupture.36 Immunodeficient scid/scid mice with CD4+ T cells have accelerated disease, and thus, CD4+ T cells may have a pathogenic role in atherosclerosis.37 Activated CD4+ T cells are primarily Th1 cells. Patients with coronary heart disease with a predominant Th1 response have been found to have enhanced systemic inflammatory activity, suggesting that Th1 cells have an important role in plaque rupture.38,39,40 In our model, although the Th1 cells proportion among splenocytes from ApoE−/− mice did not differ significantly between mice with ruptured plaques and those without ruptured plaques, the expression of T-bet, the Th1 cytokine, in the arterial wall was enhanced significantly in both vulnerable and ruptured plaques. These data suggest that combined stimulation may increase the level of inflammation in plaques and induce the formation of vulnerable plaques but does not exacerbate the systemic Th1 response.
Different subsets of CD4+ T cells have different functions in different stages of atherosclerosis. Tregs are implicated in the maintenance of self-tolerance and the control of autoimmunity.41 Patients with ACS have lower numbers of peripheral Tregs and decreased levels of IL-10, TGF-β1 and Foxp3, compared with patients with stable angina (SA) and controls,21 and the reduction in the number of Tregs is in line with the expansion of Th1 cells in patients with ACS. The opposite pattern of Tregs and Th1 cells might contribute to plaque destabilization.42,43,44,45 The proportion of CD4+CD25+Foxp3+ Tregs decreased significantly in vulnerable plaques (at 6 weeks) in our model, reflecting the exacerbation of the systemic inflammatory response. However, the proportion of Tregs was elevated with plaque rupture, and this response may be a protective mechanism when the systemic inflammatory response increases to a certain degree.
IL-17 has been linked to many autoimmune and inflammatory diseases.12,18 Patients with ACS have been found to exhibit a significant increase in number of peripheral Th17 cells and significant increases in the levels of Th17-related cytokines, such as IL-17, IL-6 and IL-23, and in the level of a transcription factor (RORγt), compared with patients with SA and controls.21 The research performed by our group and others has demonstrated that Th17 cells aggravate the development of atherosclerosis.17 However, the role of these factors in plaque rupture remains to be investigated. We found an increased proportion of Th17 cells in ApoE−/− mouse splenocytes and the upregulated expression of IL-17 in the serum and plaque lesions of mice with disrupted plaques. Th17 cells and IL-17 may be related to atherosclerotic plaque rupture.
Increasing evidence both in vitro and in vivo indicates that the apoptosis of SMCs in the fibrous cap is a major factor in plaque rupture. Cell apoptosis directly affects the structure of the atherosclerotic plaque, reduces plaque stability and leads to acute clinical events.46,47,48 Our previous research demonstrated that IL-17 can induce the apoptosis of endothelial cells49 and decrease the expression of SMCs in lesions in IL-17-interfered ApoE−/− mice during the formation of plaques.17 Therefore, we assessed the impact of IL-17 on SMC apoptosis. Consistent with previous findings, IL-17 could stimulate the apoptosis and necrosis of SMCs, suggesting that IL-17-induced VSMC apoptosis is a potential mechanism for atherosclerotic plaque rupture.
In conclusion, Th17 and IL-17 may be involved in the disruption of vulnerable plaques triggered by short-term combination stimulation in ApoE−/− mice. One of the potential mechanisms of atherosclerotic plaque rupture is the induction of VSMC apoptosis.
Acknowledgments
Our study was supported by the National ‘973' Program of China (2011CB503900), the National Natural Science Foundation of China (30628015, 30700729, and 30872309) and Natural Science foundation of Shandong (Z2008C02).
Supplementary Information
References
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc. 2009;84:917–938. doi: 10.4065/84.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part II. Mayo Clin Proc. 2009;84:1021–1036. doi: 10.1016/S0025-6196(11)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atheroscleroticlesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- Zaman AG, Helft G, Worthley SG, Badimon JJ. The role of plaque rupture and thrombosis in coronary artery disease. Atherosclerosis. 2000;149:251–266. doi: 10.1016/s0021-9150(99)00479-7. [DOI] [PubMed] [Google Scholar]
- Rekhter MD. How to evaluate plaque vulnerability in animal models of atherosclerosis. Cardiovasc Res. 2002;54:36–41. doi: 10.1016/s0008-6363(01)00537-5. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- Hosono M, de Boer OJ, van der Wal AC, van der Loos CM, Teeling P, Piek JJ, et al. Increased expression of T cell activation markers (CD25, CD26, CD40L and CD69) in atherectomy specimens of patients with unstable angina and acute myocardial infarction. Atherosclerosis. 2003;168:73–80. doi: 10.1016/s0021-9150(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Wigren M, Nisson J, Kolbus D. Lymphocytes in atherosclerosis. Clin Chim Acta. 2012;413:1562–1568. doi: 10.1016/j.cca.2012.04.031. [DOI] [PubMed] [Google Scholar]
- Vinson A, Curran JE, Johnson MP, Dyer TD, Moses EK, Blangero J, et al. Genetical genomics of Th1 and Th2 immune response in a baboon model of atherosclerosis risk factors. Atherosclerosis. 2011;217:387–394. doi: 10.1016/j.atherosclerosis.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun Rev. 2007;6:169–175. doi: 10.1016/j.autrev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Xie JJ, Wang J, Tang TT, Chen J, Gao XL, Yuan J, et al. The Th17/Treg functional imbalance during atherogenesis in ApoE−/− mice. Cytokine. 2010;49:185–193. doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T (H) 17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820–5827. doi: 10.4049/jimmunol.1000116. [DOI] [PubMed] [Google Scholar]
- Afzali GL, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Ma Y, Du Y, Liao M, Li H, Liang W, et al. The altered expression of inflammation-related microRNAs with microRNA-155 expression correlates with Th17 differentiation in patients with acute coronary syndrome. Cell Mol Immunol. 2011;8:486–495. doi: 10.1038/cmi.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wang Y, Chen K, Zhou Q, Wei W, Wang Y, et al. The role of oxidized low-density lipoprotein in breaking peripheral Th17/Treg balance in patients with acute coronary syndrome. Biochem Biophys Res Commun. 2010;394:836–842. doi: 10.1016/j.bbrc.2010.03.090. [DOI] [PubMed] [Google Scholar]
- Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122:70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Calara F, Silvestre M, Casanada F, Yuan N, Napoli C, Palinski W. Spontaneous plaque rupture and secondary thrombosis in apolipoprotein E-deficient and LDL receptor-deficient mice. J Pathol. 2001;195:257–263. doi: 10.1002/path.915. [DOI] [PubMed] [Google Scholar]
- Hu W, Polinsky P, Sadoun E, Rosenfeld ME, Schwartz SM. Atherosclerotic lesions in the common coronary arteries of ApoE knockout mice. Cardiovasc Pathol. 2005;14:120–125. doi: 10.1016/j.carpath.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Jackson CL. Atherosclerotic plaque rupture in the apolipoprotein E knockout mouse. Atherosclerosis. 2001;154:399–406. doi: 10.1016/s0021-9150(00)00515-3. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate arteryof the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- Williams H, Johnson JL, Carson KG, Jackson CL. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:788–792. doi: 10.1161/01.atv.0000014587.66321.b4. [DOI] [PubMed] [Google Scholar]
- Johnson J, Carson K, Williams H, Karanam S, Newby A, Angelini G, et al. Plaque rupture after short periods of fat feeding in the apolipoprotein E-knockout mouse: model characterization and effects of pravastatin treatment. Circulation. 2005;111:1422–1430. doi: 10.1161/01.CIR.0000158435.98035.8D. [DOI] [PubMed] [Google Scholar]
- von der Thüsen JH, van Berkel TJ, Biessen EA. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation. 2001;103:1164–1170. doi: 10.1161/01.cir.103.8.1164. [DOI] [PubMed] [Google Scholar]
- von der Thusen JH, van Vlijmen BJ, Hoeben RC, Kockx MM, Havekes LM, van Berkel TJ, et al. Induction of atherosclerotic plaque rupture in apolipoprotein E−/− mice after adenovirus-mediated transfer of p53. Circulation. 2002;105:2064–2070. doi: 10.1161/01.cir.0000015502.97828.93. [DOI] [PubMed] [Google Scholar]
- Ni M, Wang Y, Zhang M, Zhang PF, Ding SF, Liu CX, et al. Atherosclerotic plaque disruption induced by stress and lipopolysaccharide in apolipoprotein E knockout mice. Am J Physiol Heart Circ Physiol. 2009;296:H1598–H1606. doi: 10.1152/ajpheart.01202.2008. [DOI] [PubMed] [Google Scholar]
- Ni M, Chen WQ, Zhang Y. Animal models and potential mechanisms of plaque destabilization and disruption. Heart. 2009;95:1393–1398. doi: 10.1136/hrt.2008.143461. [DOI] [PubMed] [Google Scholar]
- Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41:15S–22S. doi: 10.1016/s0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- Anogeianaki A, Angelucci D, Cianchetti E, D'Alessandro M, Maccauro G, Saqqini A, et al. Atherosclerosis: a classic inflammatory disease. Int J Immunopathol Pharmacol. 2011;24:817–825. doi: 10.1177/039463201102400401. [DOI] [PubMed] [Google Scholar]
- Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37:1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- Roselaar SE, Kakkanathu PX, Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoE−/− and LDL receptor−/− mice. Decreasing density with disease progression. Arterioscler Thromb Vasc Biol. 1996;16:1013–1018. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- Fernandes JL, Mamoni RL, Orford JL, Garcia C, Selwyn AP, Coelho OR, et al. Increased Th1 activity in patients with coronary artery disease. Cytokine. 2004;26:131–137. doi: 10.1016/j.cyto.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse. Arterioscler Thromb Vasc Biol. 2006;26:2421–2432. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Han SF, Liu P, Zhang W, Bu L, Shen M, Li H, et al. The opposite-direction modulation of CD4+CD25+ Tregs and T helper 1 cells in acute coronary syndromes. Clin Immunol. 2007;124:90–97. doi: 10.1016/j.clim.2007.03.546. [DOI] [PubMed] [Google Scholar]
- Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- Foks AC, Frodermann V, ter Borg M, Habets KL, Bot I, Zhao Y, et al. Differential effects of regulatory T cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. doi: 10.1016/j.atherosclerosis.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamaté C, Merval R, et al. Inhibition of transforming growth factor-b signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Narula J, Haider N, Virmani R. Apoptosis in atherosclerosis. Does it contribute to plaque instability. Cardiol Clin. 2001;19:127–139. doi: 10.1016/s0733-8651(05)70199-5. [DOI] [PubMed] [Google Scholar]
- Kockx M. Apoptosis in the atherosclerotic plaque. Aeterioscler Thromb Vasc Biol. 1998;18:1519–1522. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J Exp Med. 2006;203:239–250. doi: 10.1084/jem.20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Wang Q, Guo C, Wang X, Cao X, Zhang L, et al. IL-17 induces apoptosis of vascular endothelial cells: a potential mechanism for human acute coronary syndrome. Clin Immunol. 2011;141:152–160. doi: 10.1016/j.clim.2011.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.